Abstract
Objectives:
To evaluate the antidiabetic, antihyperlipidemic, and antioxidant activities of the ethanolic extracts of the flowers and inflorescence stalk of Musa balbisiana Colla. in streptozotocin (STZ)-induced Type 1 diabetic rats.
Materials and Methods:
Diabetes was induced in male Wistar albino rats (150–200 g) by single intraperitoneal injection of STZ (60 mg/kg b.w. i.p.). Albino rats (n = 25) were divided into five groups, of which five animals each. Group A (normal control) and Group B (diabetic control) received normal saline (10 ml/kg/day p.o.), whereas Group C and Group D received 250 mg/kg/day p.o. of flower and inflorescence stalk ethanolic extracts, respectively, for 2 weeks. Group E (diabetic standard) received 6 U/kg/day s.c of Neutral Protamine Hagedorn insulin. Fasting blood sugar, serum insulin, catalase (CAT), malondialdehyde (MDA), and serum lipid profile were estimated at specific intervals of time. Effect of the extracts on intestinal glucose absorption was also evaluated to know the probable mechanism of action.
Results:
Diabetic control exhibited significant increase in blood glucose, serum cholesterol, triglycerides, low-density lipoprotein, serum MDA levels and decreased serum CAT, and high-density lipoprotein levels which were significantly reverted by flower and inflorescence stalk ethanolic extracts after 2 weeks. Serum insulin levels were in increased (P < 0.05), and intestinal glucose absorption decreased significantly (P < 0.01) in extract-treated groups.
Conclusion:
Flower and inflorescence stalk of M. balbisiana Colla. possess significant antidiabetic, antihyperlipidemic, and antioxidant activities in STZ-induced Type 1 diabetic rats.
Key words: Ethanolic extract, insulin, streptozotocin
Diabetes mellitus is a major health problem worldwide. In 2011, there were 366 million people with diabetes worldwide, and by 2030, this is expected to rise to 552 million.[1] It is a metabolic disorder caused by insulin deficiency, insulin resistance, or by a combination of both. Insulin deficiency may lead to Type 1 diabetes mellitus which may be autoimmune or idiopathic in nature and represents 9% cases of insulin deficiency. Type 1 diabetes mellitus is also on the increase like Type 2 diabetes mellitus with a 3–5% increase per year and India accounts for most of the children with Type 1 diabetes mellitus in Southeast Asia.[2] Type 1 diabetes mellitus begins earlier in life than Type 2 diabetes and is associated with important short- and long-term consequences.[3] Research has been directed to develop newer compounds to complement or supplement the existing treatment for Type 1 diabetes mellitus keeping in view the complications, compliance, and cost-effectiveness of prevailing therapy. Scientific studies are being conducted on a number of medicinal plants to develop promising phytochemicals. There are numerous plants in the indigenous systems of health care still awaiting scientific inquiry.[4]
Musa balbisiana Colla. (Synonym: Musa sapientum L.) is a species of the banana plant belonging to the family Musaceae.[5] The inflorescence including the flowers and stalk is a common food item in India, particularly in the northeastern states and has been used traditionally in treating diabetes. The different parts of the plant are shown to possess antiulcerative, antimicrobial, wound healing, hypoglycemic, hypolipidemic, and antiallergic properties in various studies.[6] However, existing literatures show that the effects of its flowers and inflorescence stalk have not been evaluated in streptozotocin (STZ)-induced Type 1 diabetic rats. Therefore, the present study has been designed to evaluate the antidiabetic, antihyperlipidemic, and antioxidant activities of flowers and inflorescence stalk of M. balbisiana Colla. on STZ-induced Type 1 diabetic rats.
Materials and Methods
Collection, Authentication, and Extraction of Plant Materials
The inflorescence of M. balbisiana Colla. along with the attached inflorescence stalk was collected in December 2012. The materials were identified and authenticated (V. No.DUL. Sc. 455/2013). The flowers and inflorescence stalks after drying in shade at room temperature were grounded to fine powder. The powder was then packed into Soxhlet apparatus and subjected to hot continuous percolation using ethanol (95% v/v) as a solvent. The extract was concentrated using vaccum evaporator. A final yield of 12.17% w/w for flowers and 10.57% w/w for inflorescence stalks were obtained.
