Abstract
The expression and function of microRNA-149 have been studied in numerous types of cancer. However, thus far, there are no studies of microRNA-149 in tongue squamous cell carcinoma (TSCC). The present study investigated the expression, biological function and molecular mechanism of microRNA-149 in TSCC in vitro, discussing whether it may be a therapeutic biomarker of TSCC in the future. In the present study, microRNA-149 expression in TSCC tissues, matched normal adjacent tissues, TSCC cell lines and normal gingival epithelial cells were analyzed using quantitative polymerase chain reaction. Following transfection with microRNA-149 mimics, cell proliferation, migration and invasion assays, a luciferase assay and western blotting were performed. The present study found that the expression of microRNA-149 was significantly decreased in TSCC tissues and cell lines compared with matched normal tissue and normal gingival epithelial cells, respectively. In addition, it was also demonstrated that microRNA-149 inhibited cell proliferation, migration and invasion by directly targeting specificity protein 1. Therefore, the results suggested that microRNA-149 may be a novel target for TSCC therapy in the future.
Keywords: tongue squamous cell carcinoma, specificity protein 1, microRNA-149, tongue squamous cell carcinoma
Introduction
Tongue squamous cell carcinoma (TSCC), the most common type of oral cancer, often leads to malfunctions in speech, mastication and deglutition (1). TSCC is well-known for its high rate of proliferation and nodal metastasis; metastasis is the most reliable adverse prognostic factor in TSCC patients (2). Despite being visibly located in the oral cavity, >50% patients at diagnosis present with advanced stage III or IV according to the tumor-node metastasis classification of malignant tumors system (3). Despite improvements in surgery, radiotherapy and chemotherapy, the 5-year survival rate for patients with TSCC remains poor, mainly due to regional recurrence and lymph node metastasis (4,5). An accumulating number of studies suggest that TSCC arises as a result of oncogene activation or tumor suppressor gene inactivation (6–8). However, the detailed molecular mechanism of TSCC remain unknown (5). Understanding the molecular pathways of TSCC carcinogenesis and development may be useful for improving diagnosis, treatment and prevention of the disease.
Altered expression of microRNA (miRNA) has been found in numerous types of cancer (9). miRNA is a type of small, single-stranded, non-coding RNA of between 18 and 25 nucleotides in length that performs an important role in the posttranscriptional regulation of gene expression (10). In total, >1,800 types of miRNA have been identified in miRBase version 20.0, and 1/3 of the genes in the human genome are regulated by miRNA (11,12). miRNA regulates target gene expression post-transcriptionally via incomplete base pairing with the target mRNA (13). Furthermore, miRNA performs an important role in numerous biological processes including development, differentiation, proliferation, apoptosis, angiogenesis and metabolism. Mutations of miRNA have consequently been suggested to serve an important role in carcinogenesis (14). In addition, miRNA can function as either tumor suppressors or oncogenes, depending on whether oncogenes or tumor suppressor genes are targeted (15). Thus, identifying miRNA targets is critical to understand the function of miRNA in tumorigenesis and progression. The present study also suggests that miRNAs may be a target for cancer therapy.
The expression and function of (miRNA-149) miR-149 have been investigated in a number of types of cancer. This study was aimed to investigate the expression, biological functions and molecular mechanisms of miR-149 in TSCC. The present study found that the expression of miR-149 was decreased in TSCC tissues and cell lines compared with matched normal tissue and normal gingival epithelial cells, respectively. Additionally, the present study demonstrated that miR-149 suppressed cell proliferation, migration and invasion by directly targeting specificity protein 1 (SP1). The present results improve the understanding of the mechanisms of TSCC carcinogenesis and progression, and identify new targets that may be used for the development of novel treatments of TSCC.
Materials and methods
Clinical specimens
TSCC tissue and matched normal adjacent tissue (NAT) for quantitative polymerase chain reaction (qPCR) were obtained from 62 patients who had undergone primary surgical treatment of oral tongue carcinoma at Nanfang Hospital (Guangzhou, China). None of the patients received treatment prior to the excision surgery. The tissues were flash frozen in liquid nitrogen and stored at −80°C until use. The present study was approved by the Protection of Human Subjects Committee of Nanfang Hospital (Guangzhou, China). Written informed consent was also acquired from all TSCC patients.
