Abstract
Human embryonic stem cells (hESCs) have a role in treating neurological disorders. The efficacy and safety of hESC in treating spinal cord injury (SCI) was reported in our previous study. In the present study, we have evaluated the efficacy and safety of hESC therapy in 226 patients with SCI. In the first treatment phase (T1), 0.25 mL hESCs were administered intramuscularly twice daily, 1 mL every 10 days i.v., and 1–5 mL every 7 days. Of 153 patients in the American Spinal Injury Association (ASIA) scale A at the beginning of T1, a significant number of patients (n = 80; 52.3%) moved to lower scales at the end of T1 (p = 0.01). At the end of T2, of 32 patients in ASIA scale A, 12 patients (37.5%) moved to scale B (p = 0.01). Of 19 patients, 3 patients (37.5%) moved to scale B at the end of T3 (p = 0.02). No serious adverse events (AEs) were observed. hESC transplantation is safe and effective.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ hESC therapy is viewed as a therapy with huge potential benefits but largely remains untapped because of anticipated AEs. Presently, the use of this therapy is in experimental stage and the US Food and Drug Administration has approved clinical trials for hESC therapy in the United States.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ The study used hESC therapy in patients with SCI for the very first time. The hESCs used have been well‐characterized and used in ready‐to‐inject form using patented technology.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE?
✓ The study reports that the hESC therapy can be used for patients with SCI and other incurable conditions.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS?
✓ This therapy can be revolutionizing in the treatment of patients with SCI. The therapy is simple to use, has negligible AEs, is scalable, and reproducible.
Spinal cord injury (SCI) is a challenging neurological injury and is associated with permanent disability and decreased life expectancy.1 The complex series of pathological events involved in SCI result in long‐lasting locomotor and sensory neuron degeneration below the injury.2 A recent review of epidemiology of SCI in developing countries reported the incidence to be 25.5 million cases/year.3 In India, approximately 1.5 million people live with SCI and 10,000 new cases add to this group every year.4 Neurological recovery in patients with traumatic SCI, as evaluated with American Spinal Injury Association (ASIA) scale A, is low (6–13%) and only about 2.1% of the patients have been reported to gain any functional strength.5, 6 There have been several strategies to improve neurological recovery, such as surgical intervention, physiotherapy, and pharmacological interventions, but none of these have proven to be effective.
Replacement of damaged neural tissue (neurons, oligodendrocytes), enhancement of endogenous neural regeneration (by providing neurotrophic factors or by blocking growth‐inhibiting signals), and modulation of the inflammatory response after SCI are the key elements in restoring function after SCI.7 Stem cell transplantation is a promising technology that has the potential to replace damaged neurons, reestablish lost axonal connections, and provide neuroprotective factors to allow for healing and recovery after SCI.
Human embryonic stem cells (hESCs) have a huge potential for differentiation and can provide neuronal or glial cells for transplantation.8, 9, 10 A number of studies conducted in animal models has observed hESC‐derived cells to be able to differentiate into mature oligodendrocytes and neurons in patients with SCI. Lukovic et al.2 suggested that the oligodendrocyte progenitors (OPCs) are able to “rescue” the locomotor activity because of the presence of heterogeneous cell types or multiple character of transplanted progenitors. Erceg et al.11 observed that locomotor function was significantly enhanced in adult rats transplanted with hESC‐derived OPC and/or motor neuron progenitors. Further, these progenitor cells migrated and differentiated into mature cells showing in vivo electrophysiological activity.11 Kakinohana et al.12 reported that hESC‐derived neural precursors when transplanted to ischemia‐injured lumbar spinal cord in rats or in naive immunosuppressed minipigs induced proliferation of embryoid bodies. These animals survived for 2 weeks to 4.5 months after hESC transplantation and the presence of grafted cells was confirmed after staining spinal cord sections with a combination of human‐specific (hNUMA, HO14, hNSE, and hSYN) or nonspecific (DCX, MAP2, CHAT, GFAP, and APC) antibodies.
