Abstract
Objective:
Angelica glauca Edgew (Apiaceae) is used in traditional medicine for treatment of several diseases including bronchial asthma. The present investigation was aimed to evaluate broncho-relaxant activity of A. glauca essential oil in histamine and ovalbumin (OVA)-induced broncho constriction in experimental animals.
Materials and Methods:
Airway was induced using histamine aerosol in guinea pigs (n = 24) and OVA aerosol in albino mice (n = 24). The number of inflammatory cells, namely, absolute eosinophils count in blood, total immunoglobulin E (IgE) in serum, eosinophils, and neutrophils in bronchoalveolar lavage fluid (BALF) and histopathological examination of lung tissues were investigated in A. glauca oil and dexamethasone-treated groups. A. glauca oil 200 μL/kg was given orally, and dexamethasone 2 mg/kg was given intraperitoneal. Both the treatments were repeated daily for 7 days. Results were analyzed by one-way ANOVA, and P ≤ 0.05 was considered statistically significant.
Results:
Treatment with A. glauca essential oil significantly (P < 0.001) increased the time of preconvulsive dyspnea in histamine-induced guinea pigs. Oral treatment of A. glauca oil significantly (P < 0.001) decreased absolute blood eosinophil count (from 325 ± 3.69 to 200 ± 3.05 cells/mm3), serum level of IgE (from 6.10 ± 0.05 to 0.70 ± 0.08 IU/L), and the number of eosinophils (from 11.0% ±1.41% to 3.0% ±0.51%), neutrophils (from 13.0% ±1.12% to 5.0% ±1.39%) in BALF. Histopathological changes observed in lungs of untreated group were marked suppressed by treatment with A. glauca oil.
Conclusion:
The essential oil of A. glauca has bronchorelaxation in both histamine and OVA-induced bronchoconstriction in animals. The traditional use of A. glauca against asthma could be attributed to its bronchodilator property as observed in the present study.
Key words: Angelica glauca, bronchorelaxation, histamine, ovalbumin
Bronchial asthma, a chronic disease involving the airways affects people of all ages, but it often starts during childhood. Bronchial asthma is a substantial health burden, often affecting quality of life, not only due to its physical effects but also its psychological and social effects.[1] Asthma can be classified as allergic asthma, caused by exposure to an allergen and nonallergic asthma caused by stress, exercise, illnesses like a cold or flu or exposure to extreme cold weather, irritants in the air, or some medications.[2] Bronchial asthma is characterized by inflammation of the bronchial tubes with increased production of sticky secretions inside the tubes. Currently, available treatments for asthma are managed using beta-2 agonists, anticholinergics, methylxanthines, mast cell stabilizers, leukotriene antagonist, glucocorticoids, anti-immunoglobulin E (IgE) antibody such as omalizumab are also used. Moreover, these drugs are associated with several adverse effects, such as muscle tremors, restlessness, hypotension, hyperglycemia, tachycardia, flushing, convulsions, mood changes, and adrenal crisis.[3] To minimize or possibly prevent these side effects, alternative, and complementary medicine is being sought. Some essential oils obtained from plants have been traditionally used in respiratory tract infections. Inhalation of vapors of essential oils augmented anti-inflammatory effect on trachea and reduces asthma.[4] The genus Angelica is an important medicinal and aromatic plant used in traditional medicine to treat various diseases. It is locally known as chora or gandrayan. The plant is found in Western Himalaya and ranks third on the list of 52 medicinal plants prioritized for consultation and conservation. Angelica glauca Edgew (Apiaceae) is considered to be crucially endangered and need to be conserved.[5] Traditionally, A. glauca is used in medicines, aromatic spices, and condiments. A. glauca has high global and domestic demands for the preparation of various drug formulations. In traditional medicine, the plant A. glauca is used in typhoid, bronchitis, flatulence, colic, and stomach pain.[6]
The essential oil of A. glauca has been reported to contain β-phellandrene, (Z)-ligustilide,[7] methyl octane, limonene, β-phellandrene, β-pinene, (Z)-ligustilide,[8] α-phellandrene, β-pinene, thujene, β-caryophyllene, β-bisabolene, germacrene D with oxygenated terpenes such as trans-carveol, β-caryophyllene oxide,[9] α-phellandrene, β-pinene, β-caryophyllene[10] as the major constituents. Various biological activities, namely, antioxidant, antimicrobial,[11] antifungal,[10] and insecticidal[12] have been reported. The essential oil of A. glauca has not been explored for its effect in asthma. Therefore, the aim of the present study was to evaluate effects of A. glauca in bronchoconstriction induced by histamine and ovalbumin (OVA) in experimental animals.
