Abstract
The precise mechanisms underlying the efficacy of intravenous immunoglobulin (IVIg) in autoimmune neurological disorders including Guillain-Barré syndrome (GBS) are not known. Anti-ganglioside antibodies have been reported to be pathogenic in some variants of GBS, and we have developed passive transfer animal models to study anti-ganglioside antibody mediated-endoneurial inflammation and associated neuropathological effects and to evaluate the efficacy of new therapeutic approaches. Some studies indicate that IVIg’s anti-inflammatory activity resides in a minor sialylated IVIg (sIVIg) fractions and is dependent on an innate Th2 response via binding to a specific ICAM3-grabbing nonintegrin related 1 receptor (SIGN-R1). Therefore the efficacy of IVIg, IVIg fractions with various IgG Fc sialylation status, and the involvement of Th2 pathway were examined in one of our animal model of antibody-mediated inhibition of axonal regeneration. We demonstrate that both IVIg and sIVIg ameliorated anti-glycan antibody mediated-pathological effect, whereas, the unsialylated fractions of IVIg were not beneficial in our model. Tenfold lower doses of sIVIg compared to whole IVIg provided equivalent efficacy in our studies. Moreover, we found that whole IVIg and sIVIg significantly upregulates the gene expression of IL-33, which itself can provide protection from antibody-mediated nerve injury in our model. Our results support that the SIGN-R1-Th2 pathway is involved in the anti-inflammatory effects of IVIg on endoneurium in our model and elements of this pathway including IL-33 can provide novel therapeutics in inflammatory neuropathies.
Keywords: IVIg, sIVIg, Guillain-Barré syndrome, IL-33, IL-4, Th2 pathway, SIGN-R1
Introduction
In clinical practice, Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyradiucloneuropathy (CIDP) are the commonest acute and chronic inflammatory neuropathic conditions, respectively. Understanding of the pathomechanisms of nerve inflammation and related nerve injury is incomplete but a large body of work favors synergism of cellular and humoral immune elements in the pathogenesis of these inflammatory neuropathic disorders (Dalakas, 2011;Hughes and Cornblath, 2005;Hughes et al., 1999;Ilyas et al., 1988;Quarles et al., 1990;Willison and Yuki, 2002;Yuki and Hartung, 2012). It is now widely accepted that there are two major forms of GBS: the demyelinating (Asbury et al., 1969;Prineas, 1981) and axonal (Hafer-Macko et al., 1996;McKhann et al., 1993;Ogawara et al., 2000) subtypes. Anti-ganglioside/glycan antibodies (Abs) are the most commonly recognized autoimmune effectors in this disorder and are strongly associated with the axonal forms of GBS (Hughes et al., 2005;Hughes et al., 1999;Willison et al., 2002;Yuki et al., 2004;Yuki et al., 2001).
Adaptive autoimmunity uses the powerful effector functions of cells of the innate immune system including monocytes/macrophages to induce target injury in infectious and autoimmune disorders (Nimmerjahn and Ravetch, 2008;Takai, 2002). The pathologic studies in demyelinating and axonal GBS and CIDP indicate a central role for macrophage and microglia, which are the key components of endoneurial inflammation (Griffin et al., 1990;Kiefer et al., 2001;Zhang et al., 2014). Macrophage-mediated myelin stripping and nodal and periaxonal macrophage-mediated attack on axons are pathognomonic of acquired demyelinating neuropathies (GBS and CIDP) and axonal GBS, respectively. Fc-gamma receptors (FcγRs) are critical regulators of macrophage/microglia-mediated inflammation. They are classically described as activating or inhibitory FcγRs, which signal through immunoreceptor tyrosine activation or inhibitory motifs, respectively (Hogarth, 2002;Takai, 2002). We recently demonstrated, in two separate animal models, that anti-ganglioside antibody-mediated pathological effects are dependent on Ab engaging specific axonal surface gangliosides (immune complex formation) but independent of complement mediated cytolytic injury (He et al., 2015;Zhang et al., 2014). Subsequently, we showed that specific activating FcγRs on endoneurial macrophage/microglial cells are critical inflammatory elements that mediate anti-glycan Ab-induced nerve injury.
