Abstract
To elucidate whether the pharmacokinetics (PK) and pharmacodynamics (PD) of sildenafil are influenced differently when it is coadministered with bosentan (S+B) or with ambrisentan (S+A), we evaluated the PK and PD profiles of sildenafil before and after 4–5 weeks of S+A or S+B treatment in patients with pulmonary arterial hypertension. The area under the plasma concentration–time curve of sildenafil was significantly higher in S+A treatment than in S+B treatment (165.8 ng•h/mL vs. 396.8 ng•h/mL, P = 0.018) and the oral clearance of sildenafil was significantly lower after S+A treatment than after S+B treatment (120.6 L/h/kg vs. 50.4 L/h/kg, P = 0.018). In the PD study, incremental shuttle walking distance was superior during treatment with S+A than during treatment with S+B (S+B; 280 m vs. S+A; 340 m, P = 0.042). There were no concerns about safety with either combination therapy regime.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Bosentan decreases the plasma concentration of sildenafil when coadministered in the long term, but ambrisentan has no clinically relevant pharmacokinetic interaction with sildenafil.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ This study addressed whether the pharmacokinetic and pharmacodynamic profiles of sildenafil would differ if it were administered in combination with bosentan or ambrisentan in adults with pulmonary arterial hypertension.
WHAT IS ADDED TO OUR KNOWLEDGE
✓ The plasma concentration of sildenafil when administered in combination with ambrisentan was significantly higher than that when administered in combination with bosentan. The oral clearance of sildenafil in combination with ambrisentan was significantly lower than that when sildenafil was given in combination with bosentan. Patients who received the sildenafil‐ambrisentan combination therapy had superior exercise tolerance compared with those who received sildenafil‐bosentan combination therapy.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS
✓ Our results provide evidence to support transition from bosentan to ambrisentan in patients with pulmonary arterial hypertension also treated with sildenafil.
Pulmonary arterial hypertension (PAH) is a life‐threatening disease. As it progresses, pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR) rise, leading to right ventricular failure and, ultimately, death.1 Pulmonary hypertension is divided into five clinical classifications in the latest European Society of Cardiology and European Respiratory Society (ESC/ERS) guidelines.2 PAH is identified as a clinical group 1, which includes idiopathic and familial PAH, as well as PAH associated with a variety of conditions including connective tissue disease (CTD) and human immunodeficiency virus infection. Idiopathic PAH (IPAH) has a prevalence of 10–15 cases per 1,000,000, an incidence of 2 cases per 1,000,000, and, in the Japanese population, is about twice as common in women as in men.3, 4 PAH associated with connective tissue disease is the most prevalent type. Mixed connective tissue disease, systemic sclerosis, and systemic lupus erythematosus (SLE) represent the primary CTDs associated with PAH in Japan.4 Recent data from the Registry to Evaluate Early and Long‐term Pulmonary Arterial Hypertension Disease Management (REVEAL Registry) indicated that survival of patients with PAH had dramatically improved over the past two decades because of the introduction of new therapeutic strategies, including combination therapy with phosphodiesterase 5 (PDE‐5) inhibitors, endothelin receptor antagonists (ERAs), and prostacyclin.5 Sildenafil (a PDE‐5 inhibitor) is commonly used to treat PAH, improving exercise capacity and reducing PAP and PVR.6 The ERAs, such as bosentan and ambrisentan, are also reported to improve exercise tolerance and World Health Organization (WHO) functional class, and prolong the time to clinical worsening.7, 8
A combination of a PDE‐5 inhibitor and an ERA is recommended for advanced PAH.9 Although sildenafil and bosentan are often administered in combination, it is recognized that bosentan decreases the plasma concentration of sildenafil in the long term.10, 11, 12 It was reported that combination therapy of bosentan with sildenafil did not show a beneficial effect on 6‐minute walking distance (6MWD).13 Furthermore, a recent prospective trial found that this combination did not prolong the time to first morbidity or death compared with sildenafil monotherapy.14 However, addition of sildenafil to bosentan reportedly reduced PAP and increased cardiac index and exercise tolerance15, 16, 17 Indeed, the effect of this combination therapy is still controversial. Thus, decreased sildenafil plasma concentrations, as a result of drug–drug interactions (DDI), is likely to have influenced the results of these clinical trials.