Materials and Methods
Reagents
The following reagents were commercially purchased and used: OVA (Sigma-Aldrich, USA), aluminium hydroxide (HiMedia Pvt., Ltd., Mumbai, Maharashtra, India), histamine dihydrochloride, tween 80 (HiMedia Pvt., Ltd., Mumbai, Maharashtra, India), dexamethasone (Centaur pharmaceuticals, Goa, India), Turk solution (NICE Chemical Pvt., Ltd., Cochin, Kerala, India), disodium ethylenediamine tetraacetic acid (EDTA-2Na), and A. glauca oil (Kshipra Biotech Private Limited, Indore, India).
Animals
The female Guinea pigs (400–600 g) purchased from J. N. Medical College, Belagavi, Karnataka, India and were housed in laboratory conditions (temperature 22°C ± 2°C and humidity 55% ±5%) for a week in 12:12 h light: Dark cycle before starting experimentation. The female albino mice (18–25 g) were purchased from Sri Venkateshwara Enterprises, Bengaluru, Karnataka, India and were treated similarly as Guinea pigs, before experimental study. The study was initiated after protocol approved by the Institutional Animal Ethics Committee. All the experiments were conducted in strict compliance with the ethical principles and guidelines provided by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (KLECOP/IEAC/Res. 22-10/10/2015).
Toxicity Study
Acute toxicity for this edible plant has been reported to be − 2.2 g/kg for mice and 11.2 g/kg for rat.[13] As per the IAEC advise to avoid duplication of studies, acute toxicity studies were not carried out, and two doses 200 and 400 µL/kg were used in the present study.
Chromatographic Analysis of the Essential Oil of Angelica glauca
The chemical composition of the essential oil was analyzed using a gas chromatograph (GC) (Varian 450 fitted with a fused silica capillary column TG-5 (30 m × 0.25 mm id, 0.25 μm film thickness) under the experimental conditions reported earlier.[14,15] The oven temperature was programmed from 60°C to 220°C at 3°C/min, using nitrogen as carrier gas. The injector and the flame ionization detector temperature were set at 230 and 240°C, respectively. GC-mass spectrometer (GC-MS) analysis was employed a Thermo Scientific Trace Ultra GC interfaced with a Thermo Scientific ITQ 1100 MS fitted with a BP-1 fused silica capillary column (30 m × 0.25 mm; 0,25 mm id 0.25 μm film thickness). The oven and injector temperature were same as used for GC, and helium was used as carrier gas at 1.0 mL/min for analysis. The injection volume was 0.1 μL in n-hexane, with a split ratio of 1: 50. MS was taken at 70 eV with a mass range of m/z 40–450, and other parameters used were those reported earlier.[16,17] The major constituent of the essential oil of A. glauca was identified and confirmed (co-injection of commercial sample from Sigma-Aldrich, India (≥98% purity).
Histamine-induced Bronchoconstriction in Guinea Pigs
Bronchospasm was induced in each guinea pig by exposing to 1% histamine aerosol under constant pressure (1 kg/cm2) in an aerosol chamber (24 cm × 14 cm × 24 cm) made of perplex glass, and the time for onset of dyspnea which indicates preconvulsive time (PCT) was noted on day 0 as basal value. As soon as, the preconvulsion dyspnea (PCD) was recorded, the animals were removed from the chamber and positioned in fresh air to recover. After determination of PCD, the animals were randomly divided into four groups (n = 6 in each): group I negative control, received normal saline orally; Group II received intraperitoneal (i.p.) injection of dexamethasone 2 mg/kg (standard drug) once daily for 7 days, Group III and IV received orally A. glauca oil at the dose of 200 µL/kg and 400 µL/kg once daily on 7th day 2 h after the last dose, all the groups were subjected to histamine aerosol and the time for the onset of PCT was recorded.[18] The percent increase in the time of PCT was calculated using following formula.
Percentage increase in time of PCT = (1 − T1/T2) ×100
Where; T1 = time for PCT on day 0, T2 = time for PCT on day 7.