Cell culture
TSCC Tca8113 and CAL-27 cell lines and normal gingival epithelial cells were purchased from the American Type Culture Collection (Manassas, VA, USA). The Tca8113 and CAL-27 cell lines were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), while normal gingival epithelial cells were maintained in minimum essential media (Gibco; Thermo Fisher Scientific, Inc.). All media were supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 U/ml streptomycin. All cell lines were cultured at 37°C in a humidified air atmosphere containing 5% CO2.
Cell transfection
miR-149 mimics and miRNA mimics negative control (NC), obtained from Shanghai GenePharma Co., Ltd., (Shanghai, China), were used for the upregulation of miR-149 activity in cells. Transfection was completed using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
RNA isolation, reverse transcription and qPCR
Total RNA was extracted from the TSCC tissue and NAT cells and the Tca8113, CAL-27 and normal gingival epithelial cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. For complementary DNA synthesis, 1 µg RNA was mixed with 500 ng oligo-deoxythymine (Promega Corporation, Madison, WI, USA) or microRNA specific primers (Invitrogen; Thermo Fisher Scientific, Inc.). The GoScipt/ImProm-II reverse transcription system (Promega Corporation) was then used to perform reverse transcription on the samples. The temperature protocol was as follows: 95°C for 2 min; 20 cycles of 94°C for 1 min; 55°C for 1 min and 72°C for 2 min; and 72°C for 5 min. qPCR was performed using an Applied Biosystems 7500 Real-time PCR system (Thermo Fisher Scientific, Inc.) and an SYBR premix Ex Taq kit (Takara, Biotechnology Co., Ltd., Dalian, China) following the manufacturer's protocol. All samples were amplified in triplicate. The levels of miR-149 were normalized using U6 small nuclear RNA as the endogenous reference gene. The primers were obtained from Guangzhou RiboBio Co., Ltd. (Guangzhou, China), and sequences were as follows: miR-149 forward, 5′-TCTGGCTCCGTGTCTTCACTCCC-3′ and reverse, 5′-AGTGGTTGTTCTGCTCTCTGTGTC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse, 5′-ACGCTTCACGAATTTGCGTGTC-3′. Relative expression fold changes were calculated using the 2−ΔΔCq method (16).
Cell proliferation assay
To explore the effect of miR-149 on cell proliferation, an MTT assay was used. Tca8113 and CAL-27 cells transfected with miR-149 or negative control (NC) were seeded in 96-well plates at a density of 3,000 cells/well. The MTT assay was performed by incubating the cells with 20 µl MTT (5 mg/ml; Sigma-Aldrich, Merck Millipore, Darmstadt, Germany). Subsequent to incubation for 4 h at 37°C, the formazan precipitates were dissolved in 200 µl dimethyl sulfoxide. Absorbance at 490 nm was determined using an ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). All experiments were repeated at least 3 times.
Cell migration and invasion assays
The migration and invasion of the TSCC cell lines was examined using Transwell chambers (8 µm; EMD Millipore, Billerica, MA, USA). The cell migration assay was performed with uncoated Matrigel (BD Biosciences, San Jose, CA, USA) whereas the cell invasion assays were performed with coated Matrigel (BD Biosciences). A total of 5×104 Tca8113 and CAL-27 cells transfected with miR-149 or NC in 200 µl serum-free RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.) were seeded into the upper chamber. A volume of 500 µl RPMI 1640 medium containing 10% FBS was then added to the lower chamber as a chemoattractant. The cells were then incubated for 12 h, for the migration assay, and 24 h for the invasion assay. The cells remaining on the upper surface of the membranes were scraped off using cotton swabs and the membranes were fixed with 100% methanol (Shanghai Macklin Biochemical Co., Ltd, Shanghai, China) for 10 min and stained in 0.5% crystal violet (Beyotime Institute of Biotechnology, Haimen, China). The membranes were counted under an inverted microscope (CKX41; Olympus Corporation, Tokyo, Japan) to calculate their relative numbers. Each experiment was repeated at least 3 times.
