Abstract
Stroke and traumatic brain injury (TBI) are the critical public health and socioeconomic problems throughout the world. At present, citicoline is used as a coadjuvant for the management of acute ischemic stroke (AIS) and TBI in various countries. This systemic review analyzes the beneficial role of citicoline in AIS and TBI. This systemic review is based on “PubMed” and “Science Direct” search results for citicoline role in stroke and TBI. In this systemic review, we included 12 human trials. A meta-analysis was performed on the basis of neurological evaluation, functional evaluation and Glasgow outcome scale, domestic adaptation evaluation outcomes, and cognitive outcome individually. In neurological evaluation, domestic adaptation evaluation, and cognitive outcomes, there was no significant difference in both the citicoline and placebo groups (odds ratio [OR] = 1.04 [0.9–1.2, P = 0.583]; OR = 1.1 [0.94–1.27, P = 0.209]; OR = 0.953 [0.75–1.2, P = 0.691]). In evaluation of functional outcomes, there was significant difference in both groups and OR was 1.18 (1.04–1.34, P = 0.01). Functional outcomes were significantly improved by citicoline, but the positive role of this drug in neurological recovery, domestic adaptation, and cognitive outcomes is still a topic of discussion for future.
Key words: Acute ischemic stroke recovery, Barthel Index score, citicoline, Glasgow outcome scale, modified Rankin scale, National Institutes of Health Stroke Scale, traumatic brain injury
Stroke is a devastating condition for low- and middle-income countries and the third leading condition with the highest mortality and death.[1] The estimated number of deaths due to stroke was about 5.71 million people as per the World Health Organization data, and this number will be increased to 6.3 and 7.8 million in 2015 and 2030, respectively.[2,3] Similarly, traumatic brain injury (TBI) is a critical public health and socioeconomic problem throughout the world. TBI commonly leads to neurocognitive deficits (such as impaired attention, inability to form visuospatial associations, or poor executive function) and psychological health issues.[4] Current treatments for acute ischemic stroke (AIS) include intravenous thrombolytic therapy with tissue-type plasminogen activator (t-PA) and endovascular therapies, including intra-arterial thrombolytic therapy and the use of clot retrieval devices.[5] Surgical management with hemispheric decompression in patients with middle cerebral artery territory infarction and associated life-threatening parenchymal edema has also been supported.[6] Since the approval of recombinant tissue plasminogen that acts within 3–4.5 h of its administration, no new drug is found to be as effective in the treatment of acute stroke. Despite this, the number of neuroprotectors used in the management of acute stroke has become known in the last couple of years. The ideal neuroprotective approach in the management of stroke is that the agent should allow intravenous administration, should have a greater window of convenience than recombinant t-PA (rt-PA), should be well tolerable, and must augment functional recovery after stroke.[7] In several preclinical study models of acute stroke and ischemia, many neuroprotectors exhibited biochemical benefits although their efficacy was found to be deficient in many Phase III trials.[7] New insights into the pathophysiology of severe TBI, especially the realization that brain damage develops sequentially, initiated several new treatment approaches aiming at the interruption of pathophysiological mechanisms leading to secondary brain injury. A high number of pharmacological substances have been tested for their ability to ameliorate secondary damage after TBI or are currently under clinical trial, but no drug has achieved this goal so far.[8]
Cytidine-5'-diphosphocholine (citicoline or CDP-choline) has shown beneficial effects in a number of central nervous system injury models and pathological conditions of the brain as it is an intermediate product of phosphatidylcholine (PtdCho) biosynthesis. Citicoline as an intermediate of PtdCho and sphingomyelin biosynthesis enhances the synthesis of these products and simultaneously inhibits the activation of phospholipases (destructive processes). Its neuroprotective action is due to preservation of cardiolipin and sphingomyelin, prevention of arachidonic acid content of PtdCho, inhibition of lipid peroxidation, and restoration of the level of PtdCho. All above-explained effects could be related with attenuation of phospholipase A(2) activation. Citicoline, being an endogenous compound, is available naturally. However, for clinical use of citicoline, its sodium salt is synthesized.[9]
In animal models, exogenous administration of citicoline is reported to reduce the breakdown of PtdCho, hence reducing the free fatty acid levels.[10] In various ischemia/hypoxia animal models, citicoline treatment is demonstrated to be profitable,[10,11,12,13] not to mention in recent studies with reversible focal occlusion[14] as well as in intracerebral hemorrhage model.[15] The above studies manifest the beneficial effects of citicoline in reducing the concentrations of free fatty acids, thereby improving neurological signs, reducing neurological deficits, restoring performance of animal learning, decreasing glutamate-mediated injury, preserving the levels of PtdCho, and improving the survival of neurons.
