Abstract
Objective:
The objective of the study was to investigate the effect of daidzein flavonoid on cisplatin (CP)-induced hematotoxicity and hepatotoxicity in experimental rats.
Materials and Methods:
The Wistar rats were randomly divided into four equal groups: Normal (saline 1 ml p.o.), CP (7.5 mg/kg once intraperioteneally on 16th day), test group of low dose (combination of CP and daidzein 20 mg/kg p.o. for 21 days), and test group of high dose (combination of CP and daidzein 40 mg/kg p.o. for 21 days). Blood samples were collected on 22nd day from each rat and subjected for evaluation of hematological parameters such as red blood corpuscles (RBC), white blood corpuscles, hemoglobin (Hb) and platelets, and serum biomarkers such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP). Liver of each rat was excised and subjected for antioxidants evaluation such as malonyl dialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), catalase, and histopathological study.
Results:
Daidzein had a significant (P < 0.001) beneficial role in CP-induced hemotoxicity by increasing RBC, Hb, packed cell volume, and platelets. Daidzein also exhibited a significant (P < 0.001) protection against CP-induced hepatotoxicity by decreasing ALT, AST, ALP, and MDA level and by elevating the GSH, SOD, and catalase.
Conclusions:
Daidzein attenuates CP-induced oxidative stress on blood cells and antioxidants in rats.
Key words: Cisplatin, daidzein, hematotoxicity, hepatotoxicity, oxidative stress
One of the pivot features which differentiate between antineoplastic drugs from other molecules is the intensity of adverse effects at therapeutic dose range. Most of anticancer drugs such as cisplatin (CP) target directly on multiplying cells. The main mechanisms for CP-induced toxicities attributed to the development of reactive oxygen species (ROS) and nitrogenous compounds by oxidative stress injury and diminish of antioxidant defense role. Management of variety of toxicities evoked by antitumor agents is of utmost needed as they affect on overall quality of life.[1,2] Several animal models were performed in combination of CP with various antioxidants, free radical scavengers, and natural herb constituents with antioxidant properties in prevention of toxic effects induced by CP without interfering its antitumor action.[3,4]
Now days, the development of phytochemicals/phytoconstituents for investigating the synergistic effect in combination with anticancer drugs/chemotherapeutic agents is widely raised. Flavonoids are the polyphenolic, dietary molecules that are most commonly found in fruits, flowers, and vegetables. Many studies already proved that flavonoids from fruits and vegetables have potential as antineoplastic drugs due to their antiproliferative action and were also used in combination with other drugs for greater efficacy and safety.[5]
Daidzein is a polyphenolic flavonoid belongs to isoflavone group. Daidzein is a phytoestrogen commonly found in red clover (Trifolium pratense), alfalfa (Medicago sativa), soya (soybeans and soya foods), and some legumes (Leguminosae). Daidzein has already proved the protection against the neoplasia of chest, large intestine, breast, ovary, and prostate gland. It also possesses estrogenic, anti-inflammatory, cardioprotective, hypoglycemic, neuroprotective, and free radical scavenging actions.[6,7,8]
Daidzein is a pivot circulating in people receiving more soy diet. Daidzein prevents the action of both isoenzymes of human alcohol dehydrogenase (ADH). Because this inhibitory action on both ADH isoenzymes, it may reduce the alcohol consumption in humans, and also it plays a vital role in prevention of osteoporosis and menopausal symptoms.[6,8,9]
The present study was undertaken to evaluate the effect of daidzein on CP-induced hemotoxicity and hepatotoxicity in experimental rats.
Materials and Methods
Animals
Wistar rats of either sex (200–240 g) were procured from animal house of Shree Devi College of Pharmacy, Mangalore. They were acclimatized to controlled laboratory conditions of temperature (25 ± 2°C), 30%–70% humidity, and 12 h light–dark cycles. The rats were randomized into experimental and control groups. They had free access to standard pellets as basal diet and water ad libitum. All the studies conducted were approved by the Institutional Animal Ethical Committee, Shree Devi College of Pharmacy (SDCP/IAEC-04/2014-15), Mangalore, Karnataka, according to prescribed guidelines of committee for the purpose of control and supervision of experiments in animals, Government of India.[10]
Chemicals
CP was procured from Sigma-Aldrich, Bengaluru, India, and other chemicals used were of analytical grade and purchased from standard companies. Biochemical kits for the estimation of biomarkers/enzymes were procured from Robonik India Pvt. Ltd., Mumbai, India.