In contrast, ambrisentan has no clinically relevant pharmacokinetic interaction with sildenafil,18 making combination therapy an attractive proposition. It is, however, not known whether the combination of sildenafil and ambrisentan would yield superior therapeutic benefits to those of sildenafil and bosentan. We compared the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of sildenafil when coadministered with bosentan or ambrisentan.
METHODS
Study design and participants
This was a single‐center, open‐label translational trial conducted between April 2011 and March 2012. Eligible participants were adults with PAH in WHO functional class II or III who had received combination therapy with sildenafil 20 mg t.i.d. and an ERA (bosentan 62.5 mg b.i.d. or ambrisentan 10 mg q.d.) for > 1 year. During the study period we did not change the concomitant drugs and no other drugs that might be expected to influence the PK of sildenafil and the PD study were permitted. The exclusion criteria were poor health status, apparent adverse effects of sildenafil, bosentan or ambrisentan, and difficulty taking an uninterrupted course of the study drugs.
All enrolled patients stayed on their assigned treatment regimen, as described, for the first period, and then were crossed over without a washout for the second period. Six of them received sildenafil (20 mg t.i.d.) and bosentan (62.5 mg b.i.d.) combination therapy (S+B) and were then transitioned to sildenafil (20 mg t.i.d.) and ambrisentan (10 mg q.d.) combination therapy (S+A) for at least 4–5 weeks (Figure 1). One patient received S+A and was then transitioned to S+B for at least 4–5 weeks. In all study subjects, the PK of sildenafil was measured at least 4–5 weeks after receiving each combination, and at the same time each patient's exercise tolerance was evaluated by means of the 6‐minute walking test (6MWT), the externally paced 10‐m shuttle walking test (SWT), and cardiopulmonary exercise testing (CPET), including peak oxygen consumption (pO2) and oxygen consumption at anaerobic threshold (AT). By the end of this study all patients had received combination therapy with sildenafil and ambrisentan. The primary endpoint was the difference between the PK of sildenafil in patients also taking bosentan and the PK of sildenafil on patients also taking ambrisentan. The secondary endpoint was the difference between the functional status of patients on the combination therapy of sildenafil with bosentan and those on the combination therapy of sildenafil with ambrisentan.
Figure 1.
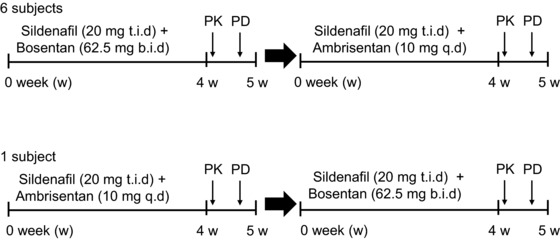
Study design. PK, pharmacokinetics; PD, pharmacodynamics; t.i.d., three times per day; b.i.d., twice per day; q.d., once per day.
Pharmacokinetic assessments
After baseline blood collection, participants were given sildenafil (20 mg) plus either bosentan (62.5 mg) or ambrisentan (10 mg), according to the protocol for each therapy period. Study drugs were administered in the morning to fasted participants and breakfast was served 2 hours later. Six participants transitioned from combination therapy of sildenafil with bosentan to combination therapy of sildenafil with ambrisentan and one from combination therapy of sildenafil with ambrisentan to combination therapy of sildenafil with bosentan. Blood samples for measurement of sildenafil concentrations were collected 0.5, 1, 1.5, 2, 3, 4, 6, and 8 hours after sildenafil administration. On the day of blood sampling, the second dose of sildenafil was administered after the 8‐hour blood sample had been collected, which matched the recommended drug administration regime and was well tolerated by the participants. Participants were confined to the study center until the final blood sample had been collected.
