Abstract
The aim of the present study was to investigate the efficacy of a potassium-competitive acid blocker (PCAB) named vonoprazan (VPZ) for improving symptoms in patients with reflux esophagitis (RE), non-erosive reflux disease (NERD), and functional dyspepsia (FD). A hospital-based, retrospective study of outpatients in our department (Department of Gastroenterology, University of Juntendo, Tokyo, Japan) between March 2015 and August 2016 was performed. The patients who were experiencing heartburn, acid regurgitation, gastric pain, and/or a heavy feeling in the stomach of at least moderate severity at baseline were treated with 20 mg VPZ once daily for 4 weeks. The patients completed the global overall symptom (GOS) scale to determine their symptom severity at baseline and after the 4 week treatment period. The proportions of patients with RE, NERD, and FD achieving improvement of their symptoms, defined as a GOS scale score of 1 (‘no problem’) or 2 (‘minimal problem’), were evaluated. During 4 weeks of VPZ therapy, changes in the gastroesophageal reflux disease (GERD) score, which was defined as the total points for heartburn and acid regurgitation on the GOS scale in patients with RE and NERD, and in the FD score, which was defined as the total points for gastric pain and a heavy feeling in the stomach on the GOS scale in patients with FD, were also evaluated. A total of 88 eligible cases were included in the present study, comprising 20 patients with RE, 25 patients with NERD, and 43 patients with FD. The rates of symptomatic improvement in patients with RE, NERD, and FD were 75.0, 60.0, and 48.8%, respectively. For the patients who were first administered VPZ, the rates of symptomatic improvement were 90.9, 66.7, and 58.8% in patients with RE, NERD, and FD, respectively. For those patients who were resistant to 8 weeks of proton pump inhibitor therapy, the rates of symptomatic improvement were 55.6, 53.8, and 42.3% in patients with RE, NERD, and FD, respectively. The GERD score in patients with RE and NERD, and the FD score in FD patients, were decreased after 4 weeks of VPZ therapy (P<0.01). In patients with RE, NERD and FD, the possibility that PCAB may be used as a novel therapeutic drug was suggested. However, the number of study subjects was small; therefore, further, larger and prospective studies are required.
Keywords: potassium-competitive acid blocker, vonoprazan, proton pump inhibitor, reflux esophagitis, non-erosive reflux disease, functional dyspepsia, global overall symptom scale, PPI-therapy resistant
Introduction
Against the background of the westernization of eating habits and the decrease in the rate of Helicobacter pylori (H. pylori) infection in Japan, gastroesophageal reflux disease (GERD) is showing a tendency to increase in incidence (1). GERD symptoms, including heartburn and regurgitation, are known to cause a decrease in the quality of life (QOL) of patients with GERD (2). Functional dyspepsia (FD) is a commonly encountered disease in clinical practice, and the symptoms of FD are also known to reduce QOL (3). Among the therapeutic drugs available for GERD and FD, proton pump inhibitors (PPIs) are predominantly used as a first-line drug (4,5); however, a substantial proportion of patients are resistant to treatment using PPIs (6). Recently, a new potassium-competitive acid blocker (PCAB) drug, termed vonoprazan (VPZ), was developed in Japan (7). A report has been published describing the effects of PCAB administration on promoting the healing of esophageal mucosal injury (8); however, there have been few reports about the efficacy of PCAB for improving the symptoms of GERD and FD. The aim of the present study was to investigate the efficacy of PCAB for producing symptomatic improvements in patients with GERD and FD.
Materials and methods
Study design
A hospital-based, retrospective study of outpatients was performed in our department (Department of Gastroenterology, University of Juntendo, Tokyo, Japan) between March 2015 and August 2016. The patients were asked to complete the global overall symptom (GOS) scale to determine symptom severity at baseline, and after 4 weeks of VPZ therapy by one specialist (D.A.), who was a member of the Japan Society of Gastroenterology. Patients also had to have heartburn, acid regurgitation, gastric pain, and/or a heavy feeling in the stomach of at least moderate severity (GOS scale score of 4) at baseline. The patients were administered with 20 mg VPZ once daily for 4 weeks. The efficacy of the improvement in the symptoms attributable to VPZ was assessed by evaluating the GOS scale scores for heartburn, acid regurgitation, gastric pain, and a heavy feeling in the stomach recorded by patients at baseline, and subsequently after the administration of VPZ for 4 weeks. The GOS scale has been validated for the assessment of upper gastrointestinal symptoms in a clinical trial setting (9), and has been used in clinical studies to assess the symptoms of GERD and FD (10,11). The GOS scale measures the severity of eight symptoms (heartburn, acid regurgitation, gastric pain, stomach feeling heavy, early satiety, feeling queasy, burping, and feeling of fullness) on a 7-point scale, from 1 [‘no problem’ (no symptoms)] to 7 [‘very severe problem’ (cannot be ignored, markedly limits my daily activities and often requires rest)] (9). The four symptoms (heartburn, acid regurgitation, gastric pain, and stomach feeling heavy) measured by the GOS scale were investigated in order to perform symptom-based evaluations in the present study.