IVIg is now the most commonly used treatment in inflammatory neuropathies such as GBS and CIDP. However, the precise mechanisms underlying its protection are not completely defined. Recent studies indicate that modulation of inflammation via innate immune effectors, i.e., FcγRs, could be a mechanism of IVIg efficacy in animal models of immune arthritis and thrombocytopenic purpura (Anthony et al., 2011;Kaneko et al., 2006;Samuelsson et al., 2001). This work from Ravetch’s group characterized SIGN-R1-Th2 pathway and showed that IVIg’s anti-inflammatory activity resides in sialylated fractions (sIVIg) and terminal α2,6 sialic acid on IgG Fc N-glycan chain (IgG FcNg) and IVIg’s efficacy is independent of IgG/IVIg competition for FcγR binding with autoAbs in their models (Anthony et al., 2011). Further, sIVIg and sialylated Fc (sFcs) were effective in suppressing inflammation at a 10- and 30-fold lower dose than whole IVIg, respectively. Moreover, sFcs or sIVIg trigger an innate Th2 response via production of IL-33 and Th2 cytokines including IL-4 that upregulate FcγRIIB on effector macrophages, which participate in suppression of inflammation. IgG Fc glycation consists of single N-linked glycan attached to each heavy chain in the Fc portion asparagine-297 (Anthony et al., 2012;Arnold et al., 2007). There is tremendous heterogeneity in IgG FcNg, including IVIg, with the variable addition of the bisecting N-acetylglucosamine, fucose to the core and sialic acid to the arms of the biantennary structure, thus affecting the efficacy of different IgG subpopulations present in the IVIg. The SIGN-R1-Th2 pathway has not been validated in neuroimmunological disorders in which IVIg is effective. Since our animal models of anti-ganglioside antibody-mediated neuropathological effects are entirely dependent on FcγR induced endoneurial inflammation, we examined the efficacy of whole IVIg and compared it with terminal α2,6 sialic acid enriched or depleted fractions of whole IVIg ( sIVIg or uIVIg, respectively) in suppressing Ab-mediated nerve injury in one of our animal models. Further, we investigated the anti-inflammatory effects of IL-33 in attenuating Ab-mediated nerve injury, as this cytokine is directly induced by sIVIg and orchestrates the anti-inflammatory effects via Th2 pathway.
Materials and Methods
Mice
Adult (8-12 weeks) wild-type C57BL/6 mice were used. All experimental procedures were complied with institutional and governmental guidelines for animal research and approved by the institutional Animal Care and Use Committee at the University of Texas Health Science Center at Houston.
Monoclonal anti-glycan antibody
Two disease relevant anti-ganglioside monoclonal antibody (mAb), GD1a/GT1b-2b (a prototypic IgG2b mAb against GD1a/GT1b) and GT1b-2b (IgG2b mAb with specificity for GT1b), were used in this study. We have demonstrated that these mAbs inhibit axon regeneration, prevent target re-innervation and induce injury to intact nerve fibers in different animal models (He et al., 2015;Lehmann et al., 2007;Zhang et al., 2011). The generation, specificity, and production, of these mAb were reported previously (Lunn et al., 2000;Schnaar et al., 2002). The hollow fiber supernatant containing anti-ganglioside mAb was used in all animal studies. An irrelevant mouse isotype matched IgG-2b mAb (Abcam, Cambridge, MA) was used as a negative control.
Preparation of sIVIg and uIVIg
Sambucus Nigra Lectin (SNL) chromatography (Vector Laboratories) was performed on whole IVIg (Octagam, Octapharma) to prepare terminal sialic acid-enriched (sIVIg) and sialic acid-depleted (uIVIg) IVIg fractions, as described (Kaneko et al., 2006). Briefly, IVIg (40mg) in Tris-buffered saline (TBS) were loaded to a column of SNL resin (4ml), and incubated for 10-15 mins at room temperature. The column was then washed with TBS, and the un-bound IVIg fractions (uIVIg) were collected. The bound proteins (sIVIg; ~2-3mg) were eluted with 0.5 M lactose in TBS. The efficiency of this lectin affinity purification is in 5-8% range. Both uIVIg and sIVIg were dialyzed against 1X PBS prior to usage.
Sciatic nerve crush model
A well-established nerve crush model was used to study the efficacy of different interventions. Nerve crush provides a convenient and well characterized system to study the regeneration of injured axons mimicking the regenerative response of degenerating axons in GBS, and it also allows us to study the factors affecting nerve repair. Briefly, the left sciatic nerve was crushed at middle thigh level on day 0, as described (Lehmann et al., 2007). Single dose of individual anti-glycan mAb or sham mAb (0.5-1mg) was injected on day 3 via intra-peritoneal route (i.p.). Daily injection of whole IVIg (2g/kg per day; i.p.), sIVIg (0.2g/kg per day; i.p.), uIVIg (2g/kg per day i.p.,), IL-33 (500 ng per day; subcutaneously; R&D Systems, Minneapolis, MN) or vehicle control (subcutaneously) were given to animals 5 days/week for 2 weeks after the nerve crush. For IVIg dose-response study, 0-2g/kg/day of whole IVIg was administered i.p. for 5 days/week for 2 weeks. Behavioral testing and electrophysiology were performed on all animals. The experiments were terminated on day 17 after the crush. All animals were perfused, sciatic and tibial nerves were harvested.
Behavioral assessment
Pinprick tests were used to evaluate sensory functional recovery. The tests were performed 1 day prior to the surgery and on indicated days post nerve crush, as described (Zhang et al., 2014). Briefly, the needle was gently applied to the lateral part of the plantar surface of the hind paw, and the animal responses to the needle prick were recorded. The Pinprick testing was performed blindly at indicated time points.