Western blotting
The TSCC cell lines were plated in 6-well plates. A total of 72 h subsequent to transfection with miR-149 or NC, the cells were washed and lysed in cold radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). Subsequent to centrifugation at 24,150 × g for 10 min at 4°C, the protein concentration was determined using a bicinchoninic acid Protein Assay kit (Beyotime Institute of Biotechnology). Equal amounts of protein were then separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Beyotime Institute of Biotechnology). The membranes were blocked with 5% skimmed milk at room temperature for 2 h and incubated with rabbit anti-human SP1 antibody (dilution, 1: 1,000; cat. no., 5931; Cell Signaling Technology, Inc., Danvers, MA, USA) according to the protocol of the manufacturer at 4°C overnight. The membranes were washed and then incubated with the corresponding horseradish peroxidase-conjugated secondary antibody (dilution, 1:1,000; cat. no., 7074; Cell Signaling Technology, Inc., Danvers, MA, USA) for 1 h. The protein bands were visualized with an enhanced chemiluminescence kit (Pierce, Thermo Fisher Scientific, Inc.) and analyzed using Quantity One software (version 4.62; Bio-Rad Laboratories, Inc.).
Luciferase assay
The human TSCC cell lines were plated in a 12-well plate at ~90% confluence and transfected with a reporter plasmid, miR-49 mimic or NC by Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) according to the protocol of the manufacturer. The activity of Photinus and Renilla luciferase in cell lysates was determined with the Dual-Luciferase Reporter Assay System (Promega Corporation) following 48 h transfection. The Photinus luciferase activity was normalized to the Renilla luciferase activity for each transfected well. All the experiments were performed in triplicate.
Statistical analysis
Data were presented as the mean ± standard deviation and compared using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
miR-149 expression in TSCC tissues and cell lines
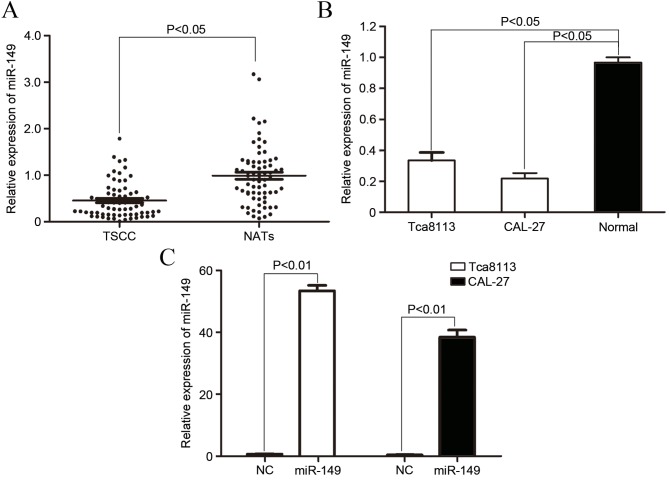
To explore the role of miR-149 in TSCC carcinogenesis and progression, the expression of miR-149 in TSCC tissue and NAT was analyzed by qPCR. As demonstrated in Fig. 1A, the expression of miR-149 was significantly downregulated in TSCC tissues compared with NAT (P=0.017).
Figure 1.
Expression of miR-149 in TSCC tissues and cell lines. (A) Expression of miR-149 was significantly downregulated in TSCC tissues compared with NAT. (B) miR-149 expression was also decreased in Tca8113 and CAL-27 cell lines compared with immortalized normal gingival epithelial cells. (C) qPCR revealed that miR-149 was significantly upregulated in Tca8113 and CAL-27 cells subsequent to transfection with miR-149 mimics. miR, mircoRNA; TSCC, tongue squamous cell carcinoma; NAT, normal adjacent tissue; qPCR, quantitative polymerase chain reaction.
The expression level of miR-149 in TSCC cell lines and normal gingival epithe-lial cells was also determined. As shown in Fig. 1B, the miR-149 expression level significantly decreased in Tca8113 (P=0.01) and CAL-27 (P=0.005) cells compared with normal gingival epithelial cells. The aforementioned results indicated that miR-149 may serve a role in TSCC carcinogenesis and progression.
To explore the function of miR-149 on TSCC cells, the present study transfected the miR-149 mimic into Tca8113 and CAL-27 cells. As demonstrated in Fig. 1C, the increased level of expression of miR-149 in Tca8113 (P=0.0000017) and CAL-27 (P=0.0000040) cells was confirmed by qPCR.
miR-149 inhibited cell proliferation in Tca8113 and CAL-27 cells
The effect of miR-149 on cell proliferation in Tca8113 and CAL-27 cells was determined by MTT assay. As shown in Fig. 2, the level of absorbance in Tca8113 and CAL-27 cells transfected with miR-149 significantly decreased compared with cells transfected with NC (P=0.022 for Tca8113; P=0.018 for CAL-27). The aforementioned results verified that miR-149 inhibited proliferation in Tca8113 and CAL-27 cell lines.