Sharp et al.13 used hESC‐derived OPCs in the adult cervical contusion rat models. The authors in the 9‐week study period observed that the transplanted hESC‐derived OPCs survived, localized to the injury site, and were able to differentiate retaining their phenotype. The forelimb locomotion was significantly improved after transplantation. Cui et al.14 transplanted embryonic stem cells as substrate adherent embryonic stem cell‐derived neural aggregates and observed an increased neuronal differentiation and neurite outgrowth and decreased astrocytic differentiation.
Cloutier et al.15 studied the survival and migration of OPCs derived from hESC in contusive SCI and locomotor outcome of transplantation in the rat model. They observed that the OPCs survived and migrated to the injury site when analyzed 2 months after implantation. Further, they did not observe histopathological changes or a decline in locomotor function. They suggested the use of OPCs derived from hESCs as a therapeutic strategy for human SCI.15 Perrotta et al.16 transplanted human motor neuron progenitor cells derived from hESCs in rats with SCI. They observed that the transplanted animals had an improved functional outcome with an early recovery rate of balance and coordination and skilled forelimb movement.16 A number of other studies have also demonstrated the “rescue” and “replace” capabilities of hESC in animal models.17, 18, 19, 20, 21
We recently reported a remarkable clinical improvement in 11 patients with SCI treated with hESC therapy in a prospective study.22 We have also reported the safety and efficacy of hESC therapy in patients with cerebral palsy, cortico‐visual impairment, Friedrich ataxia, Lyme disease, spinocerebellar ataxia, and Duchenne muscular dystrophy.23, 24, 25, 26, 27, 28 In this paper, we present the retrospective evaluation of our patients with SCI treated with hESC therapy.
METHODS
Cell culture and differentiation
An in‐house patented technology in a Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and Good Tissue Practices (GTP) compliant laboratory at Nutech Mediworld (Patent‐WO 2007/141657A PCT/1B 2007 published 13 December 2007) was used for culture and maintenance of hESCs. The cell lines were free of animal products and chromosomally stable. The process of cell culture and characterization was elaborated previously.23 The safety and efficacy of our cell line has been established and reported elsewhere.29
Study population
Inclusion and exclusion criteria
Patients with a documented diagnosis of SCI elsewhere of >3 months before the start of therapy were included in this study. All these patients had undergone other treatment(s) (such as physiotherapy, occupational therapy, etc.) before coming to our center.
The patients with acute SCI were excluded to rule out results of the natural ability of the body to repair. Patients who were pregnant, lactating, or confirmed to have received other forms of cell therapies within the last 12 months of treatment were not accepted. All the patients provided written and video informed consent before the start of the treatment.
Study design
The data of a single cohort of patients with SCI treated with hESCs conducted during 24 May 2005 to 31 August 2012 at a single site in New Delhi, India, were collected retrospectively. An independent ethics committee approved the study protocol. The institutional committee for stem cell research and therapy of Nutech Mediworld reported all the work with respect to embryonic stem cells to the National Apex Body and the Indian Council of Medical Research.
In the initial 2 years (2002–2004), the safety of hESC therapy was assessed in 33 patients (not included in this analysis) with various incurable diseases.29 Thereafter, efficacy of the therapy, dose schedule, and protocol for administration of hESCs and therapy schedule were established in a pilot study conducted on 72 patients. Thereafter, a study (validated by GVK Biosciences) was done on 108 patients with SCI that verified the safety and efficacy of hESC in patients with SCI (not included in the present analysis). The present study with 226 patients with SCI was undertaken after these two studies. The same protocol was followed in the group of patients analyzed in this study.
The study was performed under proper supervision of a team of physicians that included external consultants and validated by an external clinical research organization. The patients were scored as per ASIA scale30 by independent physicians before and after the treatment and by the in‐house physicians and the rehabilitation team.
After confirmation of diagnosis, the patients were tested for hypersensitivity reactions with hESCs (0.05 mL hESC injected subcutaneously). The study consisted of three treatment phases with gap phases in between. The treatment phases were separated by gap periods so that the hESCs could grow, repair, and regenerate the affected part.