Electrophysiology
The motor function recovery after sciatic nerve crush was monitored by a sciatic nerve conduction test, which was performed on all animals, as described (Lehmann et al., 2007). Briefly, mice were anesthetized and placed on the heating pad, and their body temperature was monitored and maintained at 35–37°C. On day 16 post sciatic nerve crush, compound muscle action potential (CMAP) amplitude was recorded from the hind paw with needle electrodes inserted in the sole of the foot and sciatic and tibial nerves were stimulated proximally at the sciatic notch.
Morphometry
The harvested sciatic and tibial nerves were post-fixed in a mixture of 3% glutaraldehyde and 4% paraformaldehyde. The fixed nerves were embedded in Epon, and stained with toluidine blue. All myelinated axons in a single whole transverse section of the nerve were counted by using a motorized stage and stereotactic imaging software (Axiovision; Zeiss, Thornwood, NY), as described (Lehmann et al., 2007;Zhang et al., 2014).
Quantitative Real-time PCR for IL-33 expression
Mice were treated with whole IVIg (2g/kg), sIVIg (0.2g/kg), or uIVIg (2g/kg), to determine their effects on modulating IL-33 expression. In subsequent studies, IVIg dose responsiveness (0-2g/kg) on IL-33 expression was examined. Spleens were removed one hour after intravenous administration of whole IVIg, sIVIg or uIVIg for further analysis, as described (Anthony et al., 2011). Total RNA was extracted from splenocytes according to manufacturer’s instruction (Invitrogen). cDNA was generated through reverse transcription from 0.2 μg of each RNA sample by using High Capacity RNA-to-cDNA master mix (Life Technologies). Quantitative real-time PCR (qPCR) was performed on ABI Step-One Plus (Applied Biosystems) using TaqMan Fast Advanced Master Mix (Life Technologies). 18s was used as normalization control. All PCR were performed in triplicate and repeated at least three times.
Ganglioside ELISA
Ganglioside ELISA was used to determine the anti-ganglioside antibody level in animal serum before and after administration of exogenous anti-glycan mAbs with or without different types of IVIg (whole IVIg; sIVIg; uIVIg). Serum samples were collected from the tail vein of animals on days 0, 5, 10, 17 after IVIg administration, and ELISA was performed, as described (Lunn et al., 2000).
Statistics
Data are reported as mean ± standard error of the mean (SEM). Differences between groups were examined by Student's t-test and two-way ANOVA with corrections for multiple comparisons, and p< 0.05 was considered statistically significant.
Results
IVIg ameliorates anti-ganglioside antibody-mediated inhibition of axon regeneration in a passive transfer animal model of GBS
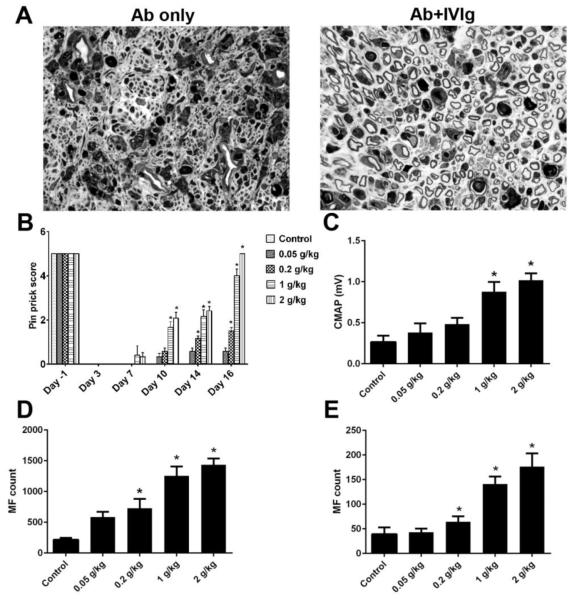
We previously demonstrated that anti-ganglioside antibodies inhibit peripheral nerve repair via targeting neuronal cell surface ganglioside in an in-vivo sciatic nerve crush model, which mimics the incomplete recovery found in some GBS patients with high titer of certain anti-ganglioside antibodies (Lehmann et al., 2007). This animal model provides a useful platform to study mechanisms of anti-ganglioside antibody mediated pathological effects, and evaluate potential therapeutic approaches. In the current study, the efficacy of whole IVIg was examined in this animal model. The WT mice passively transferred with anti-glycan mAbs were treated with IVIg or bovine serum albumin (BSA; 0.2-2 gm/kg), as 10 divided doses administered daily i.p. Our behavioral and electrophysiological data indicated that IVIg significantly improved sensory and motor nerve functional recovery in this model (Fig. 1B & C), which was further confirmed by the morphometric assessment at sciatic and tibial nerve levels (Fig. 1A, D & E). The IVIg mediated-beneficial effect was dose-dependent (Fig. 1B-E).
Figure 1. IVIg induce dose-responsive immunoprotection.