Patients of either gender who were aged ≥20 years were eligible for inclusion if information on all of the following aspects were provided from their medical records: Patient profile [age, gender, body mass index (BMI), alcohol intake, and smoking]; H. pylori infection status (negative, positive, or negative after eradication therapy); and findings of upper gastrointestinal endoscopy [hiatal hernia and endoscopic gastric mucosal atrophy (EGA)]. BMI was calculated as body weight divided by the square of body height in meters (kg/m2). H. pylori infection status was assessed by the 13C-urea breath test (12) and/or the presence of serum antibodies against H. pylori. A positive result for any of these tests was defined as being positive for H. pylori infection. A result of ‘negative after eradication’ was also defined as the 13C-urea breath test being negative for H. pylori infection at 4–8 weeks following eradication therapy. In upper gastrointestinal endoscopy, a hiatal hernia was defined as an apparent separation of the esophagogastric junction and diaphragm impression by >2 cm. EGA was classified as C-0 (normal), C-1, C-2, C-3, O-1, O-2 or O-3 using the Kimura-Takemoto classification system (13), which identifies the location of the endoscopic atrophic border. Overall, EGA was scored as 0 for C-0 type, 1 for C-1 type, 2 for C-2 type, 3 for C-3 type, 4 for O-1 type, 5 for O-2 type, and 6 for O-3 type. Patients with the following were excluded: Those who had gastrectomy, peptic ulcer, gastric or esophageal malignant disease, or successful eradication of H. pylori within the previous 6 months. Additionally, patients who were currently or previously treated with non-steroidal anti-inflammatory drugs and low-dose aspirin were excluded.
In the present study, the study patients were divided into 3 groups (RE, NERD, and FD). ‘RE’ patients were defined as those who had findings of RE of grades A, B, C, and D according to the Los Angeles Classification system (14), and also had heartburn and/or acid regurgitation of at least moderate severity (GOS scale score of 4) at baseline. ‘NERD’ patients were defined as those who had exhibited findings without RE and also had heartburn and/or acid regurgitation of at least moderate severity (GOS scale score of 4) at baseline. ‘FD’ patients were defined as those who had findings without RE, and also had gastric pain and/or a heavy feeling in the stomach of at least moderate severity (GOS scale score of 4) at baseline. However, the patients for whom the scores for heartburn and/or acid regurgitation were higher than the scores for gastric pain and/or a heavy feeling in the stomach were excluded from the FD group. As part of a subgroup analysis, in each group (RE, NERD, and FD), the patients who were administered with VPZ as an initial therapy were defined as ‘f-RE’, ‘f-NERD’, and ‘f-FD’ patients, respectively. Among the RE and NERD patients, those who had heartburn and/or acid regurgitation of at least moderate severity (GOS scale score of 4) after having received PPI therapy for >8 weeks were defined as ‘r-RE’ and ‘r-NERD’ patients, respectively. For the FD patients, the patients who had gastric pain and/or a heavy feeling in the stomach of at least moderate severity (GOS scale score of 4) after receiving PPI therapy for >8 weeks were defined as ‘r-FD’ patients. Furthermore, the total points of the GOS scale scores for both heartburn and acid regurgitation were defined as a ‘GERD score’, and the total points of the GOS scale scores of both gastric pain and a heavy feeling in the stomach were also defined as an ‘FD score’.
First, the proportions of patients with RE, NERD, and FD who achieved an improvement in their symptoms, and who were assigned a GOS scale score of 1 [‘no problem’ (no symptoms)] or 2 [‘minimal problem’ (can be easily ignored without effort)] after the administration of VPZ for 4 weeks, were evaluated. As a subgroup analysis, the proportions of f-RE, f-NERD, f-FD, r-RE, r-NERD, and r-FD patients achieving an improvement in their symptoms after the administration of VPZ were evaluated.