Figure 2.
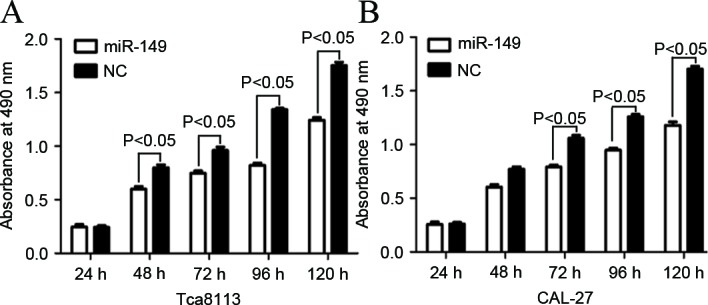
Effect of miR-149 on cell proliferation in tongue squamous cell carcinoma cells determined by MTT assay. The assay revealed that the upregulation of miR-149 significantly inhibited cell proliferation in (A) Tca8113 and (B) CAL-27 cells. miR, microRNA.
miR-149 suppressed cell migration and invasion in Tca8113 and CAL-27 cells
A Transwell apparatus assay was performed to explore the effect of miR-149 on cell migration and invasion. As demonstrated in Fig. 3, the levels of migration and invasion of Tca8113 (P=0.034 for migration; P=0.025 for invasion) and CAL-27 (P=0.023 for migration; P=0.031 for invasion) cells transfected with miR-149 significantly decreased compared with cells transfected with NC. The aforementioned results indicated that miR-149 inhibited cell migration and invasion ability in TSCC cell lines.
Figure 3.
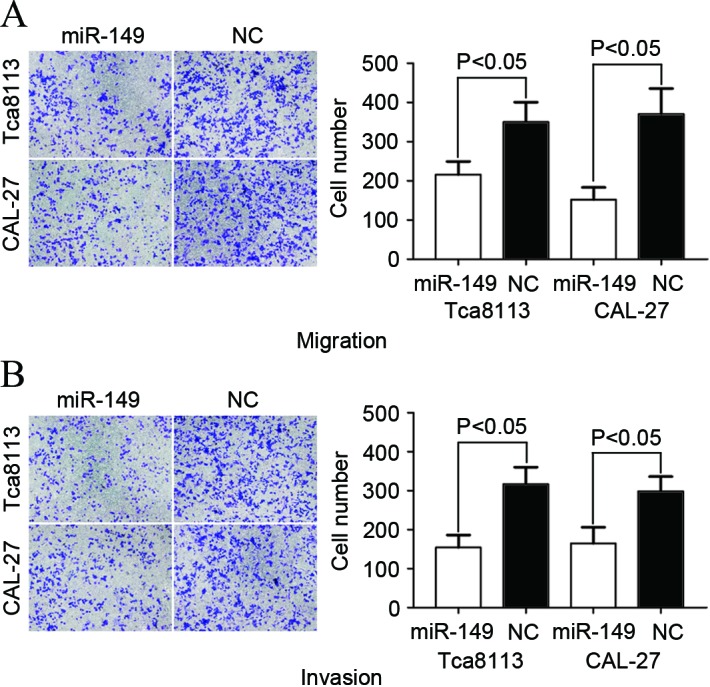
Migration and invasion assays were performed using Transwell chambers. The upregulation of miR-149 decreased the (A) migration and (B) invasion abilities of Tca8113 and CAL-27 cells.