A. IVIg-treated animals have significantly more regenerating fibers (right micrograph) compared to mice treated by anti-ganglioside antibody only (left micrograph). B-E. Enhanced nerve repair, as assessed by behavioral (B; Pin prick behavior), electrophysiological (C; CMAP amplitudes), and morphometric analysis (myelinated nerve fiber counts D, sciatic nerve; E, tibial nerve), was found in IVIg-treated animals compared to controls. *p < 0.05; Error bars, s.e.m; N=10 per group; Scale bar: 10μm.
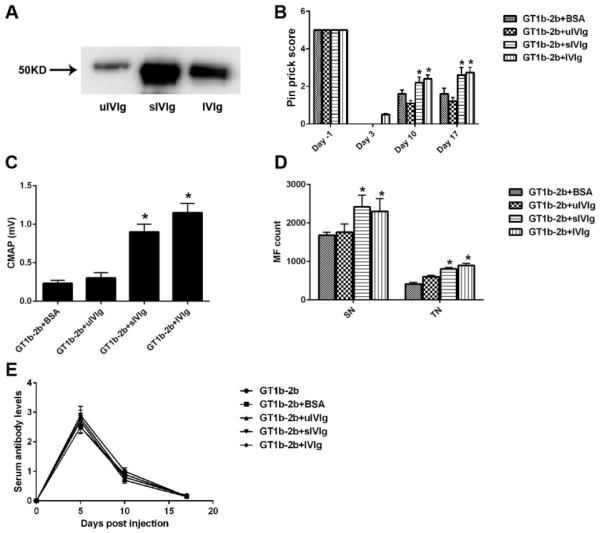
sIVIg fractions are more potent than whole IVIg in suppressing anti-glycan Ab-mediated inhibition of axon regeneration
sIVIg enriched fractions were prepared from whole IVIg by SNL chromatography. The purity of sIVIg fractions was analyzed and verified by SNL-blotting (Fig. 2A). We found that sIVIg content in IVIg is about 10% of all IgGs (data not shown). According to the dose-response study, optimal doses of whole IVIg (2 g/kg), sIVIg (0.2g/kg; 10-fold lower than whole IVIg) and uIVIg (2gm/kg) were used in conjunction with anti-glycan mAbs in our nerve crush model. We found that 10-fold lower dose of sIVIg and whole IVIg provided comparable protection from antibody-mediated inhibition of axon regeneration, whereas uIVIg was not protective in this model (Fig. 2B-D). Notably, the circulating titers of anti-glycan mAbs were comparable in all groups (Fig. 2E) suggesting that rapid catabolism of anti-glycan mAbs is not a mechanism of IVIg-mediated anti-inflammatory effects in this model. Further, these ELISA results on sera, which contain IVIg, also showed that anti-glycan mAb binding to its antigen was not altered by IVIg in this solid-phase assay, arguing against the presence of anti-idiotypic Abs to mouse anti-glycan mAbs used in this study (Fig. 2E).
Figure 2. Immunoprotection mediated by sIVIg.
A. SNL-blot showing sialylation of Fc portion of IgGs in whole IVIg, sialyl enriched (sIVIg) and sialyl-depleted (uIVIg) IVIg fractions. B-D. Significant nerve function recovery assessed by pin prick behavior (B) and nerve conduction test (C) was found in sIVIg- and IVIg-treated animals compared to controls, which was also confirmed by morphometry (D). Note: sIVIg was given 10-fold less. E. Serial ELISA on mice sera showing that circulating anti-ganglioside mAb levels are not affected by IVIg and sIVIg treatments. C-E. *p < 0.05. N = 10 per group; Error bars, s.e.m.
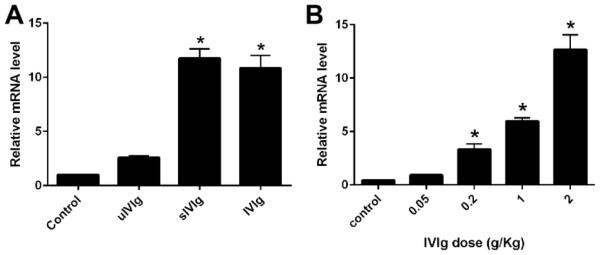
IVIg upregulates the IL-33 expression
Recent work showed that IVIg upregulates IL-33 expression, which promotes the production of IL-4 (Anthony et al., 2011), a well-known Th2 cytokine. Therefore, we examined whether IVIg and sIVIg can augment the expression of IL-33 in our animal model. WT mice were injected with various doses of whole IVIg (0.05-2g/kg) or control (BSA; 2g/kg) as a single IV injection and the splenic RNA was collected from animals 1hr after IVIg administration. IL-33 expression was determined by qPCR and 18s rRNA was used as an internal control. A significant upregulation of IL-33 gene expression was detected in mice receiving IVIg and sIVIg (Fig. 3A), and the upregulation induced by IVIg was dose-dependent (Fig. 3B). In subsequent studies with sIVIg, upregulation of IL-33 expression was observed in sIVIg-treated but not uIVIg-treated animals (Fig. 3A). These studies indicate that IVIg promotes IL-33 expression, which has been shown to induce the key Th2 cytokine, IL-4. These data support that the SIGN-R1-Th2 pathway is involved in IVIg-mediated neuroprotection in our animal model.