Experimental Animals
Healthy adult male Wistar albino rats (Rattus norvegicus) weighing 150–200 g were used in the study after obtaining ethical clearance from the Institutional Animal Ethics Committee (IAEC/AMC/04;dated 06/03/2012). The animals were housed in polypropylene cages, maintained under standard animal house condition as suggested by CPCSEA, and maintained on balanced diet and water ad libitum during the entire period of the experiment.
Drugs and Chemicals
Ethanol and normal saline (0.9% NaCl) were obtained from the Central Medical Store of the Institution. Human Neutral Protamine Hagedorn (NPH) insulin, STZ, thiobarbituric acid, citric acid, sodium citrate, sodium hydroxide, and glucose estimation kits were obtained from Hi Media Laboratories Pvt., Limited, Mumbai, Maharashtra, India. Kits for estimation of lipid profile were obtained from Crest Biosystems, Goa, India. Ether, thiopentone sodium, potassium phosphate buffer, hydrogen peroxide solution, and tricarboxylic acid were obtained from Sigma-Aldrich India, Bengaluru, Karnataka, India. Crude powder of metformin was obtained from Aventis Pharma Limited, Goa, India. RIA kits for insulin assay were supplied by Board of Radiation and Isotope Technology, Bhabha Atomic Research Centre (Navi Mumbai, Maharashtra, India).
Phytochemical Screening of Flower and Inflorescence Stalk Ethanolic Extracts
Flower and inflorescence stalk ethanolic extracts were subjected to qualitative phytochemical analysis for alkaloids, flavonoids, tannins, saponins, diterpenes, triterpenes, and phenols as per the standard methods.[7]
Acute (Oral) Toxicity Study
Acute oral toxicity test for the ethanolic extracts of flower and inflorescence stalk of M. balbisiana Colla. was carried out as per Organisation for Economic Co-operation and Development Guidelines 425 and was found to be safe up to 2000 mg/kg p.o. for each extract. One arbitrary dose of 250 mg/kg/p.o. of each extract was selected for the study.
Induction of Type 1 Diabetes in Rats
Overnight fasted Wistar rats (n = 25) were administered single intraperitoneal injection of 60 mg/kg STZ dissolved in 0.1 M cold sodium citrate buffer at pH 4.5. The rats had free access to 5% of glucose water and basal diet ad libitum during the next 24 h. Fasting blood glucose levels were estimated after 72 h. Twenty rats showing blood glucose levels more than 250 mg/dl were included in the study.[8]
Experimental Design for Study of Antidiabetic, Antihyperlipidemic, and Antioxidant Activities
Wistar albino rats (n = 25; out of which 20 rats were diabetic) weighing 150–200 g were divided into five groups, of which five animals each and treated as below.
Group A: Normal control: Normal saline; 10 ml/kg/day p.o.
Group B: Diabetic control: Normal saline; 10 ml/kg/day p.o.
Group C: Diabetic test 1: Flower ethanolic extract; 250 mg/kg/day p.o.
Group D: Diabetic test 2: Inflorescence stalk ethanolic extract; 250 mg/kg/day p.o.
Group E: Diabetic standard: Human NPH insulin; 6 U/kg/day s.c.
Fasting blood samples were collected from the retro-orbital sinus of each rat and subjected for estimation of fasting blood glucose and serum insulin on day 1, i.e., after 72 h of induction of diabetes and then on the 8th and 15th day of the experiment. Serum lipid profile, catalase (CAT), and malondialdehyde (MDA) levels were estimated on day 1 and day 15.
Body Weight Changes
The body weight of the animals in each group was recorded using a standard electronic weighing machine on day 1 and day 15 of the experiment.
Method of Blood Glucose Estimation
Fasting blood glucose was estimated by glucose oxidase method using glucose estimation kits.