Positive results of preclinical studies with animal models of neurodegenerative diseases have prompted clinical trials with citicoline as a treatment for human brain diseases[16,17,18,19,20,21,22,23,24] Although several previous small clinical studies had achieved promising results: Two recent large randomized multicenter trials – the Citicoline Brain Injury Treatment (COBRIT) trial performed in 1213 patients with TBI,[16] and the international, randomized, multicenter, placebo-controlled sequential International Citicoline Trial on Acute Stroke (ICTUS) trial performed in 2298 patients with moderate-to-severe acute ischemic stroke[17] – led to the conclusion that citicoline is not efficacious in these clinical settings. The negative outcomes of these studies were deemed surprising and prompted a few comments, which focused mostly, although not exclusively, on methodological aspects of the evaluation of the clinical effects of the drug.
Cost of therapy is the most important parameter for selecting any neuroprotector for the treatment of AIS. The incremental analysis showed that citicoline treatment was the dominant treatment when using the Cochrane study as placebo was both less effective and more expensive. The authors concluded that treatment with citicoline in acute ischemic stroke was a highly cost-effective alternative to placebo in the setting of the Spanish healthcare system.[25]
To find out some concrete results, we reviewed the currently available study evidence systematically regarding the role of citicoline in AIS and TBI, including clinical and observational studies based on neurological, functional, domestic adaptation outcome and cognitive outcome.
Material and Methods
Search Method and Selection Criteria
Potential studies were identified by searching “PubMed” and “Science Direct” for “citicoline,” “CDP-choline” in combination with “stroke,” “TBI,” “head injury,” “brain injury.” The database was searched for (nonreview) articles in English, published up to April 2015. The present review includes those trials that contain original information about the effects of the treatment with citicoline in stroke and TBI. For completeness, articles reporting observational studies of citicoline in stroke and TBI were also included. Our criteria for selection of patients were: (1) Patient of AIS, TBI, head injury, and brain injury, (2) must have been treated in 24–48 h of stroke, (3) baseline National Institutes of Health Stroke Scale (NIHSS >8), modified Rankin scale (mRS <1) immediate after stroke.
End-points
Four end-points were measured in this systemic review. A meta-analysis was performed for evaluating neurological, functional, domestic adaptation and cognitive impairment using NIHSS, mRS and Glasgow outcome scale (GOS), Barthel Index (BI) score, and cognitive impairment evaluation, respectively. All three parameters were evaluated by odds ratio (OR) and significance value P < 0.05 was decided using forest plot.
Statistical Methods
We used comprehensive meta-analysis V2 software (Biostat, Englewood, New Jersey, New Jersey) for pooling analysis of clinical trials and performed forest plot for comparison of total available clinical trials based on NIHSS, mRS/Glasgow, BI, and cognitive outcomes.
Results
Search Results
After database search as discussed in material and methods section, we found 140 studies in the first search. We excluded animal studies (n = 50) as we included only clinical studies in our systemic review. We excluded review articles (n = 42), case reports (n = 1), guidelines (n = 1), and studies other than stroke and TBI (n = 14). At the time of database search, we found some nonciticoline (n = 21) and non-English studies (n = 3), which were also excluded. Hence, finally, in this systemic review, we narrowed to 12 trials which included stroke (n = 8) and TBI (n = 4) [Figure 1].
Figure 1.