Figure 3. IVIg induce IL-33 expression.
A. Comparative IL-33 gene expression in spleens of WT mice treated with control protein (BSA; 2g/kg), uIVIg (2g/kg), sIVIg (0.2g/kg), and whole IVIg (2g/kg). B. Significant dose-dependent upregulation of IL-33 mRNA after whole IVIg treatment. *p < 0.05; Error bars, s.e.m; N=10 per group.
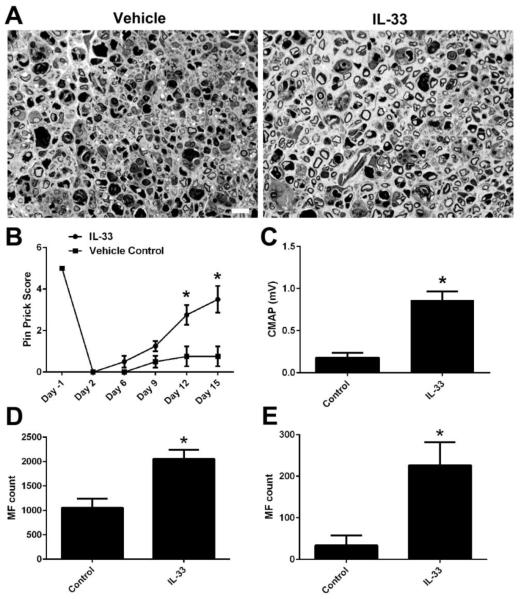
IL-33 enhance nerve repair/axonal regeneration
Next, we examined the efficacy of IL-33 in reducing the anti-glycan antibody-mediated nerve injury in the nerve crush model. WT mice passively transferred with anti-glycan mAb received daily i.p. injection of IL-33 (R&D; 500 ng) or BSA (500 ng) for 10 days. Our morphological study showed that almost no regenerating myelinated axons were found in the BSA treated animals, which is consistent with our pervious findings (Lehmann et al., 2007;Zhang et al., 2014). In contrast, there were significantly more regenerating fibers in nerves of animals treated with IL-33 (Fig. 4A). IL-33 treatment significantly enhanced both sensory and motor function recovery in this crush model as assessed by behavioral (Pin Prick test) and electrophysiology (sciatic nerve conduction test), respectively (Fig. 4B&C). Furthermore, the myelinated axon counts at sciatic (Fig. 4D) and tibial nerve (Fig. 4E) levels confirmed the effectiveness of IL-33 in significantly reducing the antibody-mediated nerve injury.
Figure 4. IL-33 enhances nerve repair.
A. Representative micrographs showing vehicle- and IL-33-treated sciatic nerves. B-D. IL-33 significantly improved the axon regeneration/nerve repair as suggested by behavioral (B), electrophysiological (C), and morphometric studies (D, sciatic nerve; E, tibial nerve). *p < 0.05. Error bars, s.e.m; N=10 per group; Scale bar: 10 μm.
Discussion
The main finding of our study is that sIVIg, a minor fraction of whole IVIg, can effectively mitigate the anti-glycan Ab-mediated inhibition of axon regeneration in a preclinical model of GBS at a much lower dose compared to whole IVIg. Autoantibodies directed against gangliosides expressed on neuronal cell surfaces have become the main focus of research in GBS (Ilyas et al., 1988;Quarles et al., 1990;Willison et al., 2002). We have demonstrated that the anti-glycan Abs induce severe neuropathological effects in different preclinical models of GBS (He et al., 2015;Lehmann et al., 2007;Zhang et al., 2014). IVIg is now the most commonly used treatment in autoimmune neuropathies including GBS and CIDP. However, there are many disadvantages including high cost, supply shortages, and multiple side effects that are usually associated with high dose and long infusion time of IVIg (Bayry et al., 2007;Kessary-Shoham et al., 1999). We compared the efficacy of whole IVIg, the terminal α2,6 sialic acid enriched- and depleted-fractions derived from whole IVIg. Our results demonstrate that the whole IVIg and sIVIg fractions significantly modulate nerve inflammation and enhance motor and sensory function recovery, whereas, the uIVIg does not alter the anti-glycan Ab mediated-pathological effects in our animal model. The data presented in this report are consistent with previous findings that the anti-inflammatory activity of IVIg is sialyation-dependent in preclinical models of autoimmune arthritis and ITP (Anthony et al., 2008a;Kaneko et al., 2006). Overall, our findings raise the possibility that sIVIg is an alternative therapeutic modality to whole IVIg in the management of immune neuropathies including GBS.