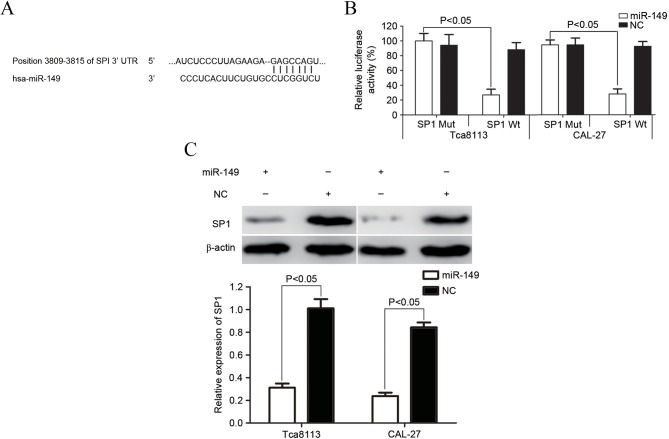
SP1 was a direct target gene of miR-149 in vitro
To predict the target gene of miR-149, bioinformatics software (TargetScan 7.1) was used (17). As demonstrated in Fig. 4A, SP1 was verified to be a direct target gene of miR-149. A luciferase assay was then performed to investigate whether miR-149 directly targets SP1. As shown in Fig. 4B, miR-149 significantly inhibited SP1 wild-type, but not SP1 mutant, luciferase activity in Tca8113 and CAL-27 cells (P=0.019 for Tca8113; P=0.015 for CAL-27).
Figure 4.
SP1 was a directly targeted gene of miR-149 in vitro. (A) Bioinformatics software assessed that SP1 mRNA contained a miR-149 seed match at position 3809–3815 of the SP1 3′-UTR. (B) miR-149 significantly inhibited the SP1 Wt but not the SP1 Mut luciferase activity in Tca8113 and CAL-27 cells. (C) Western blotting was performed to explore whether SP1 was downregulated at the protein level subsequent to transfection with miR-149 in tongue squamous cell carcinoma cells. The analysis revealed that SP1 was significantly downregulated in Tca8113 and CAL-27 cells subsequent to transfection with miR-149. SP1, specificity protein 1; miR, mircoRNA; Wt, wild-type; Mut, mutant; UTR, untranslated region; NC, microRNA mimics negative control.
Western blotting was also performed to explore whether the expression of SP1 was downregulated at the protein level subsequent to transfection with miR-149 in Tca8113 and CAL-27 cells. As demonstrated in Fig. 4C, the expression of SP1 significantly decreased in Tca8113 and CAL-27 cells subsequent to transfection with miR-149 (P=0.024 for Tca8113; P=0.012 for CAL-27). SP1 may therefore be a direct target gene of miR-149 in vitro.
Discussion
This study demonstrated that the expression levels of certain types of miRNA decrease in TSCC, and that miRNA may function as a negative regulator of oncogenes or tumor suppressors in carcinogenesis and cancer progression. miRNA is thereby a potential diagnostic and prognostic marker of TSCC with therapeutic potential (18–20). Previous studies revealed that miR-149 was downregulated in colorectal cancer, breast cancer, gastric cancer, glioma, melanoma and non-small cell lung cancer (21–26). This study investigated the expression, biological functions and molecular mechanisms of miR-149 in TSCC. The present study revealed that miR-149 was significantly downregulated in TSCC tissue and cell lines. It also suggested that miR-149 may serve an important role in TSCC carcinogenesis and development.
An accumulating number of studies indicate that miR-149 functions as a tumor suppressor in numerous types of human cancer. For example, in colorectal cancer, miR-149 inhibited cell migration and invasion by targeting Forkhead Box M1 (21). Chan et al (22) reported that miR-149 targeted G-protein-coupled receptor kinase-interacting protein 1 to suppress breast cancer cell migration, invasion and metastasis. In addition, miR-149 was reported to decrease glioma cell growth and invasion through targeting protein kinase B signaling (24). Furthermore, Wang et al (23) found that miR-149 suppressed cell proliferation and cell cycle progression via the blockade of zinc finger and BTB domain containing 2 in human gastric cancer. At present, little is known with respect to the role of miR-149 in TSCC. The present study demonstrated that the upregulation of miR-149 inhibited the proliferation, migration and invasion of TSCC cells. The present study expanded the number of known functions of miR-149 in cancer.
As miRNA functions by targeting mRNA, the identification of miR-149 target genes may contribute to the understanding of the potential role of miRNA in carcinogenesis and tumor development. In the present study, an important molecular link between miR-149 and SP1 was observed. Firstly, bioinformatics software predicted that SP1 possessed a miR-149-targeted seed sequence, suggesting that SP1 may be a putative target of miR-149. Secondly, the luciferase assay showed that miR-149 directly targeted SP1 3′-untranslated region. Finally, western blot analysis revealed that the upregulation of miR-149 suppressed the expression of SP1 at a protein level. The aforementioned findings suggested that miR-149 served a tumor suppressor role in TSCC carcinogenesis and progression through the direct targeting of SP1.