Experimental Design
The present study was designed for 22 days. Daidzein was isolated from soybeans by Soxhlet apparatus and identified by high-performance liquid chromatography method.[11] After 7–8 days of acclimatization, the experimental rats were randomly divided into four equal groups, six animals in each. Group I was used as normal and treated with saline 10 ml/kg, p.o. Groups II, III, and IV were treated with single dose of CP 7.5 mg/kg, intraperitoneally on 16th day, after successive administration of distilled water (Group II) or daidzein 20 mg/kg (Group III) or daidzein 40 mg/kg (Group IV).[12]
Samples Collection
After completion of 21 days dosing with test drug daidzein, on 22nd day (after 6 days from CP administration), the experimental rats were anesthetized by ether inhalation. Blood was collected from each rat by intracardiac puncture and divided into two samples. One sample was collected in heparinized tubes for the determination of hematological parameters and the other one left to clot at 37°C and centrifuged at 3000 rpm for 15 min. The serum (supernatant) was collected and used for the estimation of biomarkers.[12]
Hematological Parameters Estimations
The heparinized blood samples were used for the determination of erythrocytes, hemoglobin concentration (Hb%), leukocytes and thrombocytes, packed cells volume (PCV), mean corpuscle volume, mean corpuscle hemoglobin (MCH), and MCH concentration using Sysmex hemocytometer.[12]
Tissue Samples
A vertical midline thoracic and abdominal incision in each animal was made to explore their viscera. Liver of each animal was isolated, separated from their surrounding fat and connective tissue, washed, and cleaned with normal saline solution. Afterward, hepatic tissues were blotted with the help of filter paper, weighed and rinsed in ice-cold saline. Half portion of each liver was homogenized for endogenous antioxidants analysis and the other half portion was processed for histological study.[12]
Estimation of Biomarkers and Endogenous Antioxidants
Hepatic biomarkers estimation
The levels liver biomarkers such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) from one portion of heparinized serum samples were analyzed using commercial kits (Robonik India Pvt. Ltd., Mumbai, India) and semi-autoanalyzer (Robonik India Pvt. Ltd., Mumbai, India).
Endogenous Antioxidants Assessments
Half portion of liver of each rat was subjected to homogenization in ice-cold 10% trichloroacetic acid phosphate buffer and saline (0.05M, pH 7.4). The hepatic homogenates were centrifuged for 15,000 rpm for 15 min. The obtained supernatants were used for estimations of malonyl dialdehyde (MDA), GHS, superoxide dismutase (SOD), and catalase by colorimetric method.[12] The results were expressed as micromoles MDA per gram of wet tissue (µmol/g tissue).[13,14]
The glutathione (GSH) level of hepatic tissue was analyzed using Ellman's method. The amount of GSH was calculated using absorption coefficient, є = 13.6 × 10-4/M/cm.[15]
Catalase enzyme activity was measured by Aebi method. The enzyme activity of catalase was expressed as units/mg protein.[16]
SOD enzyme activity of hepatic tissue was analyzed by the method described by Kakkar et al. The results were expressed as U/mg protein.[17]
Histopathological Study
Specimens of hepatic tissues of each group were fixed in 10% buffered formalin and processed with paraffin wax. For histopathological feature examination, 5 µm sections were stained with hematoxylin and eosin for the examination using light microscope.[18]
Statistical Analysis
The data obtained by the various parameters were statistically evaluated by one-way analysis of variance (ANOVA), followed by Tukey–Kramer multiple comparison tests. The mean values ± standard error of mean (SEM) were calculated for each parameter.
Results
Hematological Parameters Evaluation
The rats pretreated with daidzein of higher and low dose were showed a significant (P < 0.001) improvement in majority of blood cell parameters compared to CP-treated rats. These findings were illustrated by elevation in the count of erythrocytes, Hb, thrombocytes, and PCV. Further, significant (P < 0.001) depletion in red blood corpuscles (RBC) and platelets and significant (P < 0.001) increase in leukocytes counts were identified in CP-treated group [Table 1 and Figure 1].
Table 1.
Effect of daidzein on hematological parameters in cisplatin-induced hemotoxicity in rats
Figure 1.
