Abstract
The aim of the present study was to investigate the effect of resveratrol on renal carcinoma cells and explore possible renin-angiotensin system-associated mechanisms. Subsequent to resveratrol treatment, the cell viability, apoptosis rate, cytotoxicity levels, caspase 3/7 activity and the levels of angiotensin II (AngII), AngII type 1 receptor (AT1R), vascular endothelial growth factor (VEGF) and cyclooxygenase-2 (COX-2) were evaluated in renal carcinoma cells. The effects of AngII, AT1R, VEGF and COX-2 on resveratrol-induced cell growth inhibition and apoptosis were also examined. The results indicated that resveratrol treatment may suppress growth, induce apoptosis, and decrease AngII, AT1R, VEGF and COX-2 levels in renal carcinoma ACHN and A498 cells. In addition, resveratrol-induced cell growth suppression and apoptosis were reversed when co-culturing with AT1R or VEGF. Thus, resveratrol may suppress renal carcinoma cell proliferation and induce apoptosis via an AT1R/VEGF pathway.
Keywords: resveratrol, renal carcinoma cells, cell proliferation, apoptosis, renin-angiotensin system
Introduction
Resveratrol (molecular formula, C14H12O3; CAS number, 501–36-0) is a non-toxic phytoalexin antioxidant and an effective anticancer compound that can be extracted from grapes, red wines, berries and peanuts (1), which imparts cancer chemopreventive and therapeutic responses (2,3). It is suggested to have potent antitumor properties against numerous human cancers (4,5). The renin-angiotensin system (RAS) is classically described as an important endocrine system that regulates blood pressure and electrolyte balance. Studies have demonstrated that RAS may be involved in numerous pathophysiological processes, such as maintaining blood pressure/blood volume homeostasis and ion-fluid balance, phylogenetic advancement, growth promotion and angiogenesis, ontogeny and phylogeny (6). RAS is also involved in several cancers, including glioblastoma multiform (7), and bladder (8) and renal (9) cancers. Previous studies have suggested that resveratrol may play anticancer roles via a RAS-dependent pathway, such as in renal (10) and bladder (8) cancer cells. Angiotensin II (AngII), which is known to be the main effector of the RAS pathway, has an important role in regulating cancer inflammation and tumor angiogenesis (11). The majority of AngII effects are mediated by 2 specific receptors subtypes, consisting of AngII type 1 receptor (AT1R) and AngII type 2 receptor (AT2R) (12), which are detected in astrocytomas (13), esophageal squamous cell carcinoma (14) and renal clear cell carcinoma (15). AT1R-mediated AngII activity plays a central role in mediating the majority of the actions of the RAS pathway (16). Vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) are important molecules in tumor-associated angiogenesis, as they activate endothelial cell metastasis and increase vascular permeability (17–19). Inhibition of VEGF suppresses tumor angiogenesis and tumor growth in vivo (20). As an important downstream regulator in RAS, VEGF is reported to be induced by AngII, by binding AT1R in pancreatic cancer cells (21). Cyclooxygenase-2 (COX-2) is a key enzyme involved in carcinogenesis and tumor progression, particularly in neoangiogenesis and lymphovascular invasion (22–24), and has also been detected as upregulated through mechanisms distinct from the VEGF axis (25–27). In the present study, the suppression of proliferation and induction of apoptosis by resveratrol was investigated in renal carcinoma cell lines. The levels of the 4 important factors in RAS, consisting of AngII, AT1R, VEGF and COX-2 were examined, and the potential mechanisms were analyzed.
Materials and methods
Cell culture and resveratrol treatment
Human renal carcinoma ACHN and A498 cell lines were purchased from Guangzhou Jennio Biological Technology Co., Ltd (Guangzhou, China). ACHN cells were cultured in high glucose Dulbecco's modified Eagle medium (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.). A498 cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v) FBS. The two cell lines were cultured at 37°C in a 5% humidified CO2 atmosphere. Resveratrol was purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). Resveratrol was dissolved in DMSO to create a stock solution at a concentration of 100 mM, which was subsequently diluted in culture medium to the desired concentration for experiments. DMSO was used as the vehicle control.
Enzyme-linked immunosorbent assay (ELISA)
Subsequent to ACHN and A498 cells being treated with resveratrol, the medium was collected by centrifugation at 400 × g for 15 min at 4°C, and the liquid supernatant was stored at −80°C until ELISA. ACHN and A498 cells were lysed with sodium dodecyl sulfate (SDS) lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China), and total proteins were extracted at 4°C. The concentrations of AngII, AT1R, VEGF and COX-2 in the culture medium and cells were determined using a human AngII ELISA kit (catalogue no., ml003766; Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China), an AT1R ELISA kit (catalogue no., ml006788; Shanghai Enzyme-linked Biotechnology Co., Ltd.), a VEGF ELISA kit (catalogue no., ml009877; Shanghai Enzyme-linked Biotechnology Co., Ltd.) and a COX-2 ELISA Kit (catalogue no., ml006532; Shanghai Enzyme-linked Biotechnology Co., Ltd.) in accordance with the manufacturers' protocols.
Cell proliferation assay
Effects of resveratrol treatment on the cell proliferation of renal carcinoma cells were detected by Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Mashikimachi, Kumamoto, Japan) assay. ACHN and A498 cells were seeded in 96-well plates (Corning Incorporated, Corning, NY, USA) at a density of 5×103 cells per well with 100 µl culture medium. Subsequent to allowing 24 h for adhering, resveratrol was added at varying concentrations for 12, 24 and 48 h. The culture medium was then removed and replaced with 100 µl medium containing CCK-8 reagent (10 µl; Dojindo Molecular Technologies, Inc.) in each well. The plates were incubated at 37°C for 2 h. Absorbance at 450 nm was recorded using a spectrophotometer (EnSpire 2300 Multilabel Reader; PerkinElmer, Waltham, MA, USA).