SP1, a sequence-specific DNA-binding protein, maps to 12q13.1 and encodes a protein of 785 amino acids (27). It was the first transcription factor identified and characterized. SP1 is widely expressed in all mammalian tissues and performs a number of important functions in normal tissue development (28). The aberrant expression of SP1 has been found in numbers types of human cancer, and the involvement of SP1 in carcinogenesis and cancer progression has also been verified (29–31). An increasing number of studies suggest that SP1 regulates a variety of biological functions, including cell survival, proliferation, differentiation, migration and invasion (32–34). Additionally, several compounds with anti-tumor effects, which target SP1 have been developed or are in development, and some of them are currently used clinically for cancer treatment (35–38). Therefore, SP1 has been suggested to be a novel target for cancer therapy due to the cancer-associated functions of the protein.
SP1 has been found to be regulated by multiple types of miRNA in a variety of types of cancer, including TSCC. miR-29b and miR-375 function as tumor suppressors in TSCC through targeting SP1 (39,40). miR-145, miR-133a, miR-133b, miR-22 and miR-335 serve an important role in the biology of gastric cancer by regulating SP1 directly (41–43). miR-145 was also found to regulate SP1 in ovarian cancer cells sensitized to paclitaxel (44). In esophageal carcinoma, Wang et al (45) found that ectopic miR-429 suppressed cell invasion and induced cell apoptosis via the blockade of SP1. Zhang et al (46) demonstrated that miR-377 decreased cell growth and invasion ability by inhibiting SP1. In hepatocellular carcinoma, the restoration of miR-1188 inhibited cell proliferation and migration, and enhanced cell apoptosis through the down-regulation of SP1 (47). In the present study, the upregulation of miR-149 in TSCC cell lines revealed that miR-149 inhibited cell proliferation, migration and invasion via the blockade of SP1. miR-149 may therefore act as a regulator of SP1.
In conclusion, the present study revealed that miR149 was significantly downregulated in TSCC tissue and cell lines. The present study also observed that miR-149 contributed to cell proliferation, migration and invasion by directly targeting SP1 in TSCC. The identified candidate target gene of miR-149 may provide an understanding of potential carcinogenic mechanisms in TSCC. The findings of the present study have therapeutic implications and may be exploited for the future treatment of TSCC.
References
- 1.Yu ZW, Zhong LP, Ji T, Zhang P, Chen WT, Zhang CP. MicroRNAs contribute to the chemoresistance of cisplatin in tongue squamous cell carcinoma lines. Oral Oncol. 2010;46:317–322. doi: 10.1016/j.oraloncology.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Yuen PW, Lam KY, Chan AC, Wei WI, Lam LK. Clinicopathological analysis of local spread of carcinoma of the tongue. Am J Surg. 1998;175:242–244. doi: 10.1016/S0002-9610(97)00282-1. [DOI] [PubMed] [Google Scholar]
- 3.Po Wing Yuen A, Lam KY, Lam LK, Ho CM, Wong A, Chow TL, Yuen WF, Wei WI. Prognostic factors of clinically stage I and II oral tongue carcinoma-A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck. 2002;24:513–520. doi: 10.1002/hed.10094. [DOI] [PubMed] [Google Scholar]
- 4.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–662. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 5.Song KB, Liu WJ, Jia SS. miR-219 inhibits the growth and metastasis of TSCC cells by targeting PRKCI. Int J Clin Exp Med. 2014;7:2957–2965. [PMC free article] [PubMed] [Google Scholar]