Secondly, changes in the GERD score in patients with RE and NERD, and in the FD score in FD patients, during 4 weeks of VPZ therapy were evaluated. The presence of adverse events was investigated throughout the administration of VPZ, and these were assessed according to whether or not they were serious.
The present study was performed in accordance with the principles of the Declaration of Helsinki. The Juntendo University Ethics Committee approved the study, and the study protocol (reference no. 16-098). With regard to the informed consent of participants, the Juntendo University Ethics Committee made a decision based on the Ethical Guidelines for Medical and Health Research Involving Human Subjects, which states that non-intervention studies are deemed exempt from patients' consent and, instead, researchers must notify the study subjects about the information regarding study contents on a home page, and guarantee an opportunity when the study subjects could refuse it. Consequently, the decision of the Juntendo University Ethics Committee was put into practice in the present study.
Statistical analysis
The clinical characteristics were evaluated using the χ2 test and Fisher's exact test. Changes in the GERD and FD scores in before and after 4-weeks of VPZ therapy were evaluated using Student's t-test. All statistical analyses were performed using SPSS version 19 software (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics
The clinical characteristics of the 88 eligible cases, in total [30 men (34.1%) and 58 women (65.9%); mean age, 60.2±14.7; and mean BMI, 21.9±3.3] are summarized in Table I. The rates of alcohol intake and smoking were 25.0 and 10.2%, respectively. Cases who were H. pylori negative, H. pylori positive, and H. pylori negative following eradication therapy numbered 52 (59.1%), 8 (9.1%), and 28 (31.8%), respectively. There were 20 (22.7%) cases of RE, and 9, 7, 3, and 1 of these cases had Los Angeles grades A, B, C, and D, respectively. Hiatal hernia was observed in 51 (58.0%) cases. The mean EGA was 1.7±1.8. The numbers of cases of EGA classified as C-0, C-1, C-2, C-3, O-1, O-2, and O-3 were 35, 16, 9, 6, 16, 3, and 3, respectively. In the present study, RE, NERD, and FD patients numbered 20, 25, and 43, respectively. During the 4-week administration of VPZ, four patients had mild constipation and one patient had mild diarrhea; however, none of these patients discontinued the treatment since their symptoms of constipation and diarrhea were only mild.
Table I.
Clinical characteristics of study subjects (n=88).
| Characteristic | Total (n=88) | RE (n=20) | NERD (n=25) | FD (n=43) |
|---|---|---|---|---|
| Patient profile | ||||
| Age (years) | 60.2 (±14.7)b | 59.1 (±15.1)b | 61.7 (±15.2)b | 59.8 (±14.4)b |
| Gender | ||||
| Male | 30 (34.1)a | 13 (65.0)a | 7 (28.0)a | 10 (23.3)a |
| Female | 58 (65.9)a | 7 (35.0)a | 18 (72.0)a | 33 (76.7)a |
| BMI (kg/m2) | 21.9 (±3.3)b | 24.0 (±2.6)b | 22.3 (±2.9)b | 20.8 (±3.4)b |
| Smoking | ||||
| Smoker | 9 (10.2)a | 4 (20.0)a | 2 (8.0)a | 3 (7.0)a |
| Non-smoker | 79 (89.8)a | 16 (80.0)a | 23 (92.0)a | 40 (93.0)a |
| Alcohol consumption | ||||
| Drinker | 22 (25.0)a | 9 (45.0)a | 6 (24.0)a | 7 (16.3)a |
| Non-drinker | 66 (75.0)a | 11 (55.0)a | 19 (76.0)a | 36 (83.7)a |
| H. pylori | ||||
| H. pylori infection | ||||
| Positive | 8 (9.1)a | 1 (5.0)a | 4 (16.0)a | 3 (7.0)a |
| Negative | 52 (59.1)a | 15 (75.0)a | 11 (44.0)a | 26 (60.5)a |
| Negative after eradication | 28 (31.8)a | 4 (20.0)a | 10 (40.0)a | 14 (32.5)a |
| Upper GI findings | ||||
| Reflux esophagitis | ||||
| Yes | 20 (22.7)a | |||
| No | 68 (77.3)a | |||
| LA-grade | ||||
| A | 9 | |||
| B | 7 | |||
| C | 3 | |||
| D | 1 | |||
| Hiatal hernia | ||||
| Yes | 51 (58.0)a | 18 (90.0)a | 7 (28.0)a | 26 (60.5)a |
| No | 37 (42.0)a | 2 (10.0)a | 18 (72.0)a | 17 (39.5)a |
| Endoscopic gastric mucosal atrophy | 1.7 (±1.8)b | 1.3 (±1.6)b | 1.8 (±1.8)b | 1.8 (±1.9)b |
Number (%)
median (mean ± standard deviation); RE, reflux esophagitis; NERD, non-erosive reflux disease; FD, functional dyspepsia; BMI, body mass index; H. pylori, Helicobacter pylori; GI, gastrointestinal; LA-grade, Los Angeles grade.