Estimation of Serum Insulin
Serum insulin was assayed by the radioimmunoassay method. The method is based on the principle that the insulin present in the test serum competes with the radiolabeled insulin for its antibody when added to a tube containing a fixed amount of antibody and a fixed amount of the radiolabeled insulin (I125-labeled). The amount of radiolabeled insulin bound to the antibody is inversely proportional to the amount of insulin in serum. Gamma scintillation counter is used to measure the bound radiolabeled insulin.[9]
Biochemical Estimations of Serum Lipid Profile
The total serum cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol estimation was done by the method described by CHOD-PAP, GPO-PAP and PEG-CHOD-PAP methods, respectively, using commercial kits. Low-density lipoprotein (LDL)-cholesterol was measured using the formula of Friedewald et al.[10]

Biochemical Estimations for Antioxidant Status of Plant Extracts
Enzymatic assay of catalase
CAT activity was measured in blood on day 1 and day 15 of the experiment by continuous spectrophotometric rate determination by Beers and Sizer method.[11] The values were expressed as µmol of H2O2/min/ml.
Estimation of serum malondialdehyde
MDA level was measured on day 1 and day 15 of the experiment by colorimeter using thiobarbituric acid reactive substance as described by Satoh.[12] The results were expressed in nmol/ml.
Evaluation of the Effect of Plant Extracts on Intestinal Glucose Absorption
The effect of flower and inflorescence stalk ethanolic extract on intestinal glucose absorption was studied by the method described by Das et al. with some modifications.[13] Das et al. used five to six intestinal loops of roughly equal size lying between proximal jejunum and distal ileum, while in the present study, an intestinal loop of 8 cm from pyloric end was made. Healthy albino male rats weighing 150–200 g; 20 in number were divided into four groups of five animals each and treated as below.
Group A1: Normal Control: Normal Saline; 10 ml/kg/day p.o.
Group B1: Test Drug 1: Flower ethanolic extract; 250 mg/kg/day p.o.
Group C1: Test Drug 2: Inflorescence stalk extract; 250 mg/kg/day p.o.
Group D1: Standard Drug: Metformin; 90 mg/kg/day p.o.
Metformin was used as the standard drug because, as an antidiabetic, it is shown to retard intestinal glucose absorption which is one of the proposed mechanisms for decreasing blood glucose.[14] The drugs were administered continuous for 7 days following which animals were kept fasting for 18 h. Subsequently, all animals were anesthetized with thiopentone sodium 40 mg/kg intraperitoneally. The abdomen of the rat was opened through midline incision and an intestinal loop of 8 cm from pyloric end was made. D-glucose 2.5 mg (1 ml of 250 mg% in normal saline at 37°C) was injected into the loop by a tuberculin syringe. The animal was bled to death by incising the aorta after an absorptive period of 15 min. After that, the intact loop was excised and weighed before and after draining the contents of loop to know the final volume. After constant dilution, resultant fluid was analyzed for glucose concentration by glucose oxidase method. The absorption was expressed in terms of mg/g dry weight/hour. Dry weight of intestinal segment was measured after dehydrating the loop in ethyl alcohol for 24 h and then drying in hot air oven at 110–120°C for 2 h.
Statistical Analysis
The results were statistically analyzed using one-way ANOVA followed by Tukey's post hoc test. P < 0.05 was considered statistically significant. Student's t-test (paired) was applied to compare body weight of animals on day 1 and day 15 of the experiment. The statistical analysis was done using computerized GraphPad Prism Software version 5.00 (GraphPad Software, Inc., San Diego, California, CA, USA).
Results
Acute Toxicity Test
No sign of toxicity and mortality was recorded among the rats at a dose of 2000 mg/kg, hence, one eighth of the dose tested, i.e., 250 mg/kg was selected arbitrarily for the study.
Qualitative Phytochemical Analysis
Both the extracts were positive for flavonoids, diterpenes, tannins, saponins, and phenols. Alkaloid was found to be present in flower extract but absent in inflorescence stalk extract. Triterpenes were found to be absent in both flower and inflorescence stalk ethanolic extract.
Effect of Extracts on Fasting Blood Glucose Level in Diabetic Rats
Table 1 shows the fasting blood glucose levels on 1st day, 8th day, and 15th day of the experiment. There was significant rise (P < 0.01) in blood glucose level in rats after 72 h (day 1) of administration of STZ. In Group C (flower extract), Group D (Inflorescence stalk extract) and Group E (Insulin-treated group), the blood glucose levels decreased significantly (P < 0.01) in comparison to diabetic control on day 8 and decreased further at the end of 2nd week. The flower ethanolic extract was found to possess higher antihyperglycemic activity (P < 0.01) than the inflorescence stalk ethanolic extract on both day 8 and day 15.