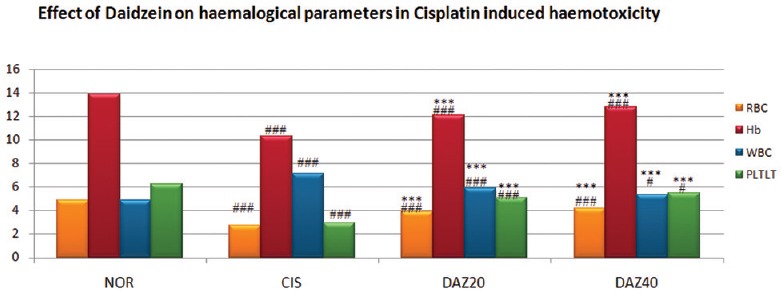
Effect of Daidzein on hematological parameters in cisplatin-induced hemotoxicity in rats. n=6, values are expressed in mean ± standard error of mean, one-way analysis of variance, followed by Tukey–Kramer multiple comparison test. *P<0.05, **P<0.01, ***P<0.001 when compared to control and #P<0.05, ##P<0.01, ###P<0.001 compared to normal
Hepatic Biomarkers Evaluation
Daidzein pretreated experimental rats of both (higher and lower dose) groups were exhibited a significant (P < 0.001) decrease in the number of ALT, AST, and ALP compared to CP administered rats. However, compared to normal group, a significant (P < 0.001) increase in the number of ALT, AST, and ALP was observed in CP-treated rats [Table 2 and Figure 2].
Table 2.
Effect of daidzein on serum biomarkers in cisplatin-induced hepatotoxicity in rats

Figure 2.
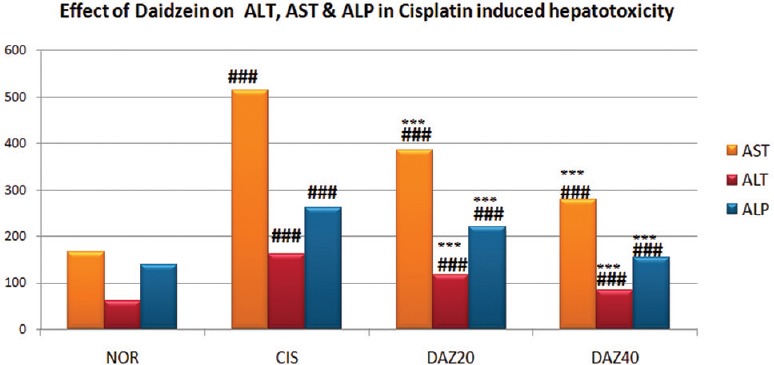
Effect of Daidzein on serum biomarkers in cisplatin-induced hepatotoxicity in rats. n=6, values are expressed in mean ± standard error of mean, one-way analysis of variance, followed by Tukey–Kramer multiple comparison test. *P<0.05, **P<0.01, ***P<0.001 when compared to control and #P<0.05,##P<0.01, ###P<0.001 compared to normal
Estimation of Endogenous Antioxidants
A significant (P < 0.001) depletion in lipid peroxide (LPO) was observed in daidzein pretreated rats of higher dose group compared to CP-treated group which was identified by diminish in the MDA level. A significant elevation in LPO was exhibited in CP-treated rats which was identified by increase in MDA level. Daidzein pretreated rats of both (higher and lower dose) groups were illustrated remarkable (P < 0.001) rise in the value of GSH compared to CP-treated rats and significant (P < 0.001) decline in GSH level was observed in CP-treated rats compared to normal group rats. There was a significant (P < 0.001) elevation in SOD, and catalase units were found in daidzein pretreated rats of both (higher and lower dose) groups compared to CP-treated group. However, compared to normal group rats, a remarkable (P < 0.001) decline in SOD and catalase was found in CP-treated rats [Table 3 and Figure 3].
Table 3.
Effect of daidzein on endogenous antioxidants in cisplatin- hepatotoxicity in rats
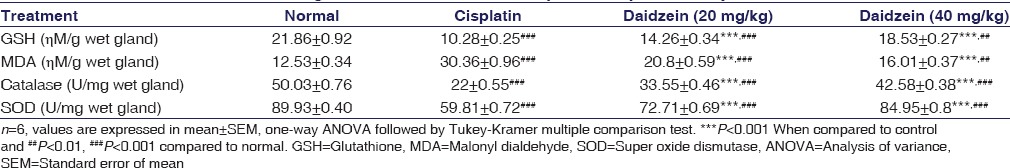
Figure 3.