RE patients
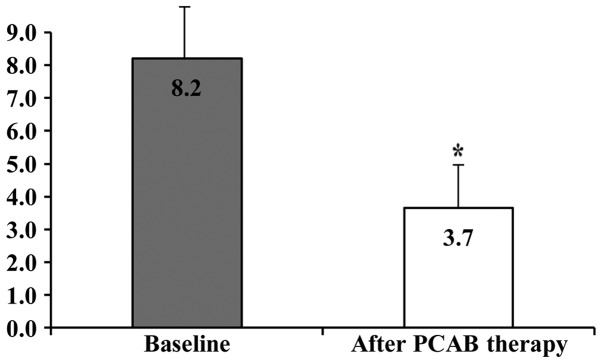
The clinical characteristics of the 20 eligible patients with RE [13 men (65.0%) and 7 women (35.0%); mean age, 59.1±15.1 years; and mean BMI, 24.0±2.6) are shown in Table I. The rates of alcohol intake and smoking were 45.0 and 20.0%, respectively. Cases who were H. pylori negative, H. pylori positive, and H. pylori negative following eradication therapy numbered 15 (75.0%), 1 (5.0%), and 4 (20.0%), respectively. Among the cases of RE, 9, 7, 3, and 1 cases had Los Angeles grades A, B, C, and D, respectively. Hiatal hernia was observed in 18 (90.0%) cases. The mean EGA was 1.3±1.6. The numbers of cases of EGA classified as C-0, C-1, C-2, C-3, O-1, O-2, and O-3 were 9, 5, 1, 2, 2, 1, and 0, respectively. Of the 20 RE patients, 11 cases had f-RE and 9 cases had r-RE. The proportion of RE patients who achieved an improvement in their reflux symptoms after 4 weeks of VPZ therapy was 75.0% (15/20). The proportions of patients with f-RE and r-RE who achieved an improvement in their reflux symptoms after 4 weeks of VPZ therapy were 90.9% (10/11) and 55.6% (5/9), respectively. The GERD scores at baseline and after 4 weeks of VPZ therapy in patients with RE were 8.2±1.7 and 3.7±1.4, respectively, and this difference was statistically significant (P<0.01; Fig. 1).
Figure 1.
Changes from baseline of the GERD scores following administration of 20 mg VPZ once daily for 4 weeks in patients with RE. The GERD scores at baseline and after 4 weeks of VPZ therapy in RE patients were 8.2±1.7 and 3.7±1.4, respectively, and this difference was statistically significant (*P<0.01). GERD, gastroesophageal reflux disease; RE, reflux esophagitis; VPZ, vonoprazan; PCAB, potassium-competitive acid blocker.
NERD patients
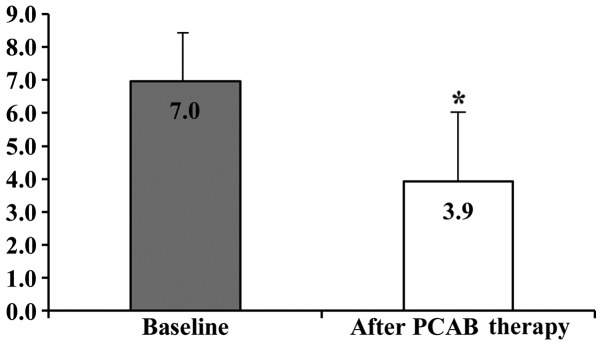
The clinical characteristics of the 25 eligible patients with NERD [7 men (28.0%) and 18 women (72.0%); mean age 61.7±15.2, and mean BMI, 22.3±2.9] are shown in Table I. The rates of alcohol intake and smoking were 24.0 and 8.0%, respectively. Cases who were H. pylori negative, H. pylori positive, and H. pylori negative following eradication therapy numbered 11 (44.0%), 4 (16.0%), and 10 (40.0%), respectively. Hiatal hernia was observed in 7 (28.0%) cases. The mean EGA was 1.8±1.8. The numbers of cases of EGA classified as C-0, C-1, C-2, C-3, O-1, O-2, and O-3 were 9, 4, 3, 2, 6, 0, and 1, respectively. Of the 25 patients with NERD, 12 cases had f-NERD and 13 cases had r-NERD. The proportion of the total NERD patients who achieved an improvement in their reflux symptoms after 4 weeks of VPZ therapy was 60.0% (15/25). The proportions of patients with f-NERD and r-NERD who achieved an improvement in their reflux symptoms after 4 weeks of VPZ therapy were 66.7% (8/12) and 53.8% (7/13), respectively. The GERD scores at baseline and after 4 weeks of VPZ therapy in patients with NERD were 7.0±1.6 and 3.9±2.0, and this difference was statistically significant (P<0.01; Fig. 2).