The plasma concentration of sildenafil was determined by liquid chromatography / mass spectrometry. Briefly, diazepam (100 μL of 1 μg/mL) was added to a 500‐μL plasma sample as an internal standard. Cold acetonitrile (500 μL) was added to each sample and tubes mixed by vortexing to enable deproteination. After centrifugation at 3,000 rpm for 10 minutes at 4°C, the supernatant was diluted with water (1.5 mL) and applied to an Oasis hydrophilic‐lipophilic balanced extraction cartridge (Waters, Milford, MA). The cartridge was washed with 5% methanol in water (1.0 mL) and eluted with acetonitrile (1.0 mL). The eluate was evaporated under a stream of nitrogen gas at 40°C. A Micromass ZQ Mass Spectrometer (Waters) was operated in positive ion mode at m/z 475. The limit of quantification was 1 ng/mL and the intra‐assay coefficient of variation was < 6.40%.
The PK parameters for sildenafil were estimated by noncompartmental analyses from the concentration–time profile in plasma. The area under the curve (AUC) from 0 to 8 hours (AUC0‐8) was calculated using the trapezoidal rule for the observed values and subsequent extrapolation to 8 hours. The oral clearance (CL/F) was calculated as dose/AUC8‐∞. The maximum concentrations (Cmax) and the time of maximum concentration (Tmax) were estimated directly from the observed plasma concentration–time data.
Pharmacodynamic assessments
Six‐minute walk test
The 6MWT was conducted in a 30‐m long internal corridor by a single physiotherapist according to current standards.19 Participants were instructed to “walk from one end of the corridor to the other at your own pace, in order to cover as much ground as possible.” Each minute the investigator encouraged the participants with standardized statements. Participants were allowed to stop and rest during the test but were instructed to resume walking as soon as they were able to do so. During the test, cardiac rhythm was continuously assessed with a wireless electrocardiogram (ECG) monitor (EC‐12RS, Labtech, Debrecen, Hungary). On cessation of exercise, we recorded the distance walked, peak heart rate (HR), systolic and diastolic blood pressures (SBP, DBP), and perceived exertion scores using a modified Borg scale.
The externally paced 10‐m shuttle walking test (SWT)
Patients performed the SWT as described by Singh et al.20 The SWT was performed in an enclosed corridor on a 10‐m long course identified by two cones placed 0.5 m from either end to avoid the need for abrupt changes in direction. The speed at which patients walked was dictated by an audio signal played on a compact disc. The start of the test was indicated by a triple beep. Thereafter, a single beep was emitted at regular intervals, at which point the subject's goal was to be at the opposite end of the course; that is, when the patient heard the signal he or she should have been rounding the cone to proceed back down the course to the start. The initial walking speed was set at 0.50 m/s; the speed for the next level was increased each minute by 0.17 m/s. A change in speed to the next level was indicated by a triple beep. The operator sat beside the course and no encouragement was given; the only verbal contact was the advice given each minute to increase the walking speed slowly. The test was stopped when the patient was no longer able to maintain the required speed. Patients were continuously assessed with a wireless ECG monitor. After the test the number of completed shuttles was recorded and the total distance walked calculated. Peak HR, SBP, DBP, modified Borg scale score, and reason(s) for test termination were also recorded.
Cardiopulmonary exercise testing: assessment of peak oxygen consumption and anaerobic threshold
All patients were screened for possible health or safety concerns and a physician was readily available during all tests. Workload was increased on a bicycle ergometer (Aerobike 75XLIII, Combi Wellness, Tokyo, Japan) using a ramp protocol (10–20 W/min) that was individually selected for each patient. Protocols were selected on the basis of health status, current level of activity, and previous exercise test performance. Patients exercised to volitional fatigue or until they demonstrated one of the safety‐related termination criteria set by the exercise laboratory. A test was considered satisfactory when a patient exceeded a respiratory exchange ratio of > 1.05, a rating of 9 or 10 out of 10 on the modified Borg scale, or both. Because a mouthpiece was used during testing, patients were given instructions on how to describe symptoms using hand signals but were encouraged to exercise until volitional fatigue or until they demonstrated one of the safety‐related termination criteria set by the laboratory. Blood pressure was measured manually at 2‐minute intervals throughout the exercise period and during recovery. Peripheral oxygen saturation (SpO2) was measured continuously using a finger sensor (WristOx2, Nonin Medical, Plymouth, MN). A 12‐lead ECG was continuously monitored using a wireless exercise ECG system (EC‐12RS, Labtech) with Mason–Likar ECG electrode placement. During testing, breath‐by‐breath ventilation was analyzed with a metabolic cart (Aero Monitor AE‐310S, Minato Medical Science, Osaka, Japan). The pVO2 was measured as the highest 20‐second average of O2 at peak exercise. AT was calculated using the Veq‐CO2 method, which has been validated in patients with congenital heart disease.21
Statistical analysis
All data are expressed as the means ± SD for parametrically distributed values or the median (interquartile range) for nonparametrically distributed values. The Wilcoxon signed rank test was used to assess differences in the PK parameters of sildenafil and PD parameters between sildenafil‐bosentan and sildenafil‐ambrisentan combination therapies. P < 0.05 was considered statistically significant. All analyses were performed using SPSS statistics software (v. 21.0; IBM, Armonk, NY).