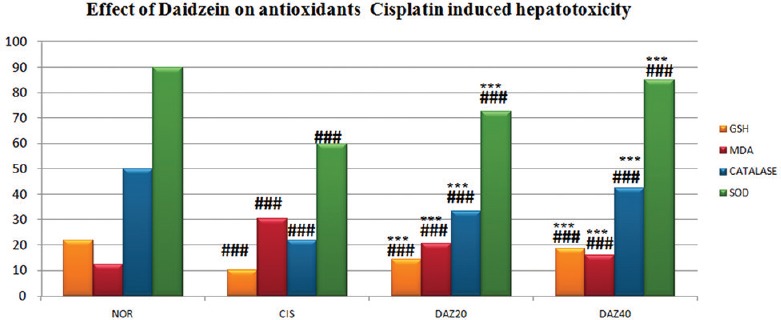
Effect of Daidzein on antioxidants in cisplatin-induced hepatotoxicity in rats. n=6, values are expressed in mean ± standard error of mean, one-way analysis of variance, followed by Tukey–Kramer multiple comparison test. *P<0.05, **P<0.01, ***P<0.001 when compared to control and #P<0.05, ##P<0.01, ###P<0.001 compared to normal
Histopathological Study
A normal histological architecture of hepatic tissue was observed with hepatocytes, central vein, Kupffer cells, liver sinusoids, and parenchymal cells in normal group. In control group treated with CP exhibits various degrees of degeneration and necrosis of parenchymal cell, inflammatory infiltration and congestion of hepatic sinusoids, and proliferation of Kupffer cells. In daidzein pretreated rats were revealed protection toward histopathological features compared to CP-treated rats by significant refinement in histological architecture of liver, hepatic sinusoids appear to closely normal and decline in Kupffer cell proliferation [Figure 4].
Figure 4.
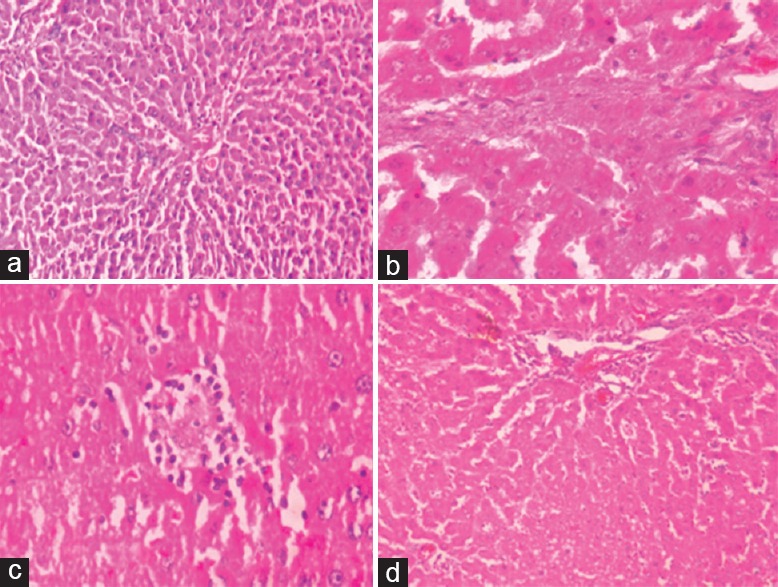
(a) Normal control group (normal appearance of hepatocytes and cords, no fatty degeneration, and no necrosis). (b) Cisplatin (7.5 mg/kg) control group (fatty degeneration and necrosis, inflammatory infiltration, and enlarged vacuolations). (c) Daidzein (20 mg/kg) low-dose group (mild inflammation, slight fatty degeneration, and less vacuolations in cytoplasm). (d) Daidzein (40 mg/kg) high-group (cytoplasm well maintained, no inflammation, and very few fatty degenerations)
Discussion
The current study was focused to investigate the effect of phytoestrogen, daidzein on CP-induced hemotoxicity and hepatotoxicity in Wistar rats.