Table 1.
Effect of flower and inflorescence stalk ethanolic extracts of Musa balbisiana Colla. on fasting blood glucose in Type 1 diabetic rats

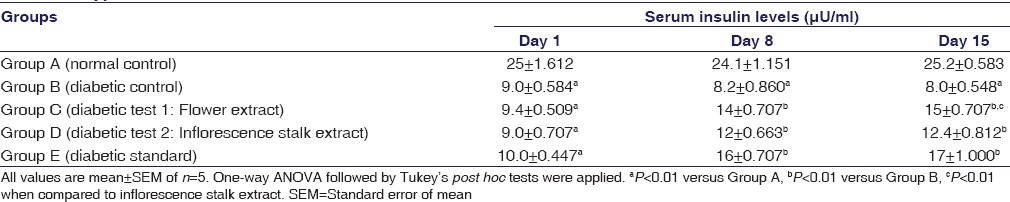
Effects of Extracts on Serum Insulin Levels
Table 2 shows the serum insulin levels on 1st day, 8th day, and 15th day of the experiment. On day 1, significant reduction (P < 0.01) of blood insulin levels were observed in all Type 1 diabetic groups compared to normal control. On day 8 and day 15 of the experiment, the diabetic control group showed significant (P < 0.01) reduction of serum insulin compared to normal control, whereas flower extract, inflorescence stalk extract-treated groups as well as diabetic standard showed significant rise (P < 0.01) of serum insulin compared to diabetic control. On day 15, the rise in serum insulin was more (P < 0.01) in flower extract-treated group compared to inflorescence stalk extract-treated group.
Table 2.
Effect of flower and inflorescence stalk ethanolic extracts of Musa balbisiana Colla. on serum insulin levels in Type 1 diabetic rats
Effect of Extracts on Body Weight
The effect of flower and inflorescence stalk ethanolic extract on body weight in Type 1 diabetic rats is depicted in [Figure 1]. There was significant reduction in the body weight of diabetic control in comparison to normal control on day 15, whereas flower extract, inflorescence stalk extract-treated groups as well as diabetic standard showed significant increase (P < 0.01) in the body weight in comparison to diabetic control. Body weight increased significantly (P < 0.05) in all the groups after 2 weeks when compared to day 1 except diabetic control group where there was a significant decrease (P < 0.01) in the body weight.
Figure 1.
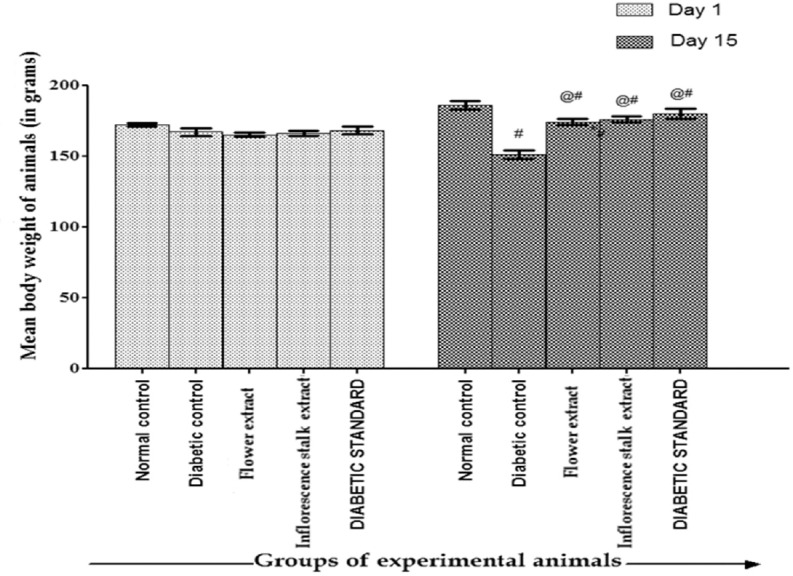
Effect of flower extract and inflorescence stalk extract of Musa balbisiana Colla. on body weight in Type 1 diabetic rats. *P < 0.01 versus normal control; @P < 0.01 versus diabetic control; #P < 0.01 when compared between initial (day 1) and final weight (day 15)
Effect of Extracts on Intestinal Glucose Absorption
The effect of extracts on intestinal glucose absorption is shown in Figure 2. The absorption of glucose from the rat intestines was decreased significantly (P < 0.01) by test drug 1 (flower extract), test drug 2 (inflorescence stalk extract), and metformin-treated groups in comparison to the normal control group. The reduction of intestinal glucose absorption (P < 0.01) was more with flower extract compared to the inflorescence stalk extract-treated group.