Ovalbumin-sensitization in Mice
Female mice were randomly divided into four groups (n = 6 in each), normal, negative control, dexamethasone, and A. glauca oil treatment groups. Except normal mice, other groups were sensitized by i.p. injection of 50 µg OVA and 1 mg aluminum hydroxide in 200 µL of phosphate-buffered saline (PBS) on day 1 and day 7. Group I was given normal saline for 14 days. After sensitization, from day 8 to day 14 group II was given only OVA aerosols (1% w/v) in PBS for 30 min every day. Group III was given injection dexamethasone (2 mg/kg) i.p. injection daily for 7 days along with OVA aerosols. A. glauca oil was given orally to group IV at a dose of 200 µL/kg for 7 days along with OVA aerosols. On day 15, 24 h after the final allergen aerosol animals were sacrificed by over anesthesia to collect cardiac blood on EDTA bulbs for differential cell counts by staining with a modified Giemsa stain. Lung lavaging was performed for collection of bronchoalveolar lavage fluid (BALF). The trachea was aspirated (3 times) with PBS until 2 mL of BALF was collected. BALF from each animal was pooled in a plastic tube, cooled in ice, and centrifuged (2 g) at 4°C for 10 min. Cell pellets were resuspended in the same buffer (1 mL). A portion of the cell suspension was mixed with Turk solution, and cells were counted in a hemocytometer. The lungs were dissected out along with bronchia for histopathological studies.
Measurement of Immunoglobulin E Levels in Serum
The serum level of OVA-specific IgE was measured by ELISA kit using commercially available reagents, according to the manufacturer's instructions. The detection limit was 0.1 ng/mL for IgE.
Histopathological Examination of Lung
Dissected out lungs were fixed in 10% buffered formalin for 24 h, dehydrated, embedded in paraffin, and the cut sections stained with hematoxylin-eosin were observed under light microscope to assess the degree of peribronchial and perivascular inflammation.
Statistical Analysis
Results were expressed as mean ± standard error of the mean and were analyzed by one-way ANOVA followed by Dunnett's multiple comparison tests (GraphPad Prism, Version 5, GraphPad Software, Inc., USA software for Windows) and P < 0.05 was considered statistically significant.
Results
Analysis of the Essential Oil
The GC-MS analysis of essential oil of A. glauca revealed that the main compound was identified as α-pinene [Figure 1].
Figure 1.
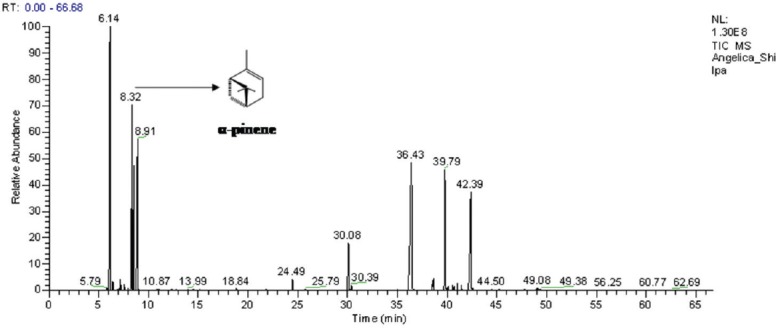
Gas chromatograph-total ion chromatogram of Angelica glauca essential oil and its major compound α-pinene
Effect of the Essential Oil of Angelica glauca on Histamine-induced Bronchoconstriction in Guinea Pigs
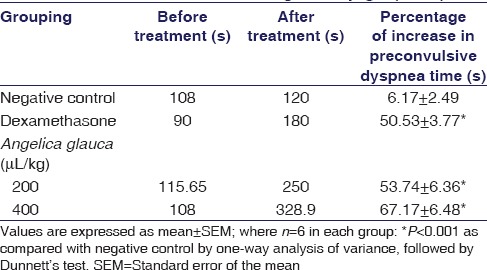
The essential oil of A. glauca significantly (P < 0.001) and dose-dependently increased the latent period for PCD. In histamine-induced bronchoconstriction at the dose of 200 and 400 µL/kg, the percentage increase in PCT was found to be 53.74 ± 6.36 and 67.17 ± 6.48, respectively. Hence, the increase of PCT at a dose of 400 µL/kg of A. glauca oil was almost comparable to that of dexamethasone (standard drug)-treated group [Table 1].
Table 1.
Effect of Angelica glauca oil on histamineinduced bronchoconstriction in guinea pigs (n=24)
Effect of the Essential Oil of Angelica glauca on Absolute Eosinophil Count in Blood
Control group showed maximum increase in eosinophil count 24 h after the final exposure of OVA. A. glauca oil-treated group at the dose of 200 µL/kg significantly (P < 0.001) decreased inflammatory cell in blood [Figure 2a].