The immunomodulatory mechanisms underlying IVIg’s efficacy, in autoimmune disorders including immune neuropathies, are not clearly established (Schwab and Nimmerjahn, 2013). The postulated mechanisms in the context of humoral autoimmunity include: anti-idiotypic/neutralizing Abs; inhibition of complement activation; increased catabolism via saturation of neonatal Fc receptor; competition with autoAbs/ICs for activating FcγRs on inflammatory cells; and upregulation of inhibitory FcγRIIB that alters the ratio of FcγR types on inflammatory cells leading to inhibitory (anti-inflammatory) signaling following interactions with immune complexes (Anthony et al., 2012). In the current context, our previous work indicates that human IVIg do not have anti-idiotypic Abs against mouse anti-ganglioside mAbs (Zhang et al., 2004), and the studies performed in C5-deficient and C3-null mice support that complement activation is not involved in Ab-mediated inhibition of axon regeneration in this model (Lehmann et al., 2007;Zhang et al., 2014). The current study examined the pharmacokinetics of anti-glycan mAbs and found that although IVIg did provide protection in our passive transfer model, it did not increase the catabolism of anti-glycan Abs. These findings would argue against enhanced clearance of pathogenic Abs and/or presence of anti-idiotypic Abs being responsible for IVIg-mediated anti-inflammatory effects in this model. Since Ab-mediated inhibition of axon regeneration in our model is dependent on activating FcγRs on circulating macrophages (Zhang et al., 2014), it is possible that IVIg’s protection in this model involves either competition for binding to activating FcγRs with anti-glycan Abs/immune complexes and/or upregulation of inhibitory FcγRIIB on inflammatory cells via the SIGN-R1-Th2 pathway (Anthony et al., 2008b).
Our study with exogenous IL-33 treatment would support that SIGN-R1-Th2 pathway is involved in IVIg-mediated neuroprotection from anti-glycan Ab-mediated nerve injury, as IVIg and anti-glycan-ganglioside immune complex competition for binding to activating FcγRs is not feasible in the IL-33 treatment paradigm. Further, we found IVIg and sIVIg induce the production of IL-33, which suggest that the anti-inflammatory effect of IVIg or sIVIg are likely through Th2 pathway. Previous studies have defined the SIGN-R1-Th2 pathway that includes sIVIg/IVIg induced production of IL-33 in spleen, which activates Fcε RI+ basophils to produce Th2 cytokines including IL-4. IL-4 upregulates inhibitory FcγRIIB receptors on the effector macrophages, which participate in suppression of inflammation (Anthony et al., 2011;Anthony et al., 2008b). Interestingly, support for the notion that increases in FcγRIIB expression on inflammatory cells is a mechanism of IVIg-mediated immunoprotection was found in CIDP. Notably, IVIg-responsive CIDP patients showed increases in FcγRIIB expression on monocytes and B cells (Tackenberg et al., 2009;Tackenberg et al., 2010).
The present study has several translational and clinical implications. IVIg has divergent immumodulation effects which may be beneficial at different stages of GBS/CIDP. Current study demonstrated the effectiveness of IVIg and sIVIg in mitigating anti-glycan antibody mediated-pathological effects on nerve regeneration. These findings suggest that IVIg or sIVIg treatment can be extended in GBS patients with delayed recovery who have persistent anti-ganglioside antibodies after the acute phase of the disease. The effects of sIVIg in GBS models of antibody-mediated injury to intact nerve fibers remain to be examined. Further, our data validate the involvement of SIGN-R1-Th2 pathway in IVIg and sIVIg mediated beneficial effects in an animal model of neuroimmunological disorder. This observation also has translational implications as substantially smaller quantities of sIVIg, at a 10-fold lower dose than IVIg, may provide equal efficacy to currently used amounts of whole IVIg requiring long infusion times spanning over days. It implicates that modifying the terminal sialic acid of IVIg could alter its anti-inflammatory activity. Notably, a hyper-sialylated IVIg had been recently developed, it has enhanced anti-inflammatory activity and can be potentially used to treat patients with autoimmune disorders (Washburn et al., 2015). Our experience with high dose IVIg in GBS patients who failed to respond to the standard dose of IVIg (2 gm/kg) is that patients with blood groups A and/or B are susceptible to hemolytic anemia due to anti-A and -B antibodies present in IVIg preparations (Nguyen et al., 2014). Use of higher effective anti-inflammatory doses of sIVIg compared to whole IVIg with reduced probability of hemolytic anemia is likely in this situation. Moreover, our studies with IL-33 suggest that different components of SIGN-R1-Th2 pathway could be potential therapeutic targets for future drug development and we show the feasibility of using IL-33 as anti-inflammatory agent.
Highlights.
IVIg and sialylated IVIg fraction (sIVIg) mitigate anti-glycan antibody mediated-pathological effect in an animal model of GBS.
Substantially smaller amount of sIVIg provides equivalent efficacy compared to whole IVIg.
IVIg and sIVIg upregulate the gene expression of IL-33, and exogenous IL-33 provides neuroprotection.