Apoptosis detection
Apoptosis cells were quantified using a fluorescein isothiocyanate Annexin V Apoptosis Detection Kit (BD Pharmingen, San Diego, CA, USA) according to the manufacturer's protocol. Cells were cultured in 6-well plates at a density of 1×105 cells per well. Following 24 h growth, cells were treated with various resveratrol concentrations and harvested for the apoptosis assay. Untreated cells were used as a negative control.
Colony formation assay
Cells (1×103 cells per well) were seeded into 6-well plates subsequent to 6 h-treatment with various concentrations of resveratrol and were cultured for 2 weeks. The number of colonies formed was counted subsequent to cells being fixed with 4% paraformaldehyde, and stained with a crystal violet staining solution (Beyotime Institute of Biotechnology).
Caspase-Glo 3/7 assays
Cells (5×103 cells per well) were seeded onto 96-well plates and exposed to different concentrations of resveratrol. Equal volume of Caspase-Glo 3/7 reagent was subsequently added into each well and incubated for 30 min at room temperature in the dark. The luminescence was determined using a luminometer (Berthold Sirius L; Titertek-Berthold, Pforzheim, Germany).
Cytotoxicity assay
The cytotoxicity of resveratrol was assessed using a lactate dehydrogenase (LDH) Cytotoxicity Assay kit (Beyotime Institute of Biotechnology) according to the manufacturer's protocol. Cells were cultured in 96-well plates (Corning Incorporated) and then treated with resveratrol for 24 h. The medium was collected by centrifugation at 400 × g for 5 min. Supernatant (120 µl/well) was transferred into another 96-well plate and 60 µl LDH detection reagent was added to each well, and then incubated for 30 min at room temperature in the dark. Absorbance was recorded at 490 nm with a spectrophotometer (EnSpire 2300 Multilabel Reader; PerkinElmer).
Western blot analysis
The expression of the apoptosis-associated proteins caspase 9, B-cell lymphoma 2 (Bcl-2) and Bcl-2-like protein 4 (Bax) were detected in renal carcinoma cells. Cells were lysed with radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology) and then total proteins were extracted at 4°C. Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). Membranes were then blocked using 5% non-fat milk in Tris-buffered saline and Tween 20 (TBS-T) at room temperature for 1 h, and the membranes were then probed with rabbit anti-human caspase 9 (catalog no., 9502; dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), Bax (catalog no., 2772; dilution, 1:1,000; Cell Signaling Technology, Inc.) and Bcl-2 rabbit monoclonal antibodies (catalog no., 2876; dilution, 1:1000; Cell Signaling Technology, Inc.). The membranes were probed with a rabbit anti-human GAPDH polyclonal antibody (catalog no., ab37168; dilution, 1:100,000; Abcam, Cambridge, UK) as a loading control. The membranes were washed 3 times with TBS-T for 5 min each time and incubated for 1.5 h with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (catalog no., E030120; dilution, 1:10,000; EarthOx Life Sciences, Millbrae, CA, USA).
Statistical analysis
ELISA, colony formation and Caspase-Glo 3/7 assays were repeated 3 times; CCK-8 and cytotoxicity assays were performed 4 times. One-way analysis of variance (SPSS 18.0; SPSS, Inc., Chicago, IL, USA) and Student's t-test (Microsoft Excel; Redmond, WA, USA) were used to evaluate the differences between 2 groups of data in all the experiments. All data were presented as the mean ± standard deviation (SD). P<0.05 was considered to indicate a statistically significant difference and P<0.01 was considered to indicate an extremely significant difference.
Results
Resveratrol suppressed growth of renal carcinoma cells
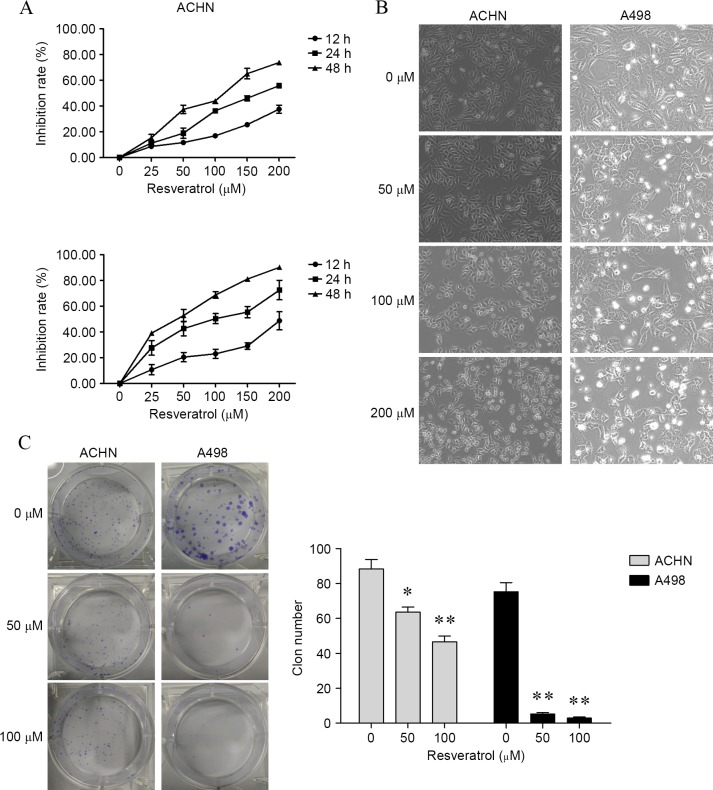
To determine the effect of resveratrol on cell proliferation in renal carcinoma cells, the present study firstly determined cell viability by CCK-8 assay. As shown in Fig. 1A, renal carcinoma cells treated with 25, 50, 100, 150 and 200 resveratrol for 12, 24 and 48 h, cell viability was significantly inhibited in a dose- and time-dependent manner compared with cells treated with 0 µM resveratrol [ACHN cells, P<0.001 for all concentrations at 12, 24 and 48 h; A498 cells, P<0.001 for all concentrations at 12, 24 and 48 h, with the exception of 25 µM for 12 h (P=0.0017)]. For morphology analysis, untreated renal carcinoma cells grew well, whereas the cells treated with resveratrol were distorted in shape and became round and underwent apoptosis (Fig. 1B). Furthermore, following 6 h of treatment with resveratrol, colony formation assay was performed and revealed a marked decrease in colony formation compared to the control group (ACHN cells, P=0.0158 by 50 µM and P=0.0026 by 100 µM; A498 cells, P=0.0002 by 50 µM and P=0.0002 by 100 µM; Fig. 1C).