Figure 2.
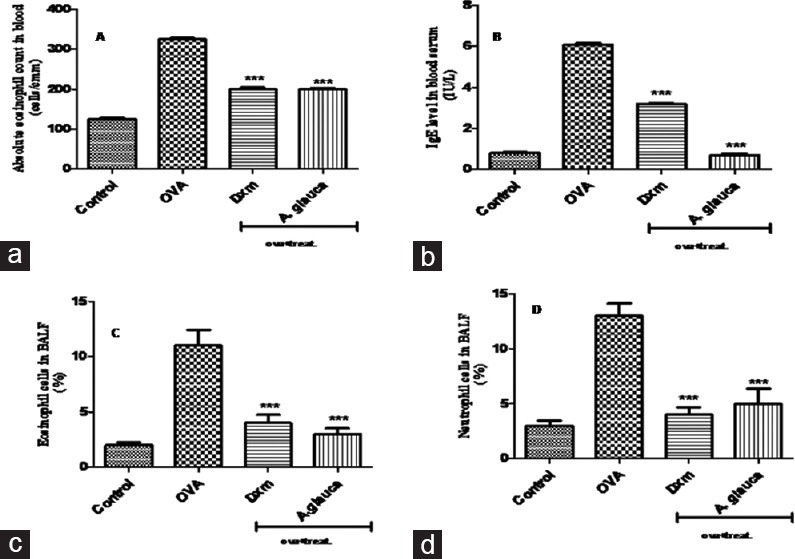
Effect of Angelica glauca essential oil on: (a) Blood eosinophils (cells/mm3); (b) serum immunoglobulin E (IU/L); (c) eosinophils in bronchoalveolar lavage fluid (percentage); (d) neutrophils in bronchoalveolar lavage fluid (percentage) values were expressed as mean ± standard error of the mean, *P < 0.001 compared with ovalbumin-treated group
Effect of the Essential Oil of Angelica glauca on Serum Immunoglobulin E Level in Ovalbumin-sensitized Mice
Antigens are known to induce antigen-specific IgE antibody production. Repeated OVA inhalation significantly (P < 0.001) increased on the day 15 (immediately after the final OVA challenge) the total serum level of IgE. The treatment with essential oil of A. glauca significantly (P < 0.001) decreased the serum IgE at a dose of 200 µL/kg [Figure 2b].
Effect of Angelica glauca Essential Oil on Inflammatory Cells in Bronchoalveolar Lavage Fluid
After challenging with OVA, the levels of inflammatory cells including eosinophils and neutrophils were significantly (P < 0.001) increased as compared with normal group. However, with A. glauca essential oil treatment, the inflammatory cells were significantly (P < 0.001) decreased as compared to that of untreated and was almost comparable to that of dexamethasone group [Figure 2c and d].
Effect of Angelica glauca on Ovalbumin-induced Histopathological Changes in Lung Tissues
The histopathological examination of lung sections of mice exposed to OVA stained with hematoxylin and eosin showed significant inflammatory changes in peribronchial and perivascular areas and also increased bronchial muscles thickening, epithelial hyperplasia [Figure 3b]. In contrast, the histological changes induced by OVA were markedly suppressed by treatment with A. glauca oil at a dose of 200 µL/kg [Figure 3d] as compared to untreated group [Figure 3a] and was almost similar to the dexamethasone [Figure 3c]-treated groups.
Figure 3.