- 6.Squarize CH, Castilho RM, Abrahao AC, Molinolo A, Lingen MW, Gutkind JS. PTEN deficiency contributes to the development and progression of head and neck cancer. Neoplasia. 2013;15:461–471. doi: 10.1593/neo.121024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knopf A, Lempart J, Bas M, SlottaHuspenina J, Mansour N, Fritsche MK. Oncogenes and tumor suppressor genes in squamous cell carcinoma of the tongue in young patients. Oncotarget. 2015;6:3443–3451. doi: 10.18632/oncotarget.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regezi JA, Dekker NP, McMillan A, RamirezAmador V, MenesesGarcia A, Ruiz-Godoy Rivera LM, Chrysomali E, Ng IO. p53, p21, Rb, and MDM2 proteins in tongue carcinoma from patients <35 versus >75 years. Oral Oncol. 1999;35:379–383. doi: 10.1016/S1368-8375(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 9.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Kozomara A, Griffiths-Jones S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–D157. doi: 10.1093/nar/gkq1027. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, Maturation, Target Recognition and Regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: The implications for cancer research. Nat Rev Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4 doi: 10.7554/eLife.05005. 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI. Mature miR-184 as Potential Oncogenic microRNA of Squamous Cell Carcinoma of Tongue. Clin Cancer Res. 2008;14:2588–2592. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 19.Jiang L, Liu X, Chen Z, Jin Y, Heidbreder CE, Kolokythas A, Wang A, Dai Y, Zhou X. MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem J. 2010;432:199–205. doi: 10.1042/BJ20100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Yu J, Jiang L, Wang A, Shi F, Ye H, Zhou X. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genomics Proteomics. 2009;6:131–139. [PMC free article] [PubMed] [Google Scholar]
- 21.Xu K, Liu X, Mao X, Xue L, Wang R, Chen L, Chu X. MicroRNA-149 suppresses colorectal cancer cell migration and invasion by directly targeting forkhead box transcription factor FOXM1. Cell Physiol Biochem. 2015;35:499–515. doi: 10.1159/000369715. [DOI] [PubMed] [Google Scholar]
- 22.Chan SH, Huang WC, Chang JW, Chang KJ, Kuo WH, Wang MY, Lin KY, Uen YH, Hou MF, Lin CM, et al. MicroRNA-149 targets GIT1 to suppress integrin signaling and breast cancer metastasis. Oncogene. 2014;33:4496–4507. doi: 10.1038/onc.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Zheng X, Zhang Z, Zhou J, Zhao G, Yang J, Xia L, Wang R, Cai X, Hu H, et al. MicroRNA-149 inhibits proliferation and cell cycle progression through the targeting of ZBTB2 in human gastric cancer. PLoS One. 2012;7:e41693. doi: 10.1371/journal.pone.0041693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan SJ, Zhan SK, Pei BG, Sun QF, Bian LG, Sun BM. MicroRNA-149 inhibits proliferation and invasion of glioma cells via blockade of AKT1 signaling. Int J Immunopathol Pharmacol. 2012;25:871–881. doi: 10.1177/039463201202500405. [DOI] [PubMed] [Google Scholar]
- 25.Jin L, Hu WL, Jiang CC, Wang JX, Han CC, Chu P, Zhang LJ, Thorne RF, Wilmott J, Scolyer RA, et al. MicroRNA-149*, a p53-responsive microRNA, functions as an oncogenic regulator in human melanoma. Proc Natl Acad Sci USA. 2011;108:15840–15845. doi: 10.1073/pnas.1019312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ke Y, Zhao W, Xiong J, Cao R. miR-149 Inhibits Non-small-cell lung cancer cells EMT by targeting FOXM1. Biochem Res Int. 2013;2013:506731. doi: 10.1155/2013/506731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang WC, Hung JJ. Functional role of post-translational modifications of Sp1 in tumorigenesis. J Biomed Sci. 2012;19:94. doi: 10.1186/1423-0127-19-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Zou WX, Chang KS. Inhibition of Sp1 functions by its sequestration into PML nuclear bodies. PLoS One. 2014;9:e94450. doi: 10.1371/journal.pone.0094450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yue L, Li L, Liu F, Hu N, Zhang W, Bai X, Li Y, Zhang Y, Fu L, Zhang X, Ye L. The oncoprotein HBXIP activates transcriptional coregulatory protein LMO4 via Sp1 to promote proliferation of breast cancer cells. Carcinogenesis. 2013;34:927–935. doi: 10.1093/carcin/bgs399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y, Li N, Qi J, Wang L, Shi Y, et al. Sp1 is involved in regulation of cystathionine γ-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell Signal. 2012;24:1229–1240. doi: 10.1016/j.cellsig.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Pathi S, Jutooru I, Chadalapaka G, Nair V, Lee SO, Safe S. Aspirin inhibits colon cancer cell and tumor growth and downregulates specificity protein (Sp) transcription factors. PLoS One. 2012;7:e48208. doi: 10.1371/journal.pone.0048208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 33.Li L, He S, Sun JM, Davie JR. Gene regulation by Sp1 and Sp3. Biochem Cell Biol. 2004;82:460–471. doi: 10.1139/o04-045. [DOI] [PubMed] [Google Scholar]