Figure 2.
Changes from baseline of the GERD scores following administration of 20 mg VPZ once daily for 4 weeks in patients with NERD. The GERD scores at baseline and after 4 weeks of VPZ therapy in NERD patients were 7.0±1.6 and 3.9±2.0, and this difference was statistically significant (*P<0.01). GERD, gastroesophageal reflux disease; VPZ, vonoprazan; NERD, non-erosive reflux disease; PCAB, potassium-competitive acid blocker.
FD patients
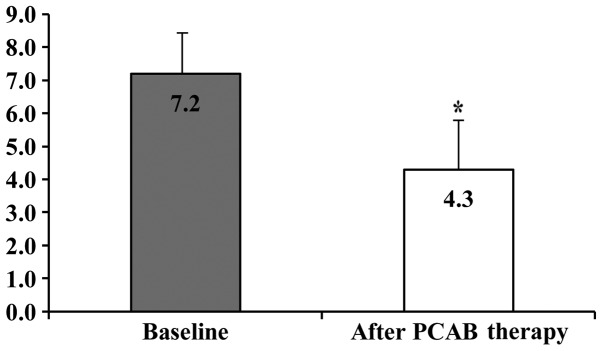
The clinical characteristics of the 43 eligible patients with FD [10 men (23.3%) and 33 women (76.7%); mean age, 59.8±14.4; and mean BMI, 20.8±3.4] are shown in Table I. Alcohol intake and smoking were 16.3 and 7.0%, respectively. Cases who were H. pylori negative, H. pylori positive, and H. pylori negative after eradication therapy numbered 26 (60.5%), 3 (7.0%), and 14 (32.6%), respectively. Hiatal hernia was observed in 26 (60.5%) cases. The mean EGA was 1.8±1.9. The numbers of cases of EGA classified as C-0, C-1, C-2, C-3, O-1, O-2, and O-3 were 17, 7, 5, 2, 8, 2, and 2, respectively. Of the 43 patients with FD, 17 cases had f-FD and 26 cases had r-FD. The proportion of FD patients who achieved an improvement in their dyspepsia symptoms after 4 weeks of VPZ therapy was 48.8% (21/43). The proportions of f-FD and r-FD patients who achieved an improvement in their dyspepsia symptoms after 4 weeks of VPZ therapy were 58.8% (10/17) and 42.3% (11/26), respectively. The FD scores at baseline and after 4 weeks of VPZ therapy in patients with FD were 7.2±1.6 and 4.3±1.8, respectively, and this difference was statistically significant (P<0.01; Fig. 3).
Figure 3.
Changes from baseline of the FD scores following administration of 20 mg VPZ once daily for 4 weeks in patients with FD. The FD scores at baseline and after 4 weeks of VPZ therapy in FD patients were 7.2±1.6 and 4.3±1.8, respectively, and this difference was statistically significant (*P<0.01). FD, functional dyspepsia; VPZ, vonoprazan; PCAB, potassium-competitive acid blocker.
Discussion
The present retrospective study is, to the best of our knowledge, the first study on efficacy of a PCAB for improving the symptoms of patients with RE, NERD, and FD. In the present study, patients were administered with a new PCAB, termed VPZ, for 4 weeks. Following this treatment, compared with baseline, the rates of symptomatic improvement in patients with RE, NERD, and FD were 75.0, 60.0, and 48.8%, respectively, and the GERD score in patients with RE and NERD, and the FD score in patients with FD, were both decreased. In cases that were administered VPZ as an initial therapy, the rates of symptomatic improvement were 90.9, 66.7, and 58.8% in patients with RE, NERD, and FD, respectively. In cases that were resistant to 8 weeks of PPI therapy, the rates of symptomatic improvement were 55.6, 53.8, and 42.3% in patients with RE, NERD, and FD, respectively. The GERD score in patients with RE and NERD, and the FD score in patients with FD, were both decreased after 4 weeks of VPZ therapy.