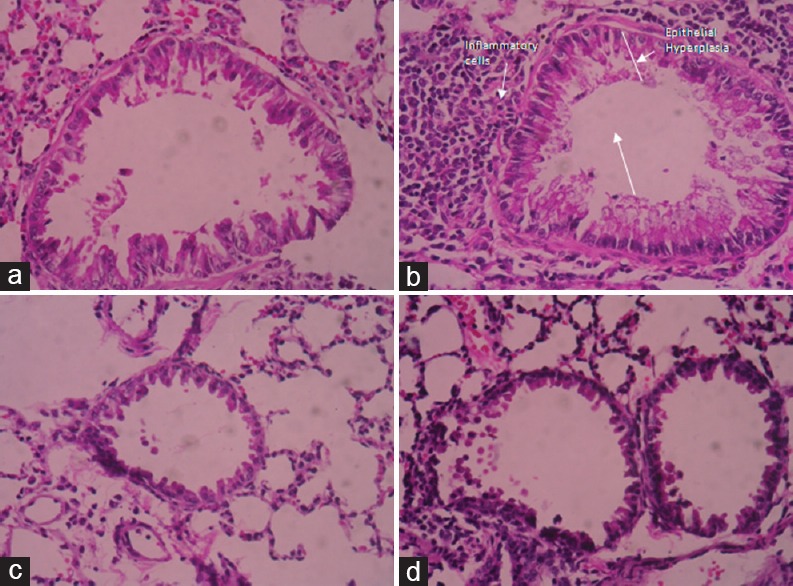
Effect of Angelica glauca oil on lung inflammatory cell infiltration in ovalbumin-induced allergic asthmatic mice (H and E, ×40,) in (a) normal group; (b) ovalbumin group; (c) ovalbumin + Dexa (2 mg/kg) group; (d) ovalbumin + Angelica glauca oil (200 μL/kg) treated
Discussion
Asthma is an allergic respiratory disease triggered by different spasmogens such as acetylcholine, leukotrienes, prostaglandins, or specific exposure of allergens, which reflect the signals of acute bronchoconstriction.[19] Histamine and other inflammatory mediators cause a host of changes in bronchial tissue by increasing the mucous secretion and simultaneous rapid constriction of bronchial smooth muscle. Bronchodilating effect of A. glauca oil was determined by its effect to increase the latency for PCT in guinea pigs. The study revealed that the latency for PCT was significantly (P < 0.001) increased suggesting bronchodilating activity of A. glauca oil against histamine.
OVA-induced model of allergic airway inflammation demonstrated that IgE levels in blood and eosinophilic infiltration in the lungs were markedly increased, as in asthmatic condition. Eosinophil count was elevated in response to the OVA exposure in untreated animals. OVA-induced asthma has been recognized as pathological condition that resembles chronic airway inflammation characteristically associated with the infiltration of lymphocytes, eosinophils, macrophages, and neutrophils into the bronchial lumen.[20]
Eosinophils become active when one has certain allergic diseases, infections, and other medical conditions. Treatment with the essential oil of A. glauca significantly reduced the total eosinophils in the blood. Asthma is almost always associated with some type of IgE-related reaction and therefore has an allergic basis.[21] Numerous epidemiologic studies have shown a highly significant relationship between asthma and sensitization to various allergens as demonstrated by skin tests or the presence of specific IgE in the serum. IgE initiates the allergic response by causing mast cells to release inflammatory mediators and by recruiting eosinophils.[22] Thus, blocking the effects of IgE is a promising strategy for preventing or ameliorating allergic symptoms.[23] This study showed significant reduction in serum IgE level by A. glauca oil-treated group. Eosinophil-mediated secretions in asthma have been reported in BALF in the form of granule-derived basic proteins.[24] Once established, the repetitive cycle of tissue damage and inflammatory cell recruitment becomes chronic, even in the absence of sustained allergen, the chronic inflammation persists. Eosinophil level in sputum is associated with the degree of chronic airway obstruction.[25] In the present studies, eosinophils in BALF were significantly (P < 0.001) decreased in A. glauca oil-treated groups and were comparable to that of dexamethasone-treated group. The effect of A. glauca essential oil was not compared with other bronchodilators. Besides eosinophils, neutrophils also have an important role in the late-phase asthmatic reaction. The findings of the present study need to be confirmed before their extrapolation to human situations.
Conclusion
The present study provides evidence that essential oil of A. glauca has bronchodilating and immunosuppressant actions in guinea pigs and mice. Efficacy of A. glauca oil in the treatment of bronchial asthma is worth exploring clinically.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to Director-in-Charge Regional Medical Research Centre (ICMR), Belagavi for encouraging the study. The authors are indebted to Kshipra Biotech Private Limited, Indore, India for providing essential oil of A. glauca.
References
- 1.GAN. The Global Asthma Report 2014. Auckland, New Zealand: Global Asthma Network; 2014. [Google Scholar]
- 2.AAAAI. American Academy of Allergy Asthma and Immunology; Asthma Overview. 2016. [Last cited on 2016 Jun 02]. Available from: http://www.aaaai.org/conditions-andtreatments/asthma .