Figure 1.
Resveratrol suppressed renal carcinoma cell proliferation. (A) Cell growth, as detected by Cell Counting Kit-8 assay, was suppressed in a dose- and time-dependent manner subsequent to resveratrol treatment. (B) Resveratrol inhibited proliferation in ACHN and A498 cells at 24 h, which was visualized by microscopy (magnification, ×100). (C) Representative pictures and quantification of resveratrol-induced colony formation suppression in renal carcinoma cells followed 6 h resveratrol treatment. *P<0.05 and **P<0.01, experimental vs. control groups.
Resveratrol induced apoptosis in renal carcinoma cells
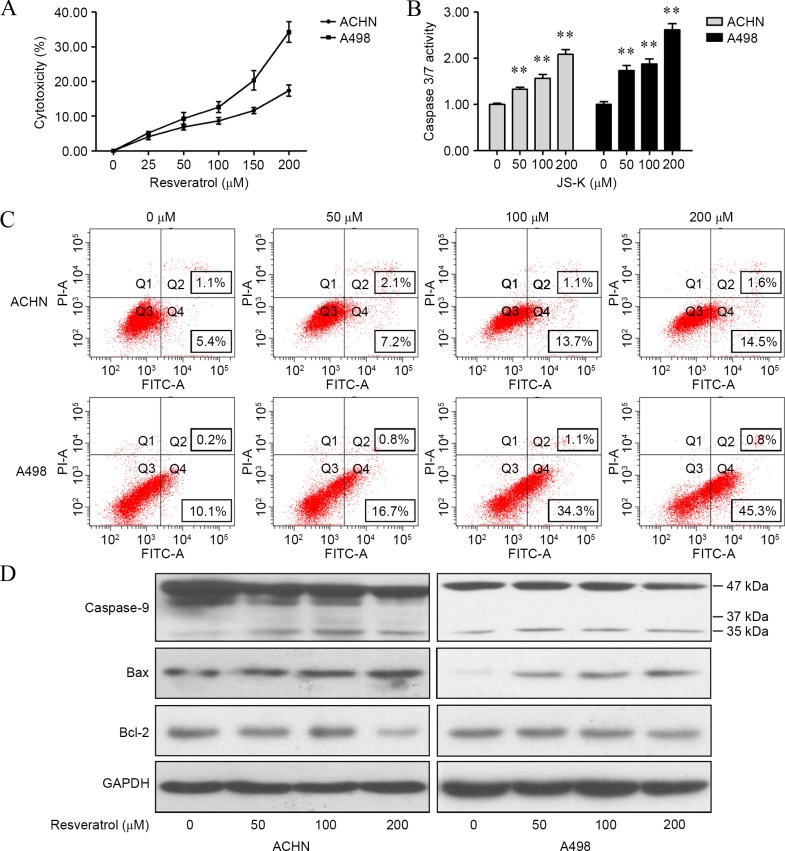
To illustrate the functions of resveratrol on apoptosis in renal carcinoma cells, cytotoxicity, caspase 3/7 activity, cell apoptosis and expression of caspase 9, Bcl-2 and Bax were determined. It was found that resveratrol significantly increased cytotoxicity (ACHN cells: 25 µM, P<0.001; 50 µM, P=0.0036; 100 µM, P=0.0314; 150 µM, P=0.0034; and 200 µM, P=0.0007; A498 cells: 25 µM, P<0.001; 50 µM, P=0.0041; 100 µM, P=0.0327; 150 µM, P=0.0032; and 200 µM P=0.0005; Fig. 2A) and caspase 3/7 activity (ACHN cells, P<0.001 for 50, 100 and 200 µM; A498 cells, P<0.001 for 50, 100 and 200 µM; Fig. 2B) in renal cancer cells compared with cells treated with 0 µM resveratrol. Results of flow cytometry assay revealed that subsequent to 24 h of treatment with resveratrol, apoptosis was induced in renal carcinoma cells (Fig. 2C). In addition, resveratrol was shown to upregulate cleaved-caspase 9 and Bax levels and downregulate Bcl-2 level in the two cell lines (Fig. 2D).
Figure 2.