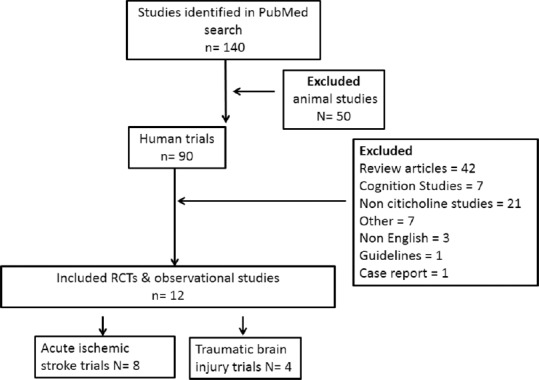
Procedure for selection of trials for systemic review
End-points
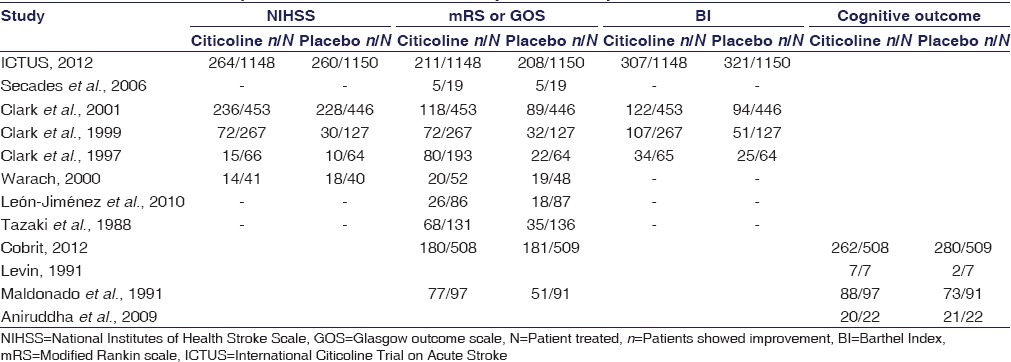
We have performed a meta-analysis of selected trials based on neurological, functional, domestic adaptation outcomes and cognitive outcomes individually. Table 1 summarizes the number of enrolled patients in citicoline and placebo group for analysis of neurological, functional, domestic adaptation outcomes and cognitive outcome in clinical trials.
Table 1.
Clinical trials and patient selected in meta-analysis for end-point evaluation
Neurological Evaluation
For analyzing the effect of citicoline for neurological outcomes, five randomized controlled trials (RCTs) were selected. In all five trials, a total of 3803 patients were randomized in two groups, citicoline (n = 1975) and placebo (n = 1825). In this meta-analysis, we found no significant difference between both the groups. In both groups, 30% (601 of 1975 and 546 of 1825) of patients showed NIHSS <1 and OR was 1.04 (0.9–1.2, P = 0.583) [Figure 2].
Figure 2.
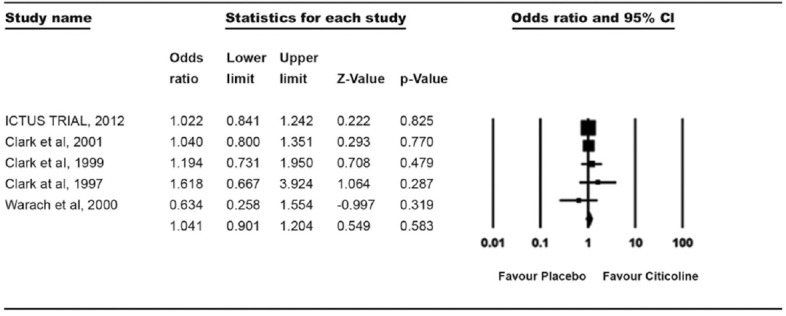
Treatment effect on the recovery of neurological assessment (National Institutes of Health Stroke Scale <1)
Functional Evaluation
Functional outcomes were measured using mRS score and Glasgow outcome assessment. In our meta-analysis, we examined eight trials with 5631 patients with AIS and TBI. In citicoline group 2954 and in placebo group 2677 patients were included. In citicoline group, 29.01% (857 of 2954) and in placebo group, 24.65% (660 of 2677) of patients showed better functional outcomes (mRS ≤1, GOS >7). There was a significant difference in both groups in our meta-analysis and OR was 1.18 (1.04–1.34, P = 0.008) [Figure 3].
Figure 3.