CP is one of the most potent cytotoxic drugs commonly used for the treatment of variety of cancers such as ovarian, testicular, lung, endometrial, bladder, germ, cervical, and head and neck carcinoma. However, besides of its clinical efficiency in carcinoma, it has severe toxicities and side effects such as liver toxicity, nephrotoxicity, myelosuppression, suppression of bone marrow, gastrointestinal toxicity, neurotoxicity, ototoxicity, and hypersensitivity reactions.[2,19]
Flavonoids are the class of polyphenolic agents and commonly distributed throughout the herbal kingdom. Flavonoids are possible protectors against CP or other cytotoxic drugs induced severe adverse effects because of its inhibitory action on ROS and iron chelating action.[20,21]
Nowadays, plant estrogen or phytoestrogen has gained interest by epidemiological and clinical evidence that intake of dietary phytoestrogen such as soybean isoflavones (daidzein) exhibits potent action toward cardiovascular disorders, atherosclerosis, osteoporosis, and cancers such as breast, colon, and prostate. These actions may be due combine estrogenic and antiestrogenic properties, inhibitory action on enzymes such as tyrosine kinase and DNA topoisomerase, or stimulation of orphan receptors and antioxidant property.[2,8,22]
Hematopoietic system is considered as sensitive to investigate the adverse effects or toxicities induced by antineoplastic agents in animals and humans. In the current work, administration of CP exhibits significant toxic effects in hematological parameters in rats 6 days after treatment, and the pretreatment with daidzein showed a significant protection against hematotoxicity. The toxic effects of CP on hematological parameters were confirmed by significant depletion in count of erythrocytes, Hb, thrombocytes, and PCV. The obtained results implicate an etiological connection between anemia disease and CP. It could be demonstrated by different mechanisms showing bone marrow suppression or elevated osmotic fragility of erythrocytes. Thus, treatment with CP might induce anemic disorders due to suppression of hematopoietic tissues, disturbance in erythropoiesis, and elevated erythrocytes reduction because of an impaired erythrocyte membrane permeability and defective iron metabolism.[12,23]
It was already evidenced that chronic administration of CP leads to decrease in thrombocytes and rise in leukocytes. The depletion of platelets may be due to myelosuppression or platelet aggregation.[24] The elevation of white blood corpuscles (WBC) count might be due to infection during CP treatment or cascade of inflammatory reactions. Moreover, it was demonstrated that CP induces oxidative stress injury in human thrombocytes and lymphocytes leads to apoptosis by affecting their life span.[25]
Daidzein pretreated rats exhibit beneficial effects on hematopoietic system by elevating RBCs, Hb%, PCV, and increase in platelet count against CP-induced hematotoxicity markers. Elevation of erythrocyte count by daidzein might be related to either stimulation of erythropoiesis or prevention of bone marrow suppression or the ability of daidzein in decreasing cholesterol effects.[26,27]
Most of the studies evidenced that hepatic damage results due to elevation in serum biomarker enzymes and oxidative stress and generation of ROS.[21]
Serum biomarkers such as ALT, AST, and ALP are regarded as sensitive parameters in hepatic injury as these are situated in cytoplasm region and entered into circulation after cellular damage and they play a vital role in evaluating function and integrity of hepatic cells. In the current work, animals administered with single dose of CP were showed a significant elevation in the levels AST, ALT, and ALP. These findings illustrate hepatic injury and cellular disruption followed by necrosis in hepatocytes due to serum enzymes.[21] Daidzein exhibits a significant decline in AST, ALT, and AST in CP-treated rats. The decline in levels of serum biomarker findings in daidzein administered animals might be due to its free radical scavenging action that reduces leakage of enzymes into bloodstream and stabilizes membrane permeability.[28]
Elevation of ROS destroys the lipid components on cytoplasmic membrane, denature of proteins and nucleic acids. CP is highly reactive with SH groups and reduces GSH content. These mechanisms lead to rise in MDA level and depletion in GSH, SOD, and catalase value in the pathogenesis of hepatic tissue injury.[19]
Several antioxidant compounds were developed and analyzed in various experimental and clinical studies to ameliorate CP-induced toxicities.[21] Daidzein administration implicates significant attenuation effects by a decline in MDA and elevation in GSH, catalase, and SOD in CP-treated rats. Moreover, previous studies had evidence that daidzein demonstrated antioxidant action on cerebral tissues, liver, and jejunum[6,28,29] Thus, daidzein significantly attenuated ROS and oxidative stress injuries in liver caused by CP by its antioxidant action.