These data suggest that SIGN-R1-Th2 pathway is involved in IVIg/sIVIg mediated anti-inflammatory effects.
Acknowledgements
This study was supported by the National Institute of Neurological Disorders and Stroke (NIH/NINDS; Grants R01 NS42888, R01 NS54962, R21NS087467), and Mr. Frank Gruen and family.
Abbreviations
- IVIg
Intravenous immunoglobulin
- GBS
Guillain-Barré syndrome
- CIDP
Chronic inflammatory demyelinating polyradiucloneuropathy
- SIGN-R1
Specific ICAM3-grabbing nonintegrin related 1 receptor
- sIVIg
α2,6 sialic acid enriched IVIg
- uIVIg
α2,6 sialic acid depleted IVIg
- FcγRs
Fc-gamma receptors
- CMAP
Compound muscle action potential
- qPCR
Quantitative real-time PCR
- BSA
Bovine serum albumin
- SNL
Sambucus Nigra Lectin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflicts of interest.
REFERENCES
- Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008a;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. U. S. A. 2008b;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Annals of the New York Academy of Science. 2012;1253:170–180. doi: 10.1111/j.1749-6632.2011.06305.x. [DOI] [PubMed] [Google Scholar]
- Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual Review of Immunology. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- Asbury AK, Arnason BG, Adams RD. The inflammatory lesion in idiopathic polyneuritis. Its role in pathogenesis. Medicine (Baltimore) 1969;48:173–215. doi: 10.1097/00005792-196905000-00001. [DOI] [PubMed] [Google Scholar]
- Bayry J, Kazatchkine MD, Kaveri SV. Shortage of human intravenous immunoglobulin--reasons and possible solutions. Nat. Clin. Pract. Neurol. 2007;3:120–121. doi: 10.1038/ncpneuro0429. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat. Rev. Neurol. 2011;7:507–517. doi: 10.1038/nrneurol.2011.121. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Stoll G, Li CY, Tyor WR, Cornblath DR. Macrophage responses in inflammatory demyelinating neuropathies. Annals of Neurology. 1990;27(suppl):S64–S68. doi: 10.1002/ana.410270717. [DOI] [PubMed] [Google Scholar]
- Hafer-Macko C, Hsieh S-T, Li CY, Ho TW, Sheikh K, Cornblath DR, McKhann GM, Asbury AK, Griffin JW. Acute motor axonal neuropathy: an antibody-mediated attack on axolemma. Annals of Neurology. 1996;40:635–644. doi: 10.1002/ana.410400414. [DOI] [PubMed] [Google Scholar]
- He L, Zhang G, Liu W, Gao T, Sheikh KA. Anti-Ganglioside Antibodies Induce Nodal and Axonal Injury via Fcgamma Receptor-Mediated Inflammation. Journal of Neuroscience. 2015;35:6770–6785. doi: 10.1523/JNEUROSCI.4926-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth PM. Fc receptors are major mediators of antibody based inflammation in autoimmunity. Current Opinion in Immunology. 2002;14:798–802. doi: 10.1016/s0952-7915(02)00409-0. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Cornblath DR. Guillain-Barré syndrome. The Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Hadden RD, Gregson NA, Smith KJ. Pathogenesis of Guillain-Barré syndrome. Journal of Neuroimmunology. 1999;100:74–97. doi: 10.1016/s0165-5728(99)00195-2. [DOI] [PubMed] [Google Scholar]
- Ilyas AA, Willison HJ, Quarles RH, Jungalwala FB, Cornblath DR, Trapp BD, Griffin DE, Griffin JW, McKhann GM. Serum antibodies to gangliosides in Guillain-Barré syndrome. Annals of Neurology. 1988;23:440–447. doi: 10.1002/ana.410230503. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Kessary-Shoham H, Levy Y, Shoenfeld Y, Lorber M, Gershon H. In vivo administration of intravenous immunoglobulin (IVIg) can lead to enhanced erythrocyte sequestration. Journal of Autoimmunity. 1999;13:129–135. doi: 10.1006/jaut.1999.0302. [DOI] [PubMed] [Google Scholar]
- Kiefer R, Kieseier BC, Stoll G, Hartung HP. The role of macrophages in immune-mediated damage to the peripheral nervous system. Progress in Neurobiology. 2001;64:109–127. doi: 10.1016/s0301-0082(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Lehmann HC, Lopez PHH, Zhang G, Ngyuen T, Zhang JY, Kieseier BC, Mori S, Sheikh KA. Passive immunization with anti-ganglioside antibodies directly inhibits axon regeneration in an animal model. Journal of Neuroscience. 2007;27:27–34. doi: 10.1523/JNEUROSCI.4017-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn MP, Johnson LA, Fromholt SE, Itonori S, Huang J, Vyas AA, Hildreth JE, Griffin JW, Schnaar RL, Sheikh KA. High-affinity anti-ganglioside IgG antibodies raised in complex ganglioside knockout mice: reexamination of GD1a immunolocalization. J. Neurochem. 2000;75:404–412. doi: 10.1046/j.1471-4159.2000.0750404.x. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Cornblath DR, Griffin JW, Ho TW, Li CY, Jiang Z, Wu HS, Zhaori G, Liu Y, Jou LP, Liu TC, Gao CY, Mao JY, Blaser MJ, Mishu B, Asbury AK. Acute motor axonal neuropathy: A frequent cause of acute flaccid paralysis in China. Annals of Neurology. 1993;33:333–342. doi: 10.1002/ana.410330402. [DOI] [PubMed] [Google Scholar]