Ethics statement
Our study protocol complied with the Declaration of Helsinki and was approved by the Institutional Research Review Board of Hamamatsu University School of Medicine, Hamamatsu, Japan. All participants gave written informed consent. The study was registered at the UMIN Clinical Trials Registry (UMIN000005464).
RESULTS
Study subjects and baseline clinical characteristics
Nine adults with PAH were enrolled; two were subsequently excluded: one whose condition had deteriorated and one who died. In addition, two subjects were excluded from the PD study (one was ineligible for PD testing and the other withdrew consent for PD testing). Ultimately, seven subjects took part in the PK study and five in the PD study. Of the seven patients with PAH, four had idiopathic PAH and three had PAH associated with other disorders (for two, SLE, and for one, systemic sclerosis). Table 1 summarizes the study participants' baseline demographic and clinical characteristics.
Table 1.
Baseline characteristics of study participants
| All patients | No 1 | No 2 | No 3 | No 4 | No 5 | No 6 | No 7 | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 51.1 ± 12.6 | 47 | 59 | 40 | 68 | 51 | 45 | 38 |
| Sex (Male / Female) | 1/ 6 | Male | Female | Female | Female | Female | Female | Female |
| Height (cm) | 156.7 ± 8.6 | 173.0 | 159.0 | 153.0 | 145.0 | 159.0 | 155.0 | 153.0 |
| Weight (kg) | 48.4 ± 7.7 | 58.7 | 38.6 | 45.7 | 38.5 | 53.6 | 48.6 | 52.8 |
| BMI (kg/m2) | 19.5 ± 2.3 | 19.6 | 15.3 | 19.5 | 18.3 | 21.2 | 20.2 | 22.6 |
| Classification | IPAH | IPAH | APAH | APAH | IPAH | IPAH | APAH | |
| (SLE) | (SSc) | (SLE) |
Data are expressed as number, or mean ± SD. BMI, body mass index; IPAH, idiopathic pulmonary arterial hypertension; APAH, associated pulmonary arterial hypertension; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Sildenafil pharmacokinetics
The sildenafil concentration‐dependent time curves for S+B and S+A treatments are shown in Figure 2. The Cmax and AUC0‐8 were significantly higher in S+A treatment than in S+B treatment (Table 2). The CL/F of sildenafil was significantly lower in those taking S+A compared with S+B treatment (Table 2).
Figure 2.
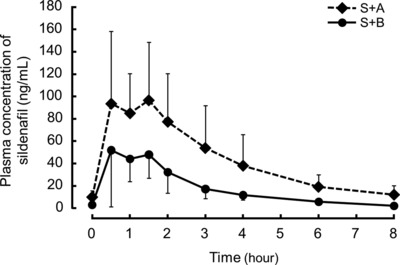
Sildenafil concentration–time curves of the two coadministration therapies. The solid line shows the sildenafil concentration when coadministered with bosentan (S+B), and the dotted line shows the sildenafil concentration when coadministered with ambrisentan (S+A). Values are expressed as mean ± SD.
Table 2.