In the present study, histopathological findings of hepatic tissues reported that CP administration induces distended hepatocytes, fatty degeneration, inflammatory infiltration, and disruption of hepatic cords and dilated blood sinusoids. It was already demonstrated that histopathological modulations occur in CP-induced liver toxicities. These observations might be correlated with an increase in serum biomarkers and oxidative stress.[21]
Daidzein-treated experimental rats were showed high potency on histopathological changes in CP-induced hepatic damage. The beneficial alterations brought in histopathological degenerations of liver tissue by daidzein might be due to its ability to diminish the free radicals in oxidative stress injury and to scavenge the peroxyl and hydroxyl radicals in hepatic tissues. Furthermore, it's demonstrated that daidzein pretreatment attenuates the liver damage and protects the antioxidant activity in D-galactosamine-induced hepatic oxidative injury in rats.[28]
Thus, oral treatment of daidzein exhibits significant efficacy in hematotoxicity and hepatotoxicity induced by CP at high dose through its free radical scavenging and antioxidant actions. The test group of high dose of daidzein exhibits more significant effects in serum biomarker levels, antioxidant levels, and blood cell parameters when compared with test group of low dose of daidzein. It shows at high-dose daidzein produced a significant potency in CP-induced toxicities.
The present study implicates that CP administration leads to hepatic damage through lipid peroxidation and oxidative evidenced by changes in liver biomarkers, MDA, GSH and antioxidant enzymes (catalase and SOD), and histopathological modulations. Further, hematological disturbances were exhibited in CP-induced rats revealed by alterations in RBC, Hb, WBC, and platelets. The oral administration with daidzein demonstrates protective role by its antioxidant and free radical scavenging actions in CP-induced hematopoietic toxicity and hepatotoxicity in rats.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Ambele R. Toxicities of anticancer drugs and its management. Int J Basic Clin Pharmacol. 2012;1:2–12. [Google Scholar]
- 2.Abdel Moneim AE. Azadirachta indica attenuates cisplatin-induced neurotoxicity in rats. Indian J Pharmacol. 2014;46:316–21. doi: 10.4103/0253-7613.132182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iseri S, Ercan F, Gedik N, Yüksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230:256–64. doi: 10.1016/j.tox.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 4.Abdelmeguid NE, Chmaisse HN, Abou Zeinab NS. Silymarin ameliorates cisplatin-induced hepatotoxicity in rats: Histopathological and ultrastructural studies. Pak J Biol Sci. 2010;13:463–79. doi: 10.3923/pjbs.2010.463.479. [DOI] [PubMed] [Google Scholar]
- 5.Hany AO, Wafaa RM, Hany HA, El-Shaimaa AA. Tangeretin alleviates cisplatin-induced acute hepatic injury in rats: Targeting MAPKs and apoptosis. PLoS ONE. 2016;11:1–18. doi: 10.1371/journal.pone.0151649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aras AB, Guven M, Akman T, Ozkan A, Sen HM, Duz U, et al. Neuroprotective effects of daidzein on focal cerebral ischemia injury in rats. Neural Regen Res. 2015;10:146–52. doi: 10.4103/1673-5374.150724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye YB, Chen AL, Lu W, Zhuo SY, Liu J, Guan JH, et al. Daidzein and genistein fail to improve glycemic control and insulin sensitivity in Chinese women with impaired glucose regulation: A double-blind, randomized, placebo-controlled trial. Mol Nutr Food Res. 2015;59:240–9. doi: 10.1002/mnfr.201400390. [DOI] [PubMed] [Google Scholar]
- 8.Guo JM, Xiao BX, Dai DJ, Liu Q, Ma HH. Effects of daidzein on estrogen-receptor-positive and negative pancreatic cancer cells in vitro. World J Gastroenterol. 2004;10:860–3. doi: 10.3748/wjg.v10.i6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Physicians Desk Reference (PDR) Health, Daidzein. [Last accessed on 2007 Jun 19]. Available from: http://www.pdrhealth.com/drug_info/nmdrugprofiles/nutsupdrugs/dai_0089.shtml .