Resveratrol induced apoptosis in renal carcinoma cells. (A) Cytotoxicity of resveratrol was determined by a lactate dehydrogenase assay. (B) Caspase 3/7 activity subsequent to resveratrol treatment was determined by a Caspase-Glo 3/7 assay. (C) Resveratrol-induced apoptosis in renal carcinoma cells with different concentrations (0, 50, 100 and 200 µM) for 24 h, which was analyzed by flow cytometry. (D) Cells were treated with resveratrol (0, 50, 100 and 200 µM) for 24 h, and the levels of caspase 9, Bax and Bcl-2 were detected by western blotting. The data showed that resveratrol regulated apoptotic proteins in renal carcinoma cells in a dose-dependent manner. **P<0.01, experimental vs. control groups. JS-K, O2-(2,4-dinitrophenyl)1-[(4-ethoxyxarbonyl) piperazin-1-yl]diazen-1-ium-1,2-diolate; FITC, fluorescein isothiocyanate; Bcl-2, B-cell lymphoma 2; Bax, bcl-2-like protein 4; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Resveratrol suppressed AngII, AT1R, VEGF and COX-2 in a dose-dependent manner
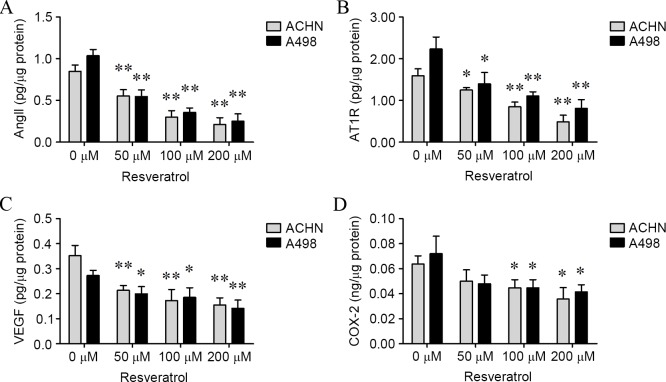
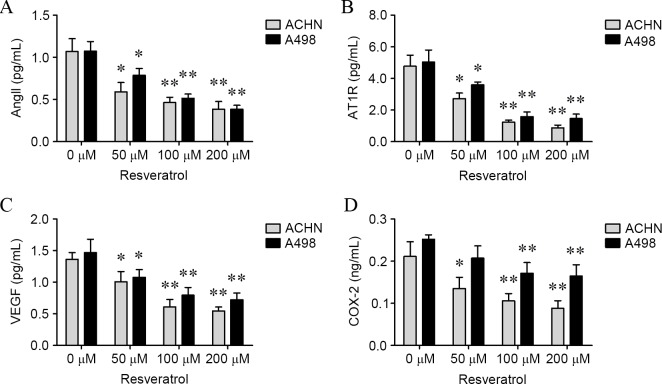
To confirm whether AngII, AT1R, VEGF and COX-2 levels would be affected by resveratrol, ACHN and A498 cells were treated with various concentrations of resveratrol for 24 h, and the levels of AngII, AT1R, VEGF and COX-2 in cells and culture medium were determined. As shown in Figs. 3 and 4, levels of AngII (ACHN cells: 50 µM, P=0.0086; 100 µM, P<0.001; and 200 µM, P<0.001; A498 cells: 50 µM, P=0.0015; 100 µM, P=0.0002; and 200 µM, P=0.0003; culture medium of ACHN cells: 50 µM, P=0.0113; 100 µM, P=0.0031; and 200 µM, P=0.0026; culture medium for A498 cells: 50 µM, P=0.0246; 100 µM, P=0.0015 and 200 µM, P=0.0007; Figs. 3A and 4A), AT1R (ACHN cells: 50 µM, P=0.0317; 100 µM, P=0.0033; and 200 µM, P=0.0013; A498 cells: 50 µM, P=0.0221; 100 µM, P=0.0031; and 200 µM, P=0.0023; culture medium for ACHN cells: 50 µM, 0.0110; 100 µM, 0.0011; and 200 µM, 0.0008; culture medium for A498 cells: 50 µM, P=0.0340; 100 µM, P=0.0019; and 200 µM, P=0.0016; Figs. 3B and 4B), VEGF (ACHN cells: 50 µM, P=0.0062; 100 µM, P=0.0068; and 200 µM, P=0.0024; A498 cells: 50 µM, P=0.0221; 100 µM, P=0.0259; and 200 µM, 0.0045; culture medium for ACHN cells: 50 µM, P=0.0341; 100 µM, P=0.0012; and 200 µM, P=0.0003; culture medium for A498 cells: 100 µM, 0.0090; and 200 µM, 0.0056; Figs. 3C and 4C) and COX-2 (ACHN cells: 100 µM, 0.0246; and 200 µM, 0.0138; A498 cells: 100 µM, 0.0398; and 200 µM, 0.0259; culture medium for ACHN cells: 50 µM, 0.0406; 100 µM, 0.0097; and 200 µM, 0.0056; culture medium for A498 cells: 100 µM, 0.0065; and 200 µM, 0.0061; Figs. 3D and 4D) were significantly decreased in the two cell lines and culture mediums in a dose-dependent manner compared with cells treated with 0 µM resveratrol.
Figure 3.
Resveratrol suppressed intracellular AngII, AT1R, VEGF and COX-2 levels in a dose-dependent manner. Subsequent to renal carcinoma cells being treated with resveratrol (0, 50, 100 and 200 µM) for 24 h, the intracellular levels of (A) AngII, (B) AT1R, (C) VEGF and (D) COX-2 were determined. *P<0.05 and **P<0.01, experimental vs. control groups. AngII, angiotensin II; AT1R, AngII type 1 receptor; VEGF, vascular endothelial growth factor; COX-2, cyclooxygenase-2.
Figure 4.