Difference in pharmacokinetic parameters of sildenafil between the two combination therapies
| S+B | S+A | P value | |
|---|---|---|---|
| tmax (h) | 0.5 (0.50–1.50) | 1.0 (0.5–2.0) | 0.336 |
| Cmax (ng/mL) | 58.3 (56.4–85.4) | 120.2 (95.6–181.1) | 0.018 |
| AUC0‐8 (ng·h /mL) | 165.8 (113.3–190.8) | 396.8 (200.8–517.8) | 0.018 |
| CL/F (L/h/kg) | 120.6 (104.8–176.6) | 50.4 (38.6–¬99.6) | 0.018 |
Data are expressed as the median (interquartile range). S+B, coadministration of sildenafil with bosentan; S+A, coadministration of sildenafil with ambrisentan; tmax, time to maximum plasma concentration; Cmax, maximum plasma concentration; AUC0‐8, area under the plasma concentration‐time curve from 0 to 8 hours; CL/F, oral clearance.
Pharmacodynamics
For the PD study, changes in 6MWD, externally paced 10 m SWD, and CPET were analyzed during transition to an ERA.
The 6MWD values were not significantly different for the two treatments (median 503.0 m [interquartile range 422.5–537.5 m] for S+A treatment compared with 454.0 m [385.5–523.0 m] for S+B treatment, P = 0.144; Figure 3 a). The SWD was greater by a median of 35.0 m during the S+A treatment periods relative to the S+B treatment period (340.0 m [290.0–625.0 m] and 280.0 m [265.0–575.0 m] for S+A and S+B treatments, respectively, P = 0.042; Figure 3 b). The pO2 was higher by a median of 1.7 mL/kg/min during the S+A treatment period relative to the S+B treatment period (17.3 mL/kg/min [15.4–19.6 mL/kg/min] and 14.0 mL/kg/min [13.3–18.6 mL/kg/min] for S+A and S+B treatments, respectively, P = 0.042; Figure 3 c). Oxygen consumption at the AT was also higher by a median of 1.5 mL/kg/min during the S+A treatment period relative to the S+B treatment period (10.8 mL/kg/min [10.0–12.7 mL/kg/min] and 12.6 mL/kg/min [11.4–14.1 mL/kg/min] for S+A and S+B treatments, respectively, P = 0.043; Figure 3 d). These results showed that exercise tolerance was more significant during treatment with S+A than during treatment with S+B, but the WHO functional class remained unchanged in all participants during the transition to an ERA.
Figure 3.
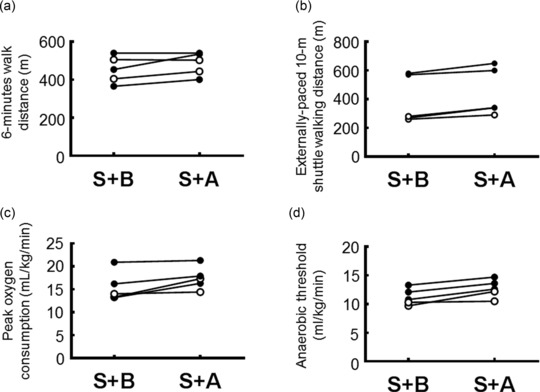
Changes in exercise tolerance between combination therapy with sildenafil plus bosentan (S+B) and sildenafil plus ambrisentan (S+A). (a,b) Changes in 6‐minute walking distance (a) and externally paced 10 m shuttle walking distance (b) for each of the two combination treatments in each study participant. (c,d) Changes in peak oxygen consumption (c) and oxygen consumption at anaerobic threshold (d) assessed by cardiopulmonary exercise testing for each of the two coadministration treatments in each study participant. Open circle: associated‐pulmonary arterial hypertension; closed circle: idiopathic pulmonary arterial hypertension.
Safety analysis
Transition from bosentan to ambrisentan or ambrisentan to bosentan was well tolerated. There were no serious adverse events in either group. All participants were able to complete the transition and take the combination therapies uninterrupted for the required duration.
DISCUSSION
We found that: (i) the Cmax and AUC0‐8 of sildenafil were significantly higher in those taking S+A than in those taking S+B; (ii) the oral clearance (CL/F) of sildenafil in the S+A treatment than in S+B treatment; and (iii) in the PD study SWD, pO2, and AT were greater during treatment with S+A than during treatment with S+B.