Figure 2.
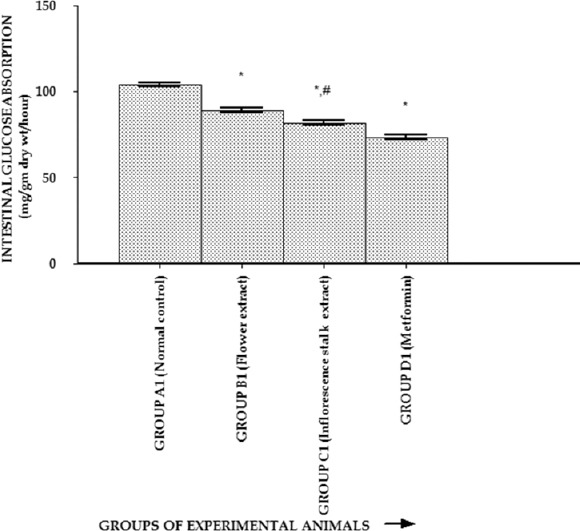
Effect of flower and inflorescence stalk ethanolic extracts of Musa balbisiana Colla. on intestinal glucose absorption. One-way ANOVA followed by Tukey's post hoc tests were applied. *P < 0.01 versus normal control; #P < 0.01 versus flower extract
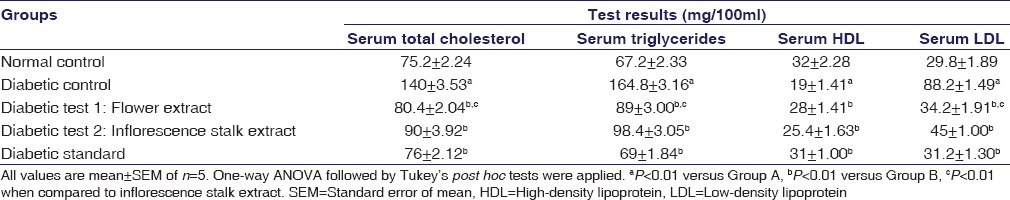
Antihyperlipidemic Activity of the Extracts
Table 3 shows the serum lipid profile of the experimental animal on day 15 of the experiment. On day 1, no statistically significant difference was noted for the serum lipid profile among the experimental groups. On day 15 of the experiment, the flower and inflorescence stalk ethanolic extract-treated diabetic animals showed significant reduction of total blood cholesterol, serum triglycerides, serum LDL, and also increased the serum HDL significantly (P < 0.01) in comparison to the diabetic control group which showed significant hyperlipidemia. The antihyperlipidemic activity was found to be significantly (P < 0.01) higher for Group C (flower extract) compared to Group D (Inflorescence extract) except for serum HDL, where both the extracts had similar effects of increasing HDL cholesterol.
Table 3.
Effect of flower and inflorescence stalk ethanolic extracts of Musa balbisiana Colla. on serum lipids in Type 1 diabetic rats on day 15 of the experiment
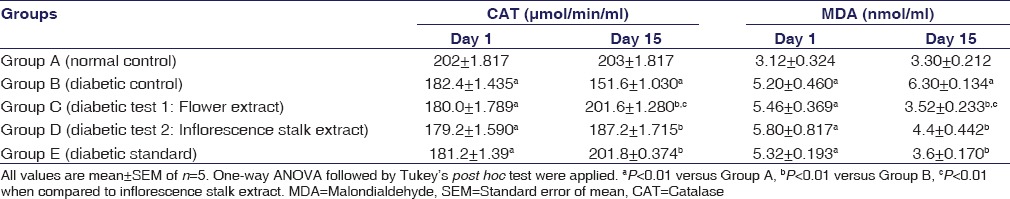
Antioxidant Activity of the Extracts
Serum CAT and MDA levels of the experimental animals are shown in the Table 4. The CAT activity was significantly reduced and MDA level was significantly increased in all diabetic rats on day 1. On day 15, Group C (flower extract) and Group D (Inflorescence extract) showed significant (P < 0.01) antioxidant activity with increased CAT and decreased MDA levels in comparison to diabetic control.