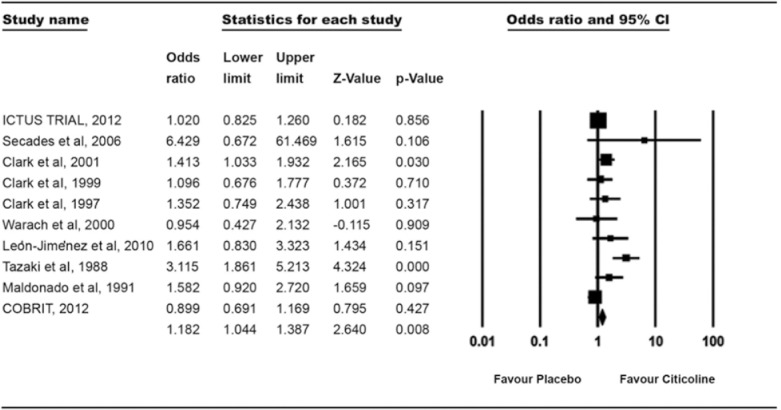
Treatment effect on the functional recovery (modified Rankin scale <1 and Glasgow outcome scale >7)
Barthel Index
We also evaluated 4 studies for assessment of domestic adaptations by BI measurement. A total of 3618 patients were enrolled in these studies and were divided into 2 groups. In both citicoline group (n = 1874) and placebo group (n = 1744), there was no significant difference in results. 28.3% patients in citicoline group and 26.7% patients in placebo group showed improvement and the OR for BI >95 (1.1 [0.94–1.27, P = 0.209]) [Figure 4].
Figure 4.
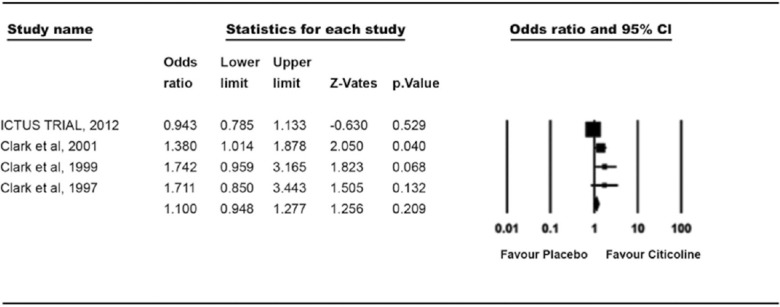
Treatment effect on the recovery of activities of daily living (Barthel Index >95)
Cognitive Outcome
For assessment of cognitive outcome, 4 trials of TBI were evaluated. A total of 3618 patients enrolled in these studies and were divided into 2 groups. In both citicoline group (n = 634) and placebo group (n = 629), there was no significant difference in results. 59.46% patients in citicoline group and 59.77% patients in placebo group showed improvement and the OR for cognitive improvement was 0.953 (0.75–1.2, P = 0.691) [Figure 5].
Figure 5.
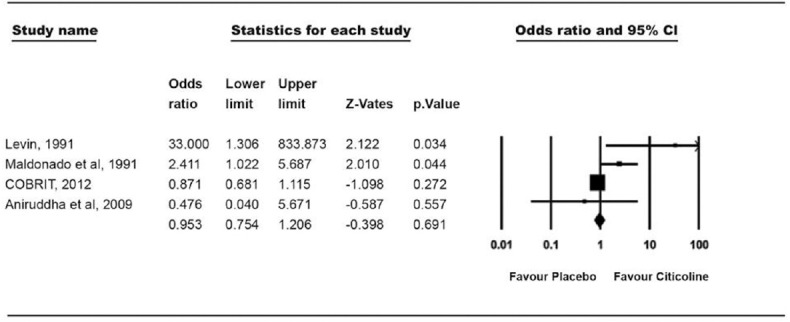
Treatment effect on recovery of cognitive functions
Discussion
In current systemic review, our pooled patient analysis was performed individually for neurological, functional, and domestic adaptation evaluation. Neurological status was measured and documented by NIHSS, a clinical stroke assessment tool for AIS. This universal stroke scale evaluates the stroke severity and lesion size. Functional outcomes were measured by mRS and Glasgow outcome in AIS and TBI trials. The mRS measures independence rather than the performance of specific tasks. In this way, mental as well as physical adaptations to the neurological deficits are incorporated. The BI is the most commonly used scale for assessing activities of daily living in patients with stroke. The reason for this individual measurement of parameters was to evaluate citicoline on neurological, functional, improvement in daily living functions and cognitive outcomes basis as well as finding out the holistic approach of citicoline in acute stroke management.