- 3.Boushey HA. Basic and clinical pharmacology. In: Akporiaye ET, Aminoff MJ, Benowitz NL, Berkowitz BA, Biaggioni I, Bikle DD, editors. Drugs Used in Asthma. 12th ed. China: McGraw-Hill Companies; 2009. p. 339. [Google Scholar]
- 4.Inouye S, Yamaguchi H, Takizawa T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J Infect Chemother. 2001;7:251–4. doi: 10.1007/s101560170022. [DOI] [PubMed] [Google Scholar]
- 5.Semwal DP, Saradhi PP, Kala CP, Sajwan BS. Medicinal plants used by local Vaidya in Ukimath block, Uttarakhand. Indian J Tradit Knowl. 2010;9:480–5. [Google Scholar]
- 6.Prajapati ND, Purohit SS, Sharma AK, Kumar T. Section II: Medicinal Plants: A to Z. Jodhpur: Agrobios (India); 2006. A Handbook of Medicinal Plants a Complete Source Book; p. 49. [Google Scholar]
- 7.Kaul PN, Mallavarapu GR, Chamoli RP. The essential oil composition of Angelica glauca roots. Planta Med. 1996;62:80–1. doi: 10.1055/s-2006-957812. [DOI] [PubMed] [Google Scholar]
- 8.Thappa RK, Kaul P, Chisti AM, Kapahi BK, Suri OP, Agarwal SG. Variability in the essential oil of Angelica glauca Edgew of different geographical regions. J Essent Oil Res. 2005;17:361–3. [Google Scholar]
- 9.Agnihotri VK, Thappa RK, Meena B, Kapahi BK, Saxena RK, Qazi GN, et al. Essential oil composition of aerial parts of Angelica glauca growing wild in North-West Himalaya (India) Phytochemistry. 2004;65:2411–3. doi: 10.1016/j.phytochem.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Irshad M, Aziz S, Rehman H, Hussain H. GC-MS analysis and antifungal activity of essential oil of Angelica glauca, Plectranthus rugosus and Valeriana wallichii. J Essent Oil Bear Plants. 2012;15:15–21. [Google Scholar]
- 11.Irshad M, Rehman HU, Shahid M, Aziz S, Ghous T. Antioxidant, antimicrobial and phytotoxic activities of essential oil of Angelica glauca. Asian J Chem. 2011;23:1947–51. [Google Scholar]
- 12.Sharma RN, Deshpande SG, Joseph M. Evaluation of natural essential oils as antifeedants against Spodoptera litura. Indian Perfum. 1990;34:196–8. [Google Scholar]
- 13.Lis-Bachin M, Gardner Z, Mugufin M. American Herbal Products Association Botanical Safety Handbook. 2nd ed. U.S: CRC Press; 2013. p. 110. [Google Scholar]
- 14.Joshi RK. Chemical composition of the essential oil of Baccharoides lilacina from India. Nat Prod Commun. 2013;8:401–2. [PubMed] [Google Scholar]
- 15.Joshi RK. Essential oil of flowers of Anaphalis contorta, an aromatic and medicinal plant from India. Nat Prod Commun. 2013;8:225–6. [PubMed] [Google Scholar]
- 16.Joshi RK. GC/MS analysis of the essential oil of Leucas indica from India. Nat Prod Commun. 2014;9:1607–8. [PubMed] [Google Scholar]
- 17.Joshi RK. GC/MS analysis of the essential oil of Vernonia cinerea. Nat Prod Commun. 2015;10:1319–20. [PubMed] [Google Scholar]
- 18.Sagar R, Sahoo HB, Kar B, Mishra NK, Mohapatra R, Sarangi SP. Pharmacological evaluation of Calendula officinalis L. on bronchial asthma in various experimental animals. Int J Nutr Pharmacol Neurol Dis. 2014;4:95–103. [Google Scholar]
- 19.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–45. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Zhang Y, Liu Q, Zhong W, Xia Z. All-trans retinoic acid attenuates airway inflammation by inhibiting Th2 and Th17 response in experimental allergic asthma. BMC Immunol. 2013;14:28. doi: 10.1186/1471-2172-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 22.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (DerP I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 23.Platts-Mills E, Thomas A. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001;164:S1–5. doi: 10.1164/ajrccm.164.supplement_1.2103024. [DOI] [PubMed] [Google Scholar]
- 24.Bloemen K, Verstraelen S, Van Den Heuvel R, Witters H, Nelissen I, Schoeters G. The allergic cascade: Review of the most important molecules in the asthmatic lung. Immunol Lett. 2007;113:6–18. doi: 10.1016/j.imlet.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: Evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–36. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]