Table 4.
Effect of flower and inflorescence stalk ethanolic extracts of Musa balbisiana Colla. on malondialdehyde and catalase activity in Type 1 diabetic rats
Discussion
Induction of Type 1 diabetes by STZ resulted in hyperglycemia in experimental groups. Oral administration of flower and inflorescence stalk ethanolic extract (250 mg/kg) for 2 weeks significantly decreased the blood glucose levels and also prevented weight loss associated with diabetes which signifies the antidiabetic activity of the extracts. The antidiabetic activity of flower and inflorescence stalk ethanolic extracts may be attributed to the insulin secreting activity as evidenced by significant increase in serum insulin in extract-treated groups. However, their insulin levels were still significantly lower compared to the normal control group. This can be explained by the fact that only very few beta cells survive in the pancreas in the process of inducing Type 1 diabetes by STZ. Therefore, antihyperglycemic action may also be due to direct insulin-like action of the extracts. Recently, ethanolic extract of the flowers of M. balbisiana Colla. (Synonym: M. sapientum) was found to contain Gallic acid as an active principle.[15] Punithavathi et al. showed that gallic acid possesses a significant antihyperglycemic and antioxidant effects on STZ-induced diabetic rats.[16] In an in vitro study, Gallic acid was shown to induce glucose transporter 4 (GLUT4) translocation and glucose uptake activity in 3T3-L1 cells. This is an evidence of insulin-like action of the flower ethanolic extract because insulin stimulates glucose transport in target tissues (muscles and adipocytes) by inducing the translocation of GLUT4 to the plasma membrane.[17]
Decreased intestinal glucose absorption shown by the extracts is another probable mechanism of antidiabetic action which can be attributed to the presence of pectins in flower ethanolic extract and presence of saponins and tannins in both the extracts. Pectins have been isolated from the juice of inflorescence stalk of M. balbisiana.[18] Pectins are soluble dietary fibers and are reported to increase the viscosity of gastrointestinal content, thereby decreasing the gastric emptying rate and suppressing or delaying the digestion and absorption of carbohydrates.[19] Tannins and saponins are found to produce marked loss in glucose transport capacity in isolated rat intestinal brush border membrane and potent S-GLUT-1-mediated inhibition of glucose absorption from the intestine respectively.[20]
Flower and inflorescence stalk ethanolic extract showed significant (P < 0.05) antioxidant activity as evidenced by increased CAT activity and decreased MDA levels in comparison to the diabetic control. The similar results with insulin-treated group may be due to correction of hyperglycemia by insulin. Because hyperglycemia itself can lead to free radical formation due to glucose oxidation, nonenzymatic glycation of proteins, and the subsequent oxidative degradation of glycated proteins.[21] Therefore, the antidiabetic activity of the extracts may partly be responsible for their antioxidant property. It can also be attributed to the phytochemicals in the extracts. Gallic acid in flower ethanolic extract is also found to possess strong antioxidant activities.[17]
The antihyperlipidemic activity of flower and inflorescence stalk ethanolic extract can be correlated to their antidiabetic activity. Insulin deficiency is associated with excess lipolysis and increased influx of free fatty acids to the liver. The extracts through their insulin-like or insulin-secreting properties might have corrected the underlying metabolic derangement responsible for hyperlipidemia. Antihyperlipidemic activity can also be because of the phytochemicals in the extracts such as saponins which may decrease cholesterol absorption from the intestinal lumen and increasing excretion of bile acids[22] and tannins by decreasing dietary absorption of cholesterol.[23] Pectins present in juice of inflorescence stalk have been shown to possess hypolipidemic effect.[19] Gallic acid recently discovered in flower extract is also shown to have antihyperlipidemic effect by cholesterol esterase inhibitory action and increasing fecal excretion of primary bile acids.[24]
Thus, the flowers and inflorescence stalk of M. balbisiana Colla. bear potential for the development of new drugs for the treatment of Type 1 diabetes and also possesses antihyperlipidemic and antioxidant property.