Results for neurological evaluation of the present meta-analysis were not in favor of citicoline, OR suggested that citicoline is just similar to placebo. The very recent trial (ICTUS, 2012) on citicoline stated that citicoline is just similar to placebo in most of the parameters for managing the acute stroke.[17] Clark et al.[20] also supported these results. Our results were in line with ICTUS trial as well as with most of the previous citicoline trials; where authors reported the neutral effect of citicoline in the improvement of neurological functions. Some contradictory results were also found in other clinical trials. Clark et al.,[18,19] in their other trials, suggested that citicoline improves neurological deficits at an initial dose of 500 mg. Another extensive meta-analysis[23] of citicoline trials also showed the positive results; however, the neurological outcomes were nonsignificantly higher in citicoline group. In spite of including these trials in our meta-analysis, the pooled patient analysis showed no significant effect of citicoline on neurological status in patients with AIS. Rao et al.[26] suggested the proper cause for the failure of citicoline and its effect on neurological outcomes in various trials. Breakdown of citicoline in choline and cytidine triphosphate (CTP) and crossing the blood brain barrier are recognized by CTP: Phosphocholine cytidylyltransferase (CCT) which is downregulated after stroke.[26] Downregulation of CCT increases fatty acid accumulation that results in significant PtdCho loss, which thus is responsible for the ineffectiveness of citicoline.[26]
For functional evaluation, we have measured mRS <1 and Glasgow >7 in our meta-analysis which is based on available RCTs and observational studies. Our results identified significant improvement in functional outcomes, which are similar to the results of a very extensive meta-analysis done by Dávalos et al. which reported full recovery observed in individual functional scales such as mRS.[23] Secades et al.[21] performed a small study which analyzed that one patient in the placebo group was categorized as having no disability (mRS <2) in comparison with five patients in the citicoline group.[19] Three continuous US-based trials done by Clark et al. also recommended the positive effect of citicoline in functional outcomes.[18,19,20] In their latest trial (2001), they found that mRS returned to baseline and showed a significant treatment effect at 6 weeks.[20] Our results are in consistency with above-mentioned trials. However, two large trials, ICTUS (2012) and COBRIT (2012), concluded the placebo effect of citicoline on functional outcome, respectively (mRs ≤1, OR 1.02 [0.83–1.26] and GOS >7, OR 0.99 [0.76–1.28]).[16,17] The reason for the failure of ICTUS trial was that they used rt-PA, a thrombolytic agent, that increases the blood flow in penumbra,[20] and COBRIT study cannot address the effects of citicoline on earlier administration in recovery or in titrated doses.[16] Improvement in functional outcome is due to the membrane stabilizing, free radical scavenging, and surfactant-precursor properties[27] of citicoline, which showed a pleiotropic effect in severely ill patients. In experimental study, it was also found that attenuation of sympathetic system could be an interpreter of better functional outcomes.[28] In brain injury animal models, citicoline has been stabilizing the immediate release of catecholamine that improves the functional outcomes directly.[29]
For improvement in daily living functions, we analyzed the trials for BI >95 and this change was not significant in our meta-analysis plot. The ORs suggested that citicoline is similar as placebo for BI improvement. ORs of ICTUS trial also support our results, which showed no marked improvement in BI when compared to placebo.[17] In the same line, two US-based RCTs[18,19] examined the effect of citicoline by BI examination and found similar results as placebo and no acceptable improvement in domestic adaptation in patients with AIS at 1000 mg and 2000 mg dose. However, another Phase III study done by Clark et al.[20] illuminated the positive effect of citicoline in daily domestic adaptations (BI >95); however, this significant difference was lost at the 12-week assessment. A meta-analysis done by Dávalos et al. summarized noticeable improvement in BI after treatment with citicoline.[23]