Resveratrol decreased AngII, AT1R, VEGF and COX-2 levels in culture medium for renal carcinoma cells. The levels of (A) AngII, (B) AT1R, (C) VEGF and (D) COX-2 in the culture medium for renal carcinoma cells subsequent to being cultured for 24 h were determined using enzyme linked immunosorbent assays. *P<0.05 and **P<0.01, experimental vs. control groups. AngII, angiotensin II; AT1R, AngII type 1 receptor; VEGF, vascular endothelial growth factor; COX-2, cyclooxygenase-2.
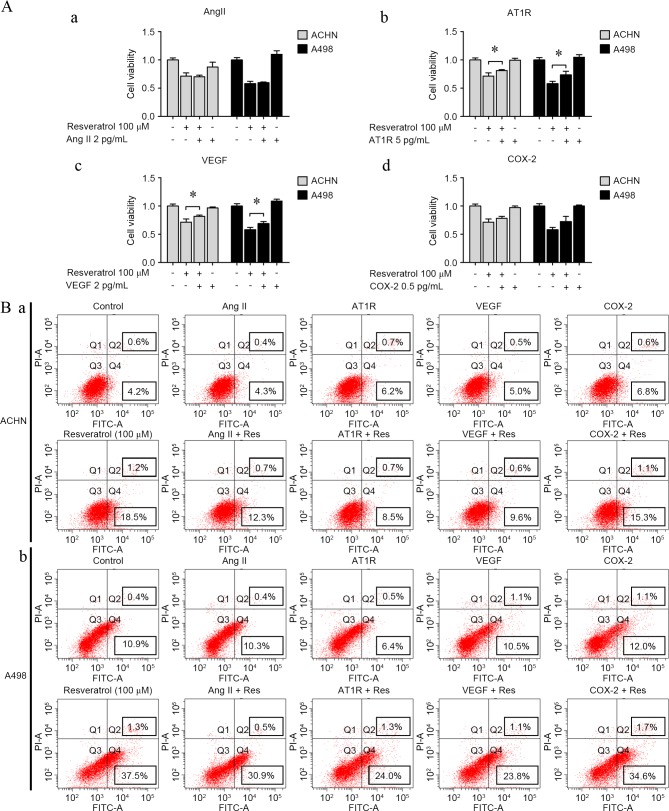
AT1R and VEGF may reverse resveratrol-induced renal carcinoma cell growth suppression and apoptosis
To determine whether AngII, AT1R, VEGF and COX-2 may reverse resveratrol-induced renal carcinoma cell regression and apoptosis, cells were incubated with AngII (2 pg/ml), AT1R (5 pg/ml), VEGF (2 pg/ml) or COX-2 (0.5 ng/ml) when exposed to resveratrol (100 µM). AT1R and VEGF were shown to reverse cell growth suppression by resveratrol (AT1R-treatment: RES vs. RES+AT1P, P=0.0442 in ACHN cells and P=0.0235 in A498A cells; VEGF-treatment: RES vs. RES+VEGF, P=0.0429 in ACHN cells and P=0.0257 in A498A cells; Fig. 5A), and cell apoptosis assay indicated a similar result with the cell growth results (AT1R-treatment: RES vs. RES+AT1P, P=0.0312 in ACHN cells and P=0.01245 in A498A cells; VEGF-treatment: RES vs. RES+VEGF, P=0.0351 in ACHN cells and P=0.0153 in A498A cells; Fig. 5B). By contrast, AngII and COX-2 played undetected roles of resveratrol-induced cell growth suppression and apoptosis.
Figure 5.
AT1R and VEGF reversed resveratrol-induced cell proliferation inhibition and apoptosis. (A) Resveratrol-induced cell proliferation inhibition in renal carcinoma cells with or without (a) AngII (2 pg/ml), (b) AT1R (5 pg/ml), (c) VEGF (2 pg/ml) and (d) COX-2 (0.5 ng/ml). (B) Apoptosis of (a) ACHN and (b) A498 cells was analyzed according to flow cytometry distributions with or without AngII (2 pg/ml), AT1R (5 pg/ml), VEGF (2 pg/ml) and COX-2 (0.5 ng/ml). *P<0.05, experimental vs. control groups. AngII, angiotensin II; AT1R, AngII type 1 receptor; VEGF, vascular endothelial growth factor; COX-2, cyclooxygenase-2; FITC, fluorescein isothiocyanate.
Discussion
As a type of free radical scavenger and antioxidant, resveratrol is recognized as an effective anticancer compound in several types of cancer, such as leukemia (28), breast (29–32), prostate (33) and ovarian cancers (34), melanoma (35) and primary brain tumors (36). In the present study, it was found that resveratrol may suppress renal carcinoma cell growth in a time- and dose-dependent manner. The present study also demonstrated that resveratrol induced renal carcinoma cell apoptosis by regulating apoptosis-associated proteins caspase 3/7/9, Bcl-2 and Bax. Reduced AngII, AT1R, VEGF and COX-2 levels were detected subsequent to resveratrol treatment and highlighted the importance of AT1R and VEGF in resveratrol-induced cell apoptosis.
Previously, the renin-angiotensin system (RAS) has been considered to be an endocrine system, and the function of which is limited to regulate blood pressure and electrolyte balance. However, recent studies have detected RAS in several tissues and indicated that local RAS may be involved in regulating a variety of physiological and pathological processes (7–9,37). In the RAS, AngII is recognized as a key biological peptide (38), with 2 major specific receptors subtypes, consisting of AT1R and AT2R (39). AngII acting on AT1R has a central role in mediating the majority of the actions of the RAS (16). Studies indicated that RAS was involved in biological activities through the AngII/AT1R pathway (39–41). In the present study, the levels of AngII and AT1R were determined subsequent to resveratrol treatment. The results demonstrated that AngII and AT1R were downregulated in the cells and the culture medium. As VEGF and COX-2 were important downstream regulators in RAS (42–45), it was found that VEGF and COX-2 were inhibited in resveratrol-treated cells and this result is similar to a previous study (29). The present data suggested that RAS suppression would be an important event during resveratrol-induced renal carcinoma cell apoptosis.