PPIs have been used as the first-line drugs for the treatment of GERD in Japan since it became evident from the results of multiple Japanese studies, and those from outside Japan, that PPIs exhibit superior protective effects against the esophageal mucosal injury associated with RE compared with those of histamine-2 receptor antagonists (4,15). However, it has become clear during the use of PPIs in patients with GERD that there are certain cases in which GERD symptoms remain following treatment with PPIs (16). It was reported that the symptoms of GERD are not proportional to the degree of the esophageal mucosal injury (17), and these symptoms reduce the QOL of GERD patients (18). Therefore, in addition to healing the esophageal mucosal injury, it is also important to achieve symptomatic relief for GERD. In a multicenter, randomized trial in Japanese patients with RE, the proportions of patients achieving sufficient relief of their reflux symptoms (heartburn and regurgitation) were similar (~80%) in 2 groups who were treated with 20 mg omeprazole and 10 mg rabeprazole, respectively, for 4 weeks (19). In the present study, patients who were administered VPZ as an initial therapy had a relatively high rate of symptomatic improvement (90.9%) in cases of RE, while the rate of improvement in patients with NERD tended to be lower (66.7%). Previous reports have revealed that not only the regurgitation of gastric acid, but also hyperesthesia and psychological factors participate in generating the subjective symptoms of NERD (20,21). Therefore, in patients with NERD, a strong suppression of gastric acid secretion might be less likely to succeed compared with that in RE patients. On the other hand, in patients with RE and NERD who were resistant to 8 weeks of PPI therapy, the rates of improvement for their GERD symptoms were 55.6 and 53.8%, respectively. PPIs are known to have certain disadvantages, including being affected by genetic polymorphisms of CYP2C19, and being inactivated easily under acidic conditions (22). In comparison with PPIs, PCABs bind to the proton pump continuously in gastric parietal cells to reduce gastric acid secretion without requiring activation or being deactivated by the gastric acid. Furthermore, it was reported that PCABs are not affected by genetic polymorphisms of CYP2C19 (23,24). In patients with GERD whose gastric acid secretion was not suppressed sufficiently well by the administration of a PPI, it may be possible to control the secretion further by administering a PCAB. PCABs may become a novel choice of therapy for patients with GERD who are resistant to PPI therapy.
The etiology of FD is complicated, and FD may be caused by various factors (25). According to the Japanese guidelines for the treatment of FD, the major therapeutic drugs for FD are gastric acid secretion inhibitors and gastroprokinetic drugs (5,26). As for gastric acid secretion inhibitors, in a multicenter, double-blinded, randomized, placebo-controlled trial in Japanese patients with FD, it was reported that treatment with 20 mg rabeprazole once daily for 8 weeks achieved a higher rate of satisfactory symptom relief compared with a placebo treatment (45.3 vs. 28.2%) (27). As for gastroprokinetic drugs, a multicenter, randomized, 4-week placebo-controlled trial in Japanese patients with FD reported that 52.2% of those receiving acotiamide, and 34.8% of those in the placebo group, were classified as responders (28). In the present study, patients with FD who were administered with VPZ for 4 weeks as an initial treatment had a relatively high response rate (58.8%), although the present study is a retrospective study, rather than a placebo-controlled study. In addition, the subgroup analysis revealed that, even for patients with FD who were resistant to 8 weeks of PPI therapy, the rate of symptomatic improvement was 42.3%. Therefore, PCABs may become novel therapeutic drugs for patients with FD who are PPI-therapy-resistant. However, for ~60% of the PPI-therapy-resistant FD patients in the present study, VPZ treatment did not succeed, and the influence of a mechanism other than gastric acid reflux was considered to be an etiological factor in these patients. In addition, the present study did not consider the disease duration of the patients with FD. The rate of symptomatic improvement elicited by the administration of PCAB to patients with FD according to the Rome III criteria (29), which categorize the disease duration of FD, has yet to be elucidated.
The present study had several limitations. First, it was a hospital-based, single-center, retrospective study of outpatients who were asked to complete a questionnaire to determine the severity of their symptoms. Secondly, the study procedures were conducted by one specialist (D.A.) who was a member of the Japan Society of Gastroenterology; therefore, the data might not represent the general population. Furthermore, the sample size of the present study was relatively small; therefore, further larger, randomized multicenter prospective studies will be required to clarify the efficacy of PCAB for improving the symptoms of patients with RE, NERD, and FD. Finally, it was not possible to investigate dietary intake, beverages, waist circumference, visceral fat area, exercise, eating habits, or sleep, which are all able to affect the pathophysiology of RE, NERD, and FD.