We measured cognitive outcomes in TBI based on four studies. Our meta-analysis clearly suggested no effect of citicoline over placebo in cognitive improvement. The very recent trial (COBRIT, 2012) on citicoline stated that citicoline is just similar to placebo in cognitive improvement of patients with TBI.[16] Similar results were submitted by Aniruddha et al.[30] in their trial on mild head injury. They found no difference between citicoline and placebo in concentration improvement in patients with mild head injury. Our results were contradictory with the findings of Levin[31] and Calatayud Maldonado et al.[32] In Levin's trial,[31] postconcussional symptoms were measured and 100% patients showed improvement in cognition after citicoline treatment compared to 29% of placebo patients. Similarly, Calatayud Maldonado et al.[32] suggested the better improvement in memory after 3 months treatment of citicoline compared to placebo. Reason behind no effect or negative effect was explained in COBRIT trial.[16] Trial explained that molecular mechanisms that may be neuroprotective by acutely promoting tissue repair and neurologic recovery in severely injured patients might be deleterious in the postacute setting or in patients with milder injuries. A therapy that affects inflammatory, lipid peroxidative, and cholinergic mechanisms may have less benefit or even an overall negative effect in those with less severe injury.[16]
After concrete analysis of all trials, we conclude that citicoline is effective in the improvement of functional outcomes, but the role of this drug in neurological recovery, improvement in domestic adaptation and cognition is still in the state of dilemma. In last few years, citicoline has become the hope of clinicians for the treatment of stroke and TBI, but the aura of this molecule faded after ICTUS and COBRIT trial, which found the negative results in neurological, domestic adaptation and cognitive outcomes. This highlights the issue on international level and suggests that large-scale clinical trials will be required to justify the use of citicoline monotherapy. This will be helpful for clinicians to decide on to the use of citicoline inpatient settings.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Baker WL, Colby JA, Tongbram V, Talati R, Silverman IE, White CM, et al. Neurothrombectomy devices for the treatment of acute ischemic stroke: State of the evidence. Ann Intern Med. 2011;154:243–52. doi: 10.7326/0003-4819-154-4-201102150-00306. [DOI] [PubMed] [Google Scholar]
- 2.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 3.Derex L, Tomsick TA, Brott TG, Lewandowski CA, Frankel MR, Clark W, et al. Outcome of stroke patients without angiographically revealed arterial occlusion within four hours of symptom onset. AJNR Am J Neuroradiol. 2001;22:685–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 5.Meyers PM, Schumacher HC, Connolly ES, Jr, Heyer EJ, Gray WA, Higashida RT. Current status of endovascular stroke treatment. Circulation. 2011;123:2591–601. doi: 10.1161/CIRCULATIONAHA.110.971564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez JC. Surgical treatment of patients with ischemic stroke decompressive craniectomy. In: Musabelliu E, Kato Y, Imizu S, Oda J, Sano H, editors. Acute Ischemic Stroke. Croatia: InTech; 2012. pp. 167–86. [Google Scholar]
- 7.Karapanayiotides T, Bogousslavsky J. Neuroprotection: What are its prospects in the stroke patient? Dialog Cardiovasc Med. 2003;8:828–34. [Google Scholar]
- 8.Clausen T, Bullock R. Medical treatment and neuroprotection in traumatic brain injury. Curr Pharm Des. 2001;7:1517–32. doi: 10.2174/1381612013397267. [DOI] [PubMed] [Google Scholar]
- 9.Adibhatla RM, Hatcher JF, Dempsey RJ. Citicoline: Neuroprotective mechanisms in cerebral ischemia. J Neurochem. 2002;80:12–23. doi: 10.1046/j.0022-3042.2001.00697.x. [DOI] [PubMed] [Google Scholar]
- 10.Trovarelli G, de Medio GE, Dorman RV, Piccinin GL, Horrocks LA, Porcellati G. Effect of cytidine diphosphate choline (CDP-choline) on ischemia-induced alterations of brain lipid in the gerbil. Neurochem Res. 1981;6:821–33. doi: 10.1007/BF00965041. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Shimizu M, Okamiya H. Pharmacological actions of a new TRH analogue, YM-14673, in rats subjected to cerebral ischemia and anoxia. Eur J Pharmacol. 1990;181:207–14. doi: 10.1016/0014-2999(90)90080-p. [DOI] [PubMed] [Google Scholar]
- 12.Hamdorf G, Cervós-Navarro J. Study of the effects of oral administration of CDP-choline on open-field behaviour under conditions of chronic hypoxia. Arzneimittelforschung. 1990;40:519–22. [PubMed] [Google Scholar]