RAS was demonstrated to be involved in tumor occurrence and development (46–48). For example, AngII was able to promote epithelial-to-mesenchymal transition in intrahepatic cholangiocarcinoma (38), and angiotensin-converting enzyme inhibitor suppresses growth of colorectal cancer cells (49). AngII was demonstrated to induce VEGF in pancreatic cancer cells through binding AT1R and extracellular signal-regulated kinase 1/2 signaling; in addition, AngII/AT1R may increase expression of VEGF (21). AT1R was identified as upregulated in renal carcinoma and in urogenital cancer in a previous study (40). COX-2 was detected as upregulated in hypoxic conditions and induced angiogenesis through mechanisms distinct from the VEGF axis (25–27). As a type of anti-cancer drug, resveratrol was reported to act via a RAS-dependent pathway (10). In the present study, the effects of AngII, AT1R, VEGF and COX-2 on resveratrol-induced cell growth suppression and apoptosis in renal carcinoma were investigated. It was found that AT1R and VEGF reverse resveratrol-induced renal carcinoma cell growth suppression and apoptosis; however, AngII and COX-2 had no significant effect on resveratrol-induced cell growth suppression and apoptosis. The present results suggest that AT1R and VEGF are the critical factors during resveratrol-induced renal carcinoma cell proliferation suppression and apoptosis.
In summary, the present study examined the effect of resveratrol on renal carcinoma cell growth, suppression and apoptosis and investigated the potential mechanism through RAS. The results indicated that resveratrol may inhibit cell growth, induce apoptosis, and decrease AngII, AT1R, VEGF and COX-2 production in renal carcinoma cells. However, AT1R and VEGF may reverse the functions of resveratrol on renal carcinoma cells. Additional studies should be conducted to demonstrate the exact mechanism underlying the resveratrol-induced cell growth, suppression and apoptosis, which may be, at least partially, through a RAS-associated pathway in renal carcinoma cells.
Acknowledgements
This study was supported in part by the following grants: Scientific Research Fund of Guangdong Medical College (grant no. M2014019); Science and Technology Planning Project of Guangdong Province (grant no. 2012B031800221); and The National Natural Science Funds (grant no. 81272833) of China.
References
- 1.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 2.Carter LG, D'Orazio JA, Pearson KJ. Resveratrol and cancer: Focus on in vivo evidence. Endocr-Relat Cancer. 2014;21:R209–R225. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ndiaye M, Kumar R, Ahmad N. Resveratrol in cancer management: Where are we and where we go from here? Ann N Y Acad Sci. 2011;1215:144–149. doi: 10.1111/j.1749-6632.2010.05851.x. [DOI] [PubMed] [Google Scholar]
- 4.Reagan-Shaw S, Mukhtar H, Ahmad N. Resveratrol imparts photoprotection of normal cells and enhances the efficacy of radiation therapy in cancer cells. Photochem Photobiol. 2008;84:415–421. doi: 10.1111/j.1751-1097.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu ML, Li H, Yu LJ, Chen XY, Kong QY, Song X, Shu XH, Liu J. Short-term resveratrol exposure causes in vitro and in vivo growth inhibition and apoptosis of bladder cancer cells. PLoS One. 2014;9:e89806. doi: 10.1371/journal.pone.0089806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura H. Renin-angiotensin system in vertebrates: Phylogenetic view of structure and function. Anat Sci Int. 2016 Oct 7; doi: 10.1007/s12565-016-0372-8. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw AR, Wickremesekera AC, Brasch HD, Chibnall AM, Davis PF, Tan ST, Itinteang T. Glioblastoma multiforme cancer stem cells express components of the renin-angiotensin system. Front Surg. 2016;3:51. doi: 10.3389/fsurg.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida T, Kinoshita H, Fukui K, Matsuzaki T, Yoshida K, Mishima T, Yanishi M, Komai Y, Sugi M, Inoue T, et al. Prognostic impact of renin-angiotensin inhibitors in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol. 2016 Oct 11; doi: 10.1245/s10434-016-5534-3. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Miyajima A, Yazawa S, Kosaka T, Tanaka N, Shirotake S, Mizuno R, Kikuchi E, Oya M. Prognostic impact of renin-angiotensin system blockade on renal cell carcinoma after surgery. Ann Surg Oncol. 2015;22:3751–3759. doi: 10.1245/s10434-015-4436-0. [DOI] [PubMed] [Google Scholar]
- 10.Yang R, Zhang H, Zhu L. Inhibitory effect of resveratrol on the expression of the VEGF gene and proliferation in renal cancer cells. Mol Med Rep. 2011;4:981–983. doi: 10.3892/mmr.2011.511. [DOI] [PubMed] [Google Scholar]