- Nguyen TP, Biliciler S, Wahed A, Sheikh K. Occurrence of hemolytic anemia in patients with GBS treated with high-dose IVIg. Neurol. Neuroimmunol. Neuroinflamm. 2014;1:e50. doi: 10.1212/NXI.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Ogawara K, Kuwabara S, Mori M, Hattori T, Koga M, Yuki N. Axonal Guillain-Barré syndrome: relation to anti-ganglioside antibodies and Campylobacter jejuni infection in Japan. Annals of Neurology. 2000;48:624–31. [PubMed] [Google Scholar]
- Prineas JW. Pathology of the Guillain-Barré syndrome. Annals of Neurology. 1981;9(Suppl):6–19. doi: 10.1002/ana.410090704. [DOI] [PubMed] [Google Scholar]
- Quarles RH, Ilyas AA, Willison HJ. Antibodies to gangliosides and myelin proteins in Guillain-Barré syndrome. Annals of Neurology. 1990;27(suppl):S48–S52. doi: 10.1002/ana.410270713. [DOI] [PubMed] [Google Scholar]
- Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, Fromholt SE, Gong Y, Vyas AA, Laroy W, Wayman DM, Heffer-Lauc M, Ito H, Ishida H, Kiso M, Griffin JW, Sheikh KA. IgG-class mouse monoclonal antibodies to major brain gangliosides. Analytic Biochemistry. 2002;302:276–284. doi: 10.1006/abio.2001.5540. [DOI] [PubMed] [Google Scholar]
- Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat. Rev. Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- Tackenberg B, Jelcic I, Baerenwaldt A, Oertel WH, Sommer N, Nimmerjahn F, Lunemann JD. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4788–4792. doi: 10.1073/pnas.0807319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackenberg B, Nimmerjahn F, Lunemann JD. Mechanisms of IVIG efficacy in chronic inflammatory demyelinating polyneuropathy. Journal of Clinical Immunology. 2010;30(Suppl 1):S65–S69. doi: 10.1007/s10875-010-9398-1. [DOI] [PubMed] [Google Scholar]
- Takai T. Roles of Fc receptors in autoimmunity. Nat. Rev. Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- Washburn N, Schwab I, Ortiz D, Bhatnagar N, Lansing JC, Medeiros A, Tyler S, Mekala D, Cochran E, Sarvaiya H, Garofalo K, Meccariello R, Meador JW, Rutitzky L, Schultes BC, Ling L, Avery W, Nimmerjahn F, Manning AM, Kaundinya GV, Bosques CJ. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E1297–E1306. doi: 10.1073/pnas.1422481112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125:2591–2625. doi: 10.1093/brain/awf272. [DOI] [PubMed] [Google Scholar]
- Yuki N, Hartung HP. Guillain-Barré syndrome. New England Journal of Medicine. 2012;366:2294–2304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, Taguchi K, Miyatake T, Furukawa K, Kobata T, Yamada M. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barré syndrome. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11404–11409. doi: 10.1073/pnas.0402391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki N, Yamada M, Koga M, Odaka M, Susuki K, Tagawa Y, Ueda S, Kasama T, Ohnishi A, Hayashi S, Takahashi H, Kamijo M, Hirata K. Animal model of axonal Guillain-Barré syndrome induced by sensitization with GM1 ganglioside. Annals of Neurology. 2001;49:712–20. [PubMed] [Google Scholar]
- Zhang G, Bogdanova N, Gao T, Song JJ, Cragg MS, Glennie MJ, Sheikh KA. Fcgamma receptor-mediated inflammation inhibits axon regeneration. PLoS. One. 2014;9:e88703. doi: 10.1371/journal.pone.0088703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Lehmann HC, Manoharan S, Hashmi M, Shim S, Ming GL, Schnaar RL, Lopez PH, Bogdanova N, Sheikh KA. Anti-Ganglioside Antibody-Mediated Activation of RhoA Induces Inhibition of Neurite Outgrowth. Journal of Neuroscience. 2011;31:1664–1675. doi: 10.1523/JNEUROSCI.3829-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Lopez PH, Li CY, Mehta NR, Griffin JW, Schnaar RL, Sheikh KA. Anti-ganglioside antibody-mediated neuronal cytotoxicity and its protection by intravenous immunoglobulin: implications for immune neuropathies. Brain. 2004;127:1085–1100. doi: 10.1093/brain/awh127. [DOI] [PubMed] [Google Scholar]