This retrospective study is the first report, to the best of our knowledge, that has examined the efficacy of PCAB for improving the symptoms of patients with GERD and FD. In GERD and FD patients, the possibility has materialized that PCAB might be useful as a novel therapeutic drug, not only as an initial therapy, but also for patients who are not satisfied with their treatment after 8 weeks of therapy with conventional PPIs. Symptomatic relief by PCABs might also increase the QOL of patients with GERD and FD. However, additional, large and prospective multicenter trials are required to further clarify the efficacy of PCABs for improving the symptoms of patients with GERD and FD.
References
- 1.Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol. 2009;44:518–534. doi: 10.1007/s00535-009-0047-5. [DOI] [PubMed] [Google Scholar]
- 2.Dimenas E. Methodological aspects of evaluation of quality of life in upper gastrointestinal diseases. Scand J Gastroenterol Suppl. 1993;199:18. doi: 10.3109/00365529309098350. [DOI] [PubMed] [Google Scholar]
- 3.Meineche-Schmidt V, Talley NJ, Pap A, Kordecki H, Schmid V, Ohlsson L, Wahlqvist P, Wiklund I, Bolling-Sternevald E. Impact of functional dyspepsia on quality of life and health care consumption after cessation of antisecretory treatment. A multicentre 3-month follow-up study. Scand J Gastroenterol. 1999;34:566–574. doi: 10.1080/003655299750026010. [DOI] [PubMed] [Google Scholar]
- 4.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: A meta-analysis. Gastroenterology. 1997;112:1798–1810. doi: 10.1053/gast.1997.v112.pm9178669. [DOI] [PubMed] [Google Scholar]
- 5.Talley NJ, Meineche-Schmidt V, Paré P, Duckworth M, Räisänen P, Pap A, Kordecki H, Schmid V. Efficacy of omeprazole in functional dyspepsia: Double-blind, randomized, placebo-controlled trials (the Bond and Opera studies) Aliment Pharmacol Ther. 1998;12:1055–1065. doi: 10.1046/j.1365-2036.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 6.Chey WD, Mody RR, Izat E. Patient and physician satisfaction with proton pump inhibitors (PPIs): Are there opportunities for improvement? Dig Dis Sci. 2010;55:3415–3422. doi: 10.1007/s10620-010-1209-2. [DOI] [PubMed] [Google Scholar]
- 7.Hori Y, Imanishi A, Matsukawa J, et al. 1- [5- (2- Fluorophenyl)- 1- (pyridin- 3- ylsulfonyl)- 1H- pyrrol- 3- yl]- N- methylmethanamine monofumarate (TAK- 438), a novel and potent potassium- competitive acid blocker for the treatment of acid- related diseases. J Pharmacol Exp Ther. 2010;335:231–238. doi: 10.1124/jpet.110.170274. [DOI] [PubMed] [Google Scholar]
- 8.Ashida K, Sakurai Y, Nishimura A, Kudou K, Hiramatsu N, Umegaki E, Iwakiri K, Chiba T. Randomised clinical trial: A dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther. 2015;42:685–695. doi: 10.1111/apt.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Zanten SJ Veldhuyzen, Chiba N, Armstrong D, Barkun AN, Thomson AB, Mann V, Escobedo S, Chakraborty B, Nevin K. Validation of a 7-point global overall symptom scale to measure the severity of dyspepsia symptoms in clinical trials. Aliment Pharmacol Ther. 2006;23:521–529. doi: 10.1111/j.1365-2036.2006.02774.x. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai K, Nagahara A, Inoue K, Akiyama J, Mabe K, Suzuki J, Habu Y, Araki A, Suzuki T, Satoh K, et al. Efficacy of omeprazole, famotidine, mosapride and teprenone in patients with upper gastrointestinal symptoms: an omeprazole-controlled randomized study (J-FOCUS) BMC Gastroenterol. 2012;12:42. doi: 10.1186/1471-230X-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Zanten SV, Armstrong D, Chiba N, Flook N, White RJ, Chakraborty B, Gasco A. Esomeprazole 40 mg once a day in patients with functional dyspepsia: The randomized, placebo-controlled ‘ENTER’ trial. Am J Gastroenterol. 2006;101:2096–2106. doi: 10.1111/j.1572-0241.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 12.Savarino V, Vigneri S, Celle G. The 13C urea breath test in the diagnosis of Helicobacter pylori infection. Gut. 1999;45:I18–I22. doi: 10.1136/gut.45.2008.i18. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87–97. doi: 10.1055/s-0028-1098086. [DOI] [Google Scholar]