- 11.Shirotake S, Miyajima A, Kosaka T, Tanaka N, Kikuchi E, Mikami S, Okada Y, Oya M. Regulation of monocyte chemoattractant protein-1 through angiotensin II type 1 receptor in prostate cancer. Am J Pathol. 2012;180:1008–1016. doi: 10.1016/j.ajpath.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res. 2012;318:1049–1056. doi: 10.1016/j.yexcr.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arrieta O, PinedaOlvera B, GuevaraSalazar P, Hernández-Pedro N, Morales-Espinosa D, Cerón-Lizarraga TL, González-De la Rosa CH, Rembao D, Segura-Pacheco B, Sotelo J. Expression of AT1 and AT2 angiotensin receptors in astrocytomas is associated with poor prognosis. Br J Cancer. 2008;99:160–166. doi: 10.1038/sj.bjc.6604431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li SH, Lu HI, Chang AY, Huang WT, Lin WC, Lee CC, Tien WY, Lan YC, Tsai HT, Chen CH. Angiotensin II type I receptor (AT1R) is an independent prognosticator of esophageal squamous cell carcinoma and promotes cells proliferation via mTOR activation. Oncotarget. 2016 Aug 24; doi: 10.18632/oncotarget.11567. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolley-Hitze T, Jouan F, Martin B, Mottier S, Edeline J, Moranne O, Le Pogamp P, Belaud-Rotureau MA, Patard JJ, Rioux-Leclercq N, Vigneau C. Angiotensin-2 receptors (AT1-R and AT2-R), new prognostic factors for renal clear-cell carcinoma? Br J Cancer. 2010;103:1698–1705. doi: 10.1038/sj.bjc.6605866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passos-Silva DG, VeranoBraga T, Santos RA. Angiotensin-(1–7): Beyond the cardio-renal actions. Clin Sci (Lond) 2013;124:443–456. doi: 10.1042/CS20120461. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 19.Cheng YD, Yang H, Chen GQ, Zhang ZC. Molecularly targeted drugs for metastatic colorectal cancer. Drug Des Devel Ther. 2013;7:1315–1322. doi: 10.2147/DDDT.S52485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding C, Li L, Yang T, Fan X, Wu G. Combined application of anti-VEGF and anti-EGFR attenuates the growth and angiogenesis of colorectal cancer mainly through suppressing AKT and ERK signaling in mice model. BMC Cancer. 2016;16:791. doi: 10.1186/s12885-016-2834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anandanadesan R, Gong Q, Chipitsyna G, Witkiewicz A, Yeo CJ, Arafat HA. Angiotensin II induces vascular endothelial growth factor in pancreatic cancer cells through an angiotensin II type 1 receptor and ERK1/2 signaling. J Gastrointest Surg. 2008;12:57–66. doi: 10.1007/s11605-007-0403-9. [DOI] [PubMed] [Google Scholar]
- 22.Hoellen F, Waldmann A, BanzJansen C, Rody A, Heide M, Köster F, Ribbat-Idel J, Thorns C, Gebhard M, Oberländer M, et al. Expression of cyclooxygenase-2 in cervical cancer is associated with lymphovascular invasion. Oncol Lett. 2016;12:2351–2356. doi: 10.3892/ol.2016.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali-Fehmi R, Morris RT, Bandyopadhyay S, Che M, Schimp V, Malone JM, Jr, Munkarah AR. Expression of cyclooxygenase-2 in advanced stage ovarian serous carcinoma: Correlation with tumor cell proliferation, apoptosis, angiogenesis, and survival. Am J Obstet Gynecol. 2005;192:819–825. doi: 10.1016/j.ajog.2004.10.587. [DOI] [PubMed] [Google Scholar]
- 24.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: A molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 25.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 26.Lee JJ, Natsuizaka M, Ohashi S, Wong GS, Takaoka M, Michaylira CZ, Budo D, Tobias JW, Kanai M, Shirakawa Y, et al. Hypoxia activates the cyclooxygenase-2-prostaglandin E synthase axis. Carcinogenesis. 2010;31:427–434. doi: 10.1093/carcin/bgp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rüegg C, Dormond O, Mariotti A. Endothelial cell integrins and COX-2: Mediators and therapeutic targets of tumor angiogenesis. Biochim Biophys Acta. 2004;1654:51–67. doi: 10.1016/j.bbcan.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Yaseen A, Chen S, Hock S, Rosato R, Dent P, Dai Y, Grant S. Resveratrol sensitizes acute myelogenous leukemia cells to histone deacetylase inhibitors through reactive oxygen species-mediated activation of the extrinsic apoptotic pathway. Mol Pharmacol. 2012;82:1030–1041. doi: 10.1124/mol.112.079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh N, Nigam M, Ranjan V, Zaidi D, Garg VK, Sharma S, Chaturvedi R, Shankar R, Kumar S, Sharma R, et al. Resveratrol as an adjunct therapy in cyclophosphamide-treated MCF-7 cells and breast tumor explants. Cancer Sci. 2011;102:1059–1067. doi: 10.1111/j.1349-7006.2011.01893.x. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Yang S, Troup S, Lu X, Callaghan S, Park DS, Xing Y, Yang X. Resveratrol induces apoptosis in breast cancer cells by E2F1-mediated up-regulation of ASPP1. Oncol Rep. 2011;25:1713–1719. doi: 10.3892/or.2011.1248. [DOI] [PubMed] [Google Scholar]
- 31.Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, Van den Berg M. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol In Vitro. 2014;28:1215–1221. doi: 10.1016/j.tiv.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Leon-Galicia I, DiazChavez J, GarciaVilla E, UribeFigueroa L, HidalgoMiranda A, Herrera LA, AlvarezRios E, GarciaMena J, Gariglio P. Resveratrol induces downregulation of DNA repair genes in MCF-7 human breast cancer cells. Eur J Cancer Prev. 2013;22:11–20. doi: 10.1097/CEJ.0b013e328353edcb. [DOI] [PubMed] [Google Scholar]