- 13.Mykita S, Golly F, Dreyfus H, Freysz L, Massarelli R. Effect of CDP-choline on hypocapnic neurons in culture. J Neurochem. 1986;47:223–31. doi: 10.1111/j.1471-4159.1986.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 14.Aronowski J, Strong R, Grotta JC. Citicoline for treatment of experimental focal ischemia: Histologic and behavioral outcome. Neurol Res. 1996;18:570–4. doi: 10.1080/01616412.1996.11740473. [DOI] [PubMed] [Google Scholar]
- 15.Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 1998;29:2136–40. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- 16.Zafonte RD, Bagiella E, Ansel BM, Novack TA, Friedewald WT, Hesdorffer DC, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT) JAMA. 2012;308:1993–2000. doi: 10.1001/jama.2012.13256. [DOI] [PubMed] [Google Scholar]
- 17.Dávalos A, Alvarez-Sabín J, Castillo J, Díez-Tejedor E, Ferro J, Martínez-Vila E, et al. Citicoline in the treatment of acute ischaemic stroke: An international, randomised, multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380:349–57. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- 18.Clark WM, Warach SJ, Pettigrew LC, Gammans RE, Sabounjian LA. A randomized dose-response trial of citicoline in acute ischemic stroke patients. Citicoline Stroke Study Group. Neurology. 1997;49:671–8. doi: 10.1212/wnl.49.3.671. [DOI] [PubMed] [Google Scholar]
- 19.Clark WM, Williams BJ, Selzer KA, Zweifler RM, Sabounjian LA, Gammans RE. A randomized efficacy trial of citicoline in patients with acute ischemic stroke. Stroke. 1999;30:2592–7. doi: 10.1161/01.str.30.12.2592. [DOI] [PubMed] [Google Scholar]
- 20.Clark WM, Wechsler LR, Sabounjian LA, Schwiderski UE. Citicoline Stroke Study Group. A phase III randomized efficacy trial of 2000 mg citicoline in acute ischemic stroke patients. Neurology. 2001;57:1595–602. doi: 10.1212/wnl.57.9.1595. [DOI] [PubMed] [Google Scholar]
- 21.Secades JJ, Alvarez-Sabín J, Rubio F, Lozano R, Dávalos A, Castillo J. Trial Investigators. Citicoline in intracerebral haemorrhage: A double-blind, randomized, placebo-controlled, multi-centre pilot study. Cerebrovasc Dis. 2006;21:380–5. doi: 10.1159/000091547. [DOI] [PubMed] [Google Scholar]
- 22.Cho HJ, Kim YJ. Efficacy and safety of oral citicoline in acute ischemic stroke: Drug surveillance study in 4,191 cases. Methods Find Exp Clin Pharmacol. 2009;31:171–6. doi: 10.1358/mf.2009.31.3.1364241. [DOI] [PubMed] [Google Scholar]
- 23.Dávalos A, Castillo J, Alvarez-Sabín J, Secades JJ, Mercadal J, López S, et al. Oral citicoline in acute ischemic stroke: An individual patient data pooling analysis of clinical trials. Stroke. 2002;33:2850–7. doi: 10.1161/01.str.0000038691.03334.71. [DOI] [PubMed] [Google Scholar]
- 24.Casado A, Secades JJ, Ibarz R, Herdman M, Brosa M. Cost-effectiveness of citicoline versus conventional treatment in acute ischemic stroke. Expert Rev Pharmacoecon Outcomes Res. 2008;8:151–7. doi: 10.1586/14737167.8.2.151. [DOI] [PubMed] [Google Scholar]
- 25.Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, et al. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Ann Neurol. 2000;48:713–22. [PubMed] [Google Scholar]
- 26.Rao AM, Hatcher JF, Dempsey RJ. CDP-choline: Neuroprotection in transient forebrain ischemia of gerbils. J Neurosci Res. 1999;58:697–705. [PubMed] [Google Scholar]
- 27.Jambou R, El-Assaad F, Combes V, Grau GE. Citicoline (CDP-choline): What role in the treatment of complications of infectious diseases. Int J Biochem Cell Biol. 2009;41:1467–70. doi: 10.1016/j.biocel.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Sander D, Winbeck K, Klingelhöfer J, Etgen T, Conrad B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology. 2001;57:833–8. doi: 10.1212/wnl.57.5.833. [DOI] [PubMed] [Google Scholar]
- 29.Hurtado O, Moro MA, Cárdenas A, Sánchez V, Fernández-Tomé P, Leza JC, et al. Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: Effects on glutamate transport. Neurobiol Dis. 2005;18:336–45. doi: 10.1016/j.nbd.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Aniruddha TJ, Pillai J, Devi BI, Sampath S, Chandramouli BA. Role of citicoline in the management of mild head injury. IJNT. 2009;6:49–52. [Google Scholar]
- 31.Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103:S39–42. doi: 10.1016/0022-510x(91)90007-t. [DOI] [PubMed] [Google Scholar]
- 32.Calatayud Maldonado V, Calatayud Pérez JB, Aso Escario J. Effects of CDP-choline on the recovery of patients with head injury. J Neurol Sci. 1991;103:S15–8. doi: 10.1016/0022-510x(91)90003-p. [DOI] [PubMed] [Google Scholar]