It is well recognized that there is a significant DDI between sildenafil and bosentan. Bosentan induces expression of cytochrome P450 3A4 (CYP3A4) in the liver and intestinal wall by activating the pregnane X receptor,22 and this induction of CYP3A4 is considered responsible for the DDI between bosentan and sildenafil. In previous reports, long‐term treatment with bosentan resulted in ∼50–60% reduction in sildenafil AUC.11, 12, 14 In contrast, ambrisentan has the advantage of having no clinically important DDI with sildenafil, because it is only a weak activator of the pregnane X receptor.23 Indeed, the sildenafil AUC was essentially unchanged (by < 2.5%) when administered with ambrisentan, as compared with sildenafil monotherapy.18 It is reasonable, therefore, to predict that the AUC of sildenafil would rise with transition from bosentan to ambrisentan, but, to the best of our knowledge, this had not been tested before. In our study, we show for the first time that combination therapy of sildenafil with ambrisentan was associated with a 2.3‐fold increase in sildenafil AUC0‐8, a 1.7‐fold increase in Cmax, and a 51% decrease in CL/F compared with combination therapy with bosentan. Our PK data support the findings of previous studies.
Although the safety and efficacy of transitioning from bosentan to ambrisentan has not been fully established, there is a small body of evidence suggesting that the safety and efficacy of ambrisentan might be superior to that of bosentan. The clinical effects of transitioning to ERAs in patients with PAH, after prior discontinuation of bosentan or sitaxentan, was reported by McGoon et al.24 In their study, 94.4% of study participants had previously received bosentan therapy and 69.4% were taking a prostanoid and/or sildenafil at baseline. In patients after switching the ERA to ambrisentan, significant improvements in 6MWD and symptoms (measured using WHO functional class and the Borg dyspnea index) were observed. Takatsuki et al. reported that, in children with PAH, the transition from bosentan to ambrisentan reduced the mean PAP and improved WHO functional class.25 Furthermore, they also reported that no patients had aminotransferase abnormalities when switched from bosentan to ambrisentan. In contrast, another study reported that addition of sildenafil to bosentan therapy did not improve 6MWD in patients with Eisenmenger syndrome.26 In our study, we compared efficacy and safety of sildenafil plus ambrisentan with those of sildenafil plus bosentan. Our results strongly suggested that combination therapy with sildenafil and ambrisentan resulted in better exercise tolerance than that with sildenafil and bosentan, as evaluated by SWD and CPET. SWD showed a small but statistically significant difference (median of 35 m, 12.5%) between the two treatment groups, a clinically relevant effect. We did not observe any significant difference in 6MWD between the two treatment regimens. Although the 6MWD is regarded as the gold standard for measuring exercise capacity, we previously reported that SWD showed a greater correlation than 6MWD with both pO2 and AT. Thus, the shuttle walking test (SWT) is a better reflection of exercise tolerance in PAH than is 6MWD.27
Combination therapy is recommended for treatment of PAH.2 Some clinical trials established supporting evidence for the value of combination therapy. Recently, it was reported that initial combination therapy with ambrisentan and tadalafil reduced clinical‐failure events, as compared with ambrisentan or tadalafil monotherapies.28 Porhownik et al. reported that addition of sildenafil to background bosentan treatment led to significantly improved 6MWD.16 However, in the COMPASS‐2 study, treating PAH patients already taking sildenafil with additional bosentan did not reduce first morbidity and mortality events.29 Decreased sildenafil plasma concentrations due to a DDI with bosentan may have contributed to the PD results in this study. Our results confirm those of previous clinical studies.