- 10. [Last accessed on 2016 Agg 08, 2016 Nov 09, 2016 Dec 02, 2016 Dec 27]. Available from: http://icmr.nic.in/bioethics/final_cpcsea.pdf .
- 11.Kale MS, Laddha KS. Isolation, characterization and quantification of isoflavone in Momordica dioica Roxb. ex wild (Cucurbitaceae) fruits. Int J Appl Res Nat Prod. 2013;5:28–36. [Google Scholar]
- 12.Ashraf YN. Protective effect of aged garlic extract against the oxidative stress induced by cisplatin on blood cells parameters and hepatic antioxidant enzymes in rats. Toxicol Rep. 2014;1:682–91. doi: 10.1016/j.toxrep.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saharan RK, Sharma SC. Correlation studies of trehalose with oxidative stress in ethanol stressed yeast pachysolen tannophilus. Curr Res J Biol Sci. 2010;2:300–5. [Google Scholar]
- 14.Mihara M, Uchiyama M. Determination of malonyl dialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 15.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 16.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods in Enzymatic Analysis. Vol. 3. New York: Academic Press; 1983. pp. 276–86. [Google Scholar]
- 17.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 18.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 5th ed. Edinburgh, New York, London, Philadelphia: Churchill Livingstone Pub.; 2002. pp. 125–38.pp. 172–5.pp. 184–93.pp. 593–620. [Google Scholar]
- 19.Amany AT, Ahmed MA, Ahmed EA, Romissaa HS. Cinnamic acid attenuates cisplatin-induced hepatotoxicity and nephrotoxicity. J Basic Environ Sci. 2016;3:1–9. [Google Scholar]
- 20.Agarwal AD. Pharmacological activities of flavonoids – A review. Int J Pharm Nanotech. 2011;4:1394–8. [Google Scholar]
- 21.Arhoghro EM, Keh CI, Prohp TP. Cymbopogon citratus aqueous extract alleviates cisplatin-induced hepatic oxidative stress and toxicity in albino rats. Int J Curr Microbiol Appl Sci. 2014;3:586–604. [Google Scholar]
- 22.Hintz KK, Ren J. Phytoestrogenic isoflavones daidzein and genistein reduce glucose-toxicity-induced cardiac contractile dysfunction in ventricular myocytes. Endocr Res. 2004;30:215–23. doi: 10.1081/erc-120037730. [DOI] [PubMed] [Google Scholar]
- 23.Yuan G, Dai S, Yin Z, Lu H, Jia R, Xu J, et al. Toxicological assessment of combined lead and cadmium: Acute and sub-chronic toxicity study in rats. Food Chem Toxicol. 2014;65:260–8. doi: 10.1016/j.fct.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 24.Sirage HM. Biochemical and hematological studies for the protective effect of Oyster Mushroom (Pleurotus ostreatus) against glycerol-induced acute renal failure in rats. J Biol Sci. 2009;9:746–52. [Google Scholar]
- 25.Olas B, Wachowicz B, Majsterek I, Blasiak J. Resveratrol may reduce oxidative stress induced by platinum compounds in human plasma, blood platelets and lymphocytes. Anticancer Drugs. 2005;16:659–65. doi: 10.1097/00001813-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Somjen D, Katzburg S, Kohen F, Gayer B, Livne E. Daidzein but not other phytoestrogens preserves bone architecture in ovariectomized female rats in vivo. J Cell Biochem. 2008;103:1826–32. doi: 10.1002/jcb.21565. [DOI] [PubMed] [Google Scholar]
- 27.Mona AL, Naglaa HM, Nahed LZ, Mohamed SA, Hassan MS. Effects of soybean isoflavone on lipid profiles and antioxidant enzyme activity in streptozotocin induced diabetic rats. Glob J Pharmacol. 2014;8:378–84. [Google Scholar]
- 28.Max YW, Bernad P, Roy S, Onni N, Heidi K, Seppo P, et al. The cytoprotective effect of α-tocopherol and daidzein against D-galactosamine-induced oxidative damage in the rat liver. Metabolism. 2007;56:865–75. doi: 10.1016/j.metabol.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa K, Adachi J, Wong MC, Ueno Y. Protective effect of daidzein against acute ethanol-induced lipid peroxidation in rat jejunum. Kobe J Med Sci. 2006;52:141–9. [PubMed] [Google Scholar]