- 14.Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ, et al. The endoscopic assessment of esophagitis: A progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 15.Soga T, Matsuura M, Kodama Y, Fujita T, Sekimoto I, Nishimura K, Yoshida S, Kutsumi H, Fujimoto S. Is a proton pump inhibitor necessary for the treatment of lower-grade reflux esophagitis? J Gastroenterol. 1999;34:435–440. doi: 10.1007/s005350050292. [DOI] [PubMed] [Google Scholar]
- 16.El-Serag H, Becher A, Jones R. Systematic review: Persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32:720–737. doi: 10.1111/j.1365-2036.2010.04406.x. [DOI] [PubMed] [Google Scholar]
- 17.Fennerty MB, Johnson DA. Heartburn severity does not predict disease severity in patients with erosive esophagitis. MedGenMed. 2006;8:6. [PMC free article] [PubMed] [Google Scholar]
- 18.Pace F, Negrini C, Wiklund I, Rossi C, Savarino V. ITALIAN ONE INVESTIGATORS STUDY GROUP: Quality of life in acute and maintenance treatment of non-erosive and mild erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2005;22:349–356. doi: 10.1111/j.1365-2036.2005.02558.x. [DOI] [PubMed] [Google Scholar]
- 19.Nagahara A, Suzuki T, Nagata N, Sugai N, Takeuchi Y, Sakurai K, Miyamoto M, Inoue K, Akiyama J, Mabe K, et al. A multicentre randomised trial to compare the efficacy of omeprazole versus rabeprazole in early symptom relief in patients with reflux esophagitis. J Gastroenterol. 2014;49:1536–1547. doi: 10.1007/s00535-013-0925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fass R. Erosive esophagitis and nonerosive reflux disease (NERD): Comparison of epidemiologic, physiologic, and therapeutic characteristics. J Clin Gastroenterol. 2007;41:131–137. doi: 10.1097/01.mcg.0000225631.07039.6d. [DOI] [PubMed] [Google Scholar]
- 21.Nagahara A, Miwa H, Minoo T, Hojo M, Kawabe M, Osada T, Kurosawa A, Asaoka D, Terai T, Ohkusa T, et al. Increased esophageal sensitivity to acid and saline in patients with nonerosive gastro-esophageal reflux disease. J Clin Gastroenterol. 2006;40:891–895. doi: 10.1097/01.mcg.0000225673.76475.9d. [DOI] [PubMed] [Google Scholar]
- 22.Adachi K, Katsube T, Kawamura A, Takashima T, Yuki M, Amano K, Ishihara S, Fukuda R, Watanabe M, Kinoshita Y. CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment Pharmacol Ther. 2000;14:1259–1266. doi: 10.1046/j.1365-2036.2000.00840.x. [DOI] [PubMed] [Google Scholar]
- 23.Shin JM, Inatomi N, Munson K, Strugatsky D, Tokhtaeva E, Vagin O, Sachs G. Characterization of a novel potassium-competitive acid blocker of the gastric H, K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438) J Pharmacol Exp Ther. 2011;339:412–420. doi: 10.1124/jpet.111.185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hori Y, Matsukawa J, Takeuchi T, Nishida H, Kajino M, Inatomi N. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther. 2011;337:797–804. doi: 10.1124/jpet.111.179556. [DOI] [PubMed] [Google Scholar]
- 25.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/S0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 26.Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: Results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut. 2008;57:740–746. doi: 10.1136/gut.2007.132449. [DOI] [PubMed] [Google Scholar]
- 27.Iwakiri R, Tominaga K, Furuta K, Inamori M, Furuta T, Masuyama H, Kanke K, Nagahara A, Haruma K, Kinoshita Y, et al. Randomised clinical trial: Rabeprazole improves symptoms in patients with functional dyspepsia in Japan. Aliment Pharmacol Ther. 2013;38:729–740. doi: 10.1111/apt.12444. [DOI] [PubMed] [Google Scholar]
- 28.Matsueda K, Hongo M, Tack J, Saito Y, Kato H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut. 2012;61:821–828. doi: 10.1136/gutjnl-2011-301454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]