Limitation of the Study
A dose-dependent study was not possible because of limitation of resources and constrains from the IAEC to limit the number of animals used in the study. Isolation of active ingredients and extraction of percentage value of phytochemicals using high-performance liquid chromatography would have been more predictive of the probable mechanism of action of the extracts.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Das AK. Type 1 diabetes in India: Overall insights. Indian J Endocrinol Metab. 2015;19(Suppl 1):S31–3. doi: 10.4103/2230-8210.155372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–58. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 4.Donga JJ, Surani VS, Sailor GU, Chauhan SP, Seth AK. A systematic review on natural medicine used for therapy of diabetes mellitus of some Indian medicinal plants. Pharma Sci Monit. 2011;2:36–72. [Google Scholar]
- 5.Consensus Document on the Biology of Bananas and Plantains (Musa spp.) Vol. 43. OECD Environment, Health and Safety Publications; 2009. Organisation for Economic Co-operation and Development; pp. 1–87. 2009 November 24. Report No. ENV/JM/MONO. [Google Scholar]
- 6.Imam MZ, Akter S. Musa paradisiaca L. and Musa sapientum L.: A phytochemical and pharmacological review. J Appl Pharm Sci. 2011;1:14–20. [Google Scholar]
- 7.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: A review. Int Pharm Sci. 2011;1:98–106. [Google Scholar]
- 8.Gurney AM, Howarth FC. Effects of streptozotocin-induced diabetes on the pharmacology of rat conduit and resistance intrapulmonary arteries. Cardiovasc Diabetol. 2009;8:1–10. doi: 10.1186/1475-2840-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel J, Goyal R, Bhatt P. Beneficial effects of levo-carnitine on lipid metabolism and cardiac function in neonatal streptozotocin rat model of diabetes. Int J Diabetes Metabol. 2008;16:29–34. [Google Scholar]
- 10.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 11.Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40. [PubMed] [Google Scholar]
- 12.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 13.Das S, Yadav RK, Nagchoudhuri J. Effect of fasting on the intestinal absorption of D-glucose and D-xylose in rats in vivo. Indian J Physiol Pharmacol. 2001;45:451–6. [PubMed] [Google Scholar]
- 14.Davis SN. Insulin, oral hypoglycemic agents and the pharmacology of the endocrine pancreas. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 11th ed. USA: McGraw Hill, Medical Publishing Division; 2006. pp. 1039–60. [Google Scholar]
- 15.Chaudhari SA, Khatwani PF, Kulkarni SR. Assessment of antidiabetic potential of flowers of Musa sapientum and development of tablet dosage form. Inventi impact. Ethnopharmacology 2013. 2013. [Last cited on 2013 Aug 12]. pp. 52–8. Available from: http://www.inventi.in/article/pep/733/12.aspx .
- 16.Punithavathi VR, Prince PS, Kumar R, Selvakumari J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur J Pharmacol. 2011;650:465–71. doi: 10.1016/j.ejphar.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 17.Prasad CN, Anjana T, Banerji A, Gopalakrishnapillai A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010;584:531–6. doi: 10.1016/j.febslet.2009.11.092. [DOI] [PubMed] [Google Scholar]
- 18.Gomathy R, Vijayalekshmi NR, Kurup PA. Hypolipidemic principle of the inflorescence stalk of plantain (Musa sapientum) J Biosci. 1989;14:301–9. [Google Scholar]
- 19.Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2:1266–89. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das S, Barman S. Antidiabetic and antihyperlipidemic effects of ethanolic extract of leaves of Punica granatum in alloxan-induced non-insulin-dependent diabetes mellitus albino rats. Indian J Pharmacol. 2012;44:219–24. doi: 10.4103/0253-7613.93853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016;24:547–53. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuura H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr. 2001;131:1000S–5S. doi: 10.1093/jn/131.3.1000S. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Bok SH, Jeon SM, Park YB, Lee SJ, Jeong TS, et al. Effect of rutin and tannic acid supplements on cholesterol metabolism in rats. Nutr Res. 2002;22:283–95. [Google Scholar]
- 24.Ngamukote S, Mäkynen K, Thilawech T, Adisakwattana S. Cholesterol-lowering activity of the major polyphenols in grape seed. Molecules. 2011;16:5054–61. doi: 10.3390/molecules16065054. [DOI] [PMC free article] [PubMed] [Google Scholar]