- 34.Mukhopadhyay D, Datta K. Multiple regulatory pathways of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in tumors. Semin Cancer Biol. 2004;14:123–130. doi: 10.1016/j.semcancer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Liu Z, Xie Z, Pang J, Yu J, Lehmann E, Huynh L, Vukosavljevic T, Takeki M, Klisovic RB, et al. Bortezomib induces DNA hypomethylation and silenced gene transcription by interfering with Sp1/NF-kappaB-dependent DNA methyltransferase activity in acute myeloid leukemia. Blood. 2008;111:2364–2373. doi: 10.1182/blood-2007-08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R, III, Li X, Safe S. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–5354. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pathi SS, Jutooru I, Chadalapaka G, Sreevalsan S, Anand S, Thatcher GR, Safe S. GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation of a reactive oxygen species-microRNA-27a: ZBTB10-specificity protein pathway. Mol Cancer Res. 2011;9:195–202. doi: 10.1158/1541-7786.MCR-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu TI, Wang MC, Chen SY, Huang ST, Yeh YM, Su WC, Chang WC, Hung JJ. Betulinic acid decreases specificity protein 1 (Sp1) level via increasing the sumoylation of sp1 to inhibit lung cancer growth. Mol Pharmacol. 2012;82:1115–1128. doi: 10.1124/mol.112.078485. [DOI] [PubMed] [Google Scholar]
- 39.Jia L, Huang Y, Zheng Y, Lyu M, Zhang C, Meng Z, Gan Y, Yu G. miR-375 inhibits cell growth and correlates with clinical outcomes in tongue squamous cell carcinoma. Oncol Rep. 2015;33:2061–2071. doi: 10.3892/or.2015.3759. [DOI] [PubMed] [Google Scholar]
- 40.Jia LF, Huang YP, Zheng YF, Lyu MY, Wei SB, Meng Z, Gan YH. miR-29b suppresses proliferation, migration, and invasion of tongue squamous cell carcinoma through PTEN-AKT signaling pathway by targeting Sp1. Oral Oncol. 2014;50:1062–1071. doi: 10.1016/j.oraloncology.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang Z, Zhu W, Shu Y, Liu P. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett. 2014;588:1168–1177. doi: 10.1016/j.febslet.2014.02.054. [DOI] [PubMed] [Google Scholar]
- 42.Guo MM, Hu LH, Wang YQ, Chen P, Huang JG, Lu N, He JH, Liao CG. miR-22 is down-regulated in gastric cancer, and its overexpression inhibits cell migration and invasion via targeting transcription factor Sp1. Med Oncol. 2013;30:542. doi: 10.1007/s12032-013-0542-7. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang X, Jiang L, Sun Z, Miao Z, Xu H. MicroRNA-335 acts as a metastasis suppressor in gastric cancer by targeting Bcl-w and specificity protein 1. Oncogene. 2012;31:1398–1407. doi: 10.1038/onc.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu X, Li Y, Xie C, Yin X, Liu Y, Cao Y, Fang Y, Lin X, Xu Y, Xu W, et al. miR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6. Int J Cancer. 2014;135:1286–1296. doi: 10.1002/ijc.28774. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Li M, Zang W, Ma Y, Wang N, Li P, Wang T, Zhao G. MiR-429 up-regulation induces apoptosis and suppresses invasion by targeting Bcl-2 and SP-1 in esophageal carcinoma. Cell Oncol (Dordr) 2013;36:385–394. doi: 10.1007/s13402-013-0144-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhang R, Luo H, Wang S, Chen W, Chen Z, Wang HW, Chen Y, Yang J, Zhang X, Wu W, et al. MicroRNA-377 inhibited proliferation and invasion of human glioblastoma cells by directly targeting specificity protein 1. Neuro Oncol. 2014;16:1510–1522. doi: 10.1093/neuonc/nou111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui W, Huang Z, He H, Gu N, Qin G, Lv J, Zheng T, Sugimoto K, Wu Q. MiR-1188 at the imprinted Dlk1-Dio3 domain acts as a tumor suppressor in hepatoma cells. Mol Biol Cell. 2015;26:1416–1427. doi: 10.1091/mbc.E14-11-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]