Our study has some limitations. First, we did not evaluate the changes in PAP and PVR by right heart catheterization (RHC). Invasive hemodynamic assessment by RHC is recommended (class I) to evaluate disease severity and clinical response to therapy. There is a consensus among experts that RHC could be useful for making therapeutic decisions. However, clinical assessment and exercise capacity also provide potentially useful prognostic information in PAH patients.2 Second, while 125 mg b.i.d. is the recommended bosentan dose for maintenance therapy, patients in our study were treated with 62.5 mg b.i.d. In Japan, physicians often prescribe bosentan at 62.5 mg b.i.d. because of concerns about dose‐dependent hepatic toxicity, even though the pharmacokinetics of bosentan and its metabolites are broadly comparable in Japanese and Caucasian individuals.30, 31 Although it has been suggested that there is no bosentan dose‐dependent association with 6MWD,8 our data do not address the possible physiological effects of transitioning to an ERA in patients taking sildenafil with bosentan at the 125 mg dose. Third, it is not clear whether the increase in sildenafil concentration or the therapeutic effects of ambrisentan—or both—contributed to the improvements in exercise tolerance that we observed. Finally, our sample size was small, reducing the power of statistical tests and increasing the risk that a type II error could have occurred.
In conclusion, the plasma concentration of sildenafil was about twice as high in adults with PAH taking sildenafil and ambrisentan (10 mg q.d.) compared with those taking sildenafil and bosentan (62.5 mg b.i.d.). Exercise tolerance was significantly better during treatment with S+A than during treatment with S+B. There were no concerns about safety with either combination therapy regime. The long‐term efficacy and safety of the combination of sildenafil with ambrisentan should be examined in large‐scale prospective randomized studies.
Conflict of Interest
K.O. has received lecture fees from Pfizer and GlaxoSmithKline. S.U. has received research funding from the Ministry of Education, Culture, Sports, Science and Technology. H.W. has received research funding from the Ministry of Health, Labour and Welfare of Japan, Teika Seiyaku, Sawai Seiyaku, Kaken Seiyaku, Nippon Shinyaku, Pfizer, Acterion, and Daiichi Sankyo, and lecture fees from Pfizer, Acterion, Astellas Pharmaceuticals, GlaxoSmithKline, and MSD. These funders had no role in the preparation of the manuscript or the decision to publish it. The remaining authors have no competing interests to declare.
Author Contributions
A.H. wrote the article. S.M., K.T., and H.W. designed the research. S.M. and H.I. performed the research. A.H., K.O., N.I., H.I., S.T., S.U., and H.W. analyzed the data. S.U. contributed new reagents and analytical tools.
Acknowledgments
We would like to thank M. Kageyama for assistance with the sample analysis. We also thank the study participants for their time and dedication to the study.
References
- 1. D'Alonzo, G.E. et al Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 115, 343–349 (1991). [DOI] [PubMed] [Google Scholar]
- 2. Galiè, N. et al 2015. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. [Epub ahead of print] 2015. [DOI] [PubMed] [Google Scholar]
- 3. Satoh, T. Epidemiology, diagnostic criteria, prognosis of pulmonary arterial hypertension with qualification criteria for the obstinate disease in Japan. Nihon Rinsho. 66, 2063–2070 (2008). [PubMed] [Google Scholar]
- 4. Japanese Circulation Society . Guidelines for Treatment of Pulmonary Hypertension (JCS2012). Date last updated: February 1, 2012. Date last accessed: November 18, 2014 (2012).
- 5. Benza, R.L. et al An evaluation of long‐term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 142, 448–456 (2012). [DOI] [PubMed] [Google Scholar]
- 6. Galie, N. et al Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 353, 2148–2157 (2005). [DOI] [PubMed] [Google Scholar]
- 7. Galie, N. et al Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double‐blind, placebo‐controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 117, 3010–3019 (2008). [DOI] [PubMed] [Google Scholar]
- 8. Rubin, L.J. et al Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 346, 896–903 (2002). [DOI] [PubMed] [Google Scholar]
- 9. Galie, N. et al Updated treatment algorithm of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 62, D60–D72 (2013). [DOI] [PubMed] [Google Scholar]
- 10. Paul, G.A. et al Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br. J. Clin. Pharmacol. 60, 107–112 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgess, G. et al Mutual pharmacokinetic interactions between steady‐state bosentan and sildenafil. Eur. J. Clin. Pharmacol. 64, 43–50 (2008). [DOI] [PubMed] [Google Scholar]
- 12. Miyakawa, S. et al Short‐term drug‐drug interaction between sildenafil and bosentan under long‐term use in patients with pulmonary arterial hypertension. Pharmacol. Pharm. 4, 542–548 (2013). [Google Scholar]
- 13. Clincaltrials.gov . Assess the efficacy and safety of sildenafil when added to bosentan in the treatment of pulmonary arterial hypertension. NCT00323297 Date last updated: July 17, 2014. Date last accessed: September 30, 2014.