- 33.Fraser SP, Peters A, FlemingJones S, Mukhey D, Djamgoz MB. Resveratrol: Inhibitory effects on metastatic cell behaviors and voltage-gated Na+ channel activity in rat prostate cancer in vitro. Nutr Cancer. 2014;66:1047–1058. doi: 10.1080/01635581.2014.939291. [DOI] [PubMed] [Google Scholar]
- 34.Mikula-Pietrasik J, Sosińska P, Książek K. Resveratrol inhibits ovarian cancer cell adhesion to peritoneal mesothelium in vitro by modulating the production of α5β1 integrins and hyaluronic acid. Gynecol Oncol. 2014;134:624–630. doi: 10.1016/j.ygyno.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Chen YJ, Chen YY, Lin YF, Hu HY, Liao HF. Resveratrol inhibits alpha-melanocyte-stimulating hormone signaling, viability, and invasiveness in melanoma cells. Evid Based Complement Alternat Med. 2013;2013:632121. doi: 10.1155/2013/632121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen S, Li H, Wu ML, Fan SH, Wang Q, Shu XH, Kong QY, Chen XY, Liu J. Inhibition of NF-κB signaling commits resveratrol-treated medulloblastoma cells to apoptosis without neuronal differentiation. J Neurooncol. 2011;104:169–177. doi: 10.1007/s11060-010-0496-y. [DOI] [PubMed] [Google Scholar]
- 37.Magliano DC, Penna-de-Carvalho A, Vazquez-Carrera M, Mandarim-de-Lacerda CA, Aguila MB. Short-term administration of GW501516 improves inflammatory state in white adipose tissue and liver damage in high-fructose-fed mice through modulation of the renin-angiotensin system. Endocrine. 2015;50:355–367. doi: 10.1007/s12020-015-0590-1. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto K, Tajima H, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi H, Nakamura K, Oyama K, Nakagawara H, et al. Angiotensin II enhances epithelial-to-mesenchymal transition through the interaction between activated hepatic stellate cells and the stromal cell-derived factor-1/CXCR4 axis in intrahepatic cholangiocarcinoma. Int J Oncol. 2012;41:573–582. doi: 10.3892/ijo.2012.1499. [DOI] [PubMed] [Google Scholar]
- 39.Kosaka T, Miyajima A, Takayama E, Kikuchi E, Nakashima J, Ohigashi T, Asano T, Sakamoto M, Okita H, Murai M, Hayakawa M. Angiotensin II type 1 receptor antagonist as an angiogenic inhibitor in prostate cancer. Prostate. 2007;67:41–49. doi: 10.1002/pros.20486. [DOI] [PubMed] [Google Scholar]
- 40.Miyajima A, Kikuchi E, Kosaka T, Oya M. Angiotensin II type 1 receptor antagonist as an angiogenic inhibitor in urogenital cancer. Rev Recent Clin Trials. 2009;4:75–78. doi: 10.2174/157488709788185996. [DOI] [PubMed] [Google Scholar]
- 41.Miyajima A, Kosaka T, Asano T, Seta K, Kawai T, Hayakawa M. Angiotensin II type I antagonist prevents pulmonary metastasis of murine renal cancer by inhibiting tumor angiogenesis. Cancer Res. 2002;62:4176–4179. [PubMed] [Google Scholar]
- 42.de Haas S, Delmar P, Bansal AT, Moisse M, Miles DW, Leighl N, Escudier B, Van Cutsem E, Carmeliet P, Scherer SJ, et al. Genetic variability of VEGF pathway genes in six randomized phase III trials assessing the addition of bevacizumab to standard therapy. Angiogenesis. 2014;17:909–920. doi: 10.1007/s10456-014-9438-1. [DOI] [PubMed] [Google Scholar]
- 43.Mittal K, Koon H, Elson P, Triozzi P, Dowlati A, Chen H, Borden EC, Rini BI. Dual VEGF/VEGFR inhibition in advanced solid malignancies: Clinical effects and pharmacodynamic biomarkers. Cancer Biol Ther. 2014;15:975–981. doi: 10.4161/cbt.29187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang WS, Liao CH, Miao CE, Wu HC, Hou LL, Hsiao CL, Ji HX, Tsai CW, Bau DT. The role of functional polymorphisms of cyclooxygenase 2 in renal cell carcinoma. Anticancer Res. 2014;34:5481–5486. [PubMed] [Google Scholar]
- 45.He W, Zhang M, Zhao M, Davis LS, Blackwell TS, Yull F, Breyer MD, Hao CM. Increased dietary sodium induces COX2 expression by activating NFkB in renal medullary interstitial cells. Pflugers Arch. 2014;466:357–367. doi: 10.1007/s00424-013-1328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan L, Feng Y, Wan HY, Ni L, Qian YR, Guo Y, Xiang Y, Li QY. Hypoxia induces dysregulation of local renin-angiotensin system in mouse Lewis lung carcinoma cells. Genet Mol Res. 2014;13:10562–10573. doi: 10.4238/2014.December.12.19. [DOI] [PubMed] [Google Scholar]
- 47.Ino K, Shibata K, Kajiyama H, Nawa A, Nomura S, Kikkawa F. Manipulating the angiotensin system-new approaches to the treatment of solid tumours. Expert Opin Biol Ther. 2006;6:243–255. doi: 10.1517/14712598.6.3.243. [DOI] [PubMed] [Google Scholar]
- 48.Araújo WF, Naves MA, Ravanini JN, Schor N, Teixeira VP. Renin-angiotensin system (RAS) blockade attenuates growth and metastatic potential of renal cell carcinoma in mice. Urol Oncol. 2015;33:389.e1–e7. doi: 10.1016/j.urolonc.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 49.Childers WK. Interactions of the renin-angiotensin system in colorectal cancer and metastasis. Int J Colorectal Dis. 2015;30:749–752. doi: 10.1007/s00384-014-2118-1. [DOI] [PubMed] [Google Scholar]