- 14. McLaughlin, V. et al Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur. Respir. J. 46, 405–413 (2015). [DOI] [PubMed] [Google Scholar]
- 15. Hatano, M. et al Acute effect of sildenafil is maintained in pulmonary arterial hypertension patients chronically treated with bosentan. Int. Heart J. 52, 233–239 (2011). [DOI] [PubMed] [Google Scholar]
- 16. Porhownik, N.R. et al Addition of sildenafil in patients with pulmonary arterial hypertension with inadequate response to bosentan monotherapy. Can. Respir. J. 15, 427–430 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathai, S.C. et al Addition of sildenafil to bosentan monotherapy in pulmonary arterial hypertension. Eur. Respir. J. 29, 469–475 (2007). [DOI] [PubMed] [Google Scholar]
- 18. Spence, R. et al Pharmacokinetics and safety of ambrisentan in combination with sildenafil in healthy volunteers. J. Clin. Pharmacol. 48, 1451–1459 (2008). [DOI] [PubMed] [Google Scholar]
- 19. Guyatt, G.H. et al The 6‐minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can. Med. Assoc. J. 132, 919–923 (1985). [PMC free article] [PubMed] [Google Scholar]
- 20. Singh, S.J. et al Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 47, 1019–1024 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohuchi, H. et al Measurement and validity of the ventilatory threshold in patients with congenital heart disease. Pediatr. Cardiol. 17, 7–14 (1996). [DOI] [PubMed] [Google Scholar]
- 22. Van Giersbergen, P.L. et al Bosentan, a dual endothelin receptor antagonist, activates the pregnane X nuclear receptor. Eur. J. Pharmacol. 450, 115–121 (2002). [DOI] [PubMed] [Google Scholar]
- 23. Weiss, J. et al Influence of sildenafil and tadalafil on the enzyme‐ and transporter‐inducing effects of bosentan and ambrisentan in LS180 cells. Biochem. Pharmacol. 85, 265–273 (2013). [DOI] [PubMed] [Google Scholar]
- 24. McGoon, M.D. et al Ambrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalities. Chest. 135, 122–129 (2009). [DOI] [PubMed] [Google Scholar]
- 25. Takatsuki, S. et al Clinical safety, pharmacokinetics, and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension. Pediatr. Pulmonol. 48, 27–34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iversen, K. et al Combination therapy with bosentan and sildenafil in Eisenmenger syndrome: a randomized, placebo‐controlled, double‐blinded trial. Eur. Heart. J. 31, 1124–1131 (2010). [DOI] [PubMed] [Google Scholar]
- 27. Irisawa, H. et al Incremental shuttle walk test as a valuable assessment of exercise performance in patients with pulmonary arterial hypertension. Circ. J. 78, 215–221 (2014). [DOI] [PubMed] [Google Scholar]
- 28. Galie, N. et al Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N. Engl. J. Med. 373, 834–844 (2015). [DOI] [PubMed] [Google Scholar]
- 29. McLaughlin, V. et al Effect of bosentan and sildenafil combination therapy on morbidity and mortality in pulmonary arterial hypertensino (PAH): results from the COMPASS‐2 study. Chest. 146, 860A (2015). [Google Scholar]
- 30. Roustit, M. et al CYP2C9, SLCO1B1, SLCO1B3, and ABCB11 polymorphisms in patients with bosentan‐induced liver toxicity. Clin. Pharmacol. Ther. 95, 583–585 (2014). [DOI] [PubMed] [Google Scholar]
- 31. van Giersbergen, P.L. et al Comparative investigation of the pharmacokinetics of bosentan in Caucasian and Japanese healthy subjects. J. Clin. Pharmacol. 45, 42–47 (2005). [DOI] [PubMed] [Google Scholar]