After the hypersensitivity testing, the patients entered the first treatment phase (T1, 8 weeks for paraplegics and 12 weeks for quadriplegics); wherein 0.25 mL (<4 million cells) hESCs were administered through i.m. route twice daily to “prime” the body and allow for the recipient immune system not to reject the stem cells. Additionally, 1 mL hESCs (<16 million cells) were administered every 10 days through i.v. route to “home in” to the required area and for systemic reach. To introduce the stem cells as close to the injured site as possible (local action); 1–5 mL hESCs (depending on the route of administration) were administered every 5–7 days by any of the supplemental routes (Figure 1). The duration of treatment and gap phases varied in quadriplegic patients and paraplegic patients as quadriplegic are generally more difficult to treat.31 After a gap period of 4–8 months, the patients entered the subsequent treatment phases (second‐T2 and third‐T3) in which they were administered the same dosage regime as T1. Each treatment phase lasted 4–6 weeks and was 4–8 months apart. In T2 and T3, an additional dose of hESCs was administered through any of the supplemental routes.
Figure 1.
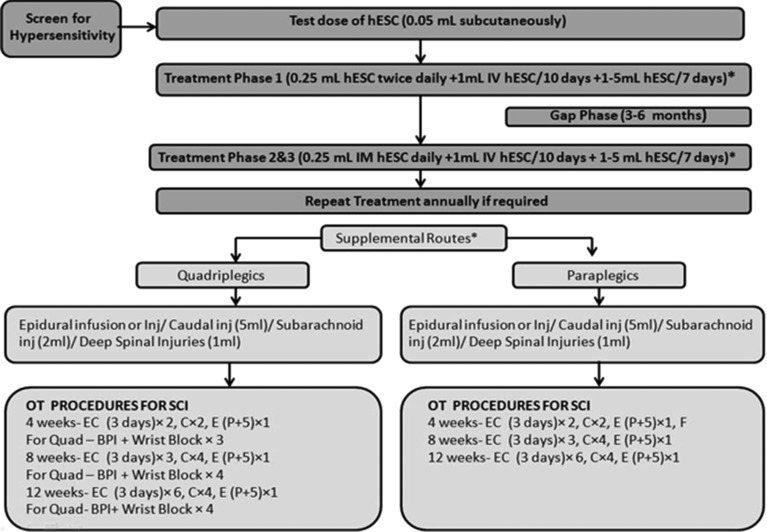
Study design. hESC, human embryonic stem cells.
No immunosuppressants were given to the patients. All the patients received physiotherapy and/or occupational therapy in addition to hESC therapy. The rehabilitation focused on overall improvement of the patient and mobilization of the patient was performed using different ambulatory aids depending on the requirement (e.g., a patient with paraplegia was made to stand with full support on a hip‐knee‐ankle‐foot orthosis (HKAFO) and as connectivity was regained, the support was reduced to knee‐ankle‐foot orthosis (KAFO), then knee brace and ankle support, and then just ankle support. The walking aids also reduced from manual support and walker to just walker, to crutches, then to walking stick, and finally to no aid.
The patients had to undergo a detailed examination by the physicians and the rehabilitation team before, during, and after each treatment cycle. All the patients had their condition videographed before, during, and after the treatment periods and had their radiological examination (magnetic resonance imaging [MRI] and lately tractography) done elsewhere and biochemical investigations done before the start of the treatment and then at regular intervals. They were referred back to their own neurologists and were reviewed by an external neurologist. A separate team of physicians (not involved with patient care) examined the observations documented by the various teams and further graded the patients.
Assessment
Each patient was assessed at admission to determine the pretherapy status of the patient. The percentage of patients with changes or no changes were calculated after each session of the therapy and reported. Statistical tests or tests of significance were performed.
Data validation
The data for all the patients were validated by Moody's International (document number NH‐hESC‐10‐1), GVK Biosciences (NM‐Hesc‐10‐1, 18 November 2010), and Quality of Austria Central Asia Pvt. Ltd. Accreditation Company (document number QACA/OCT/2013/26). These companies examined the medical and statistical data present at the institute and met the patients.
Statistical analysis
Descriptive statistics were performed to summarize data. SPSS software version 19.0 (IBM Corporation, Armonk, NY) was used for the data analysis. Chi‐squared test was used to compare AIS score at baseline and at the end of the therapy. A p value of < 0.05 was considered significant.
RESULTS
Patients
A total of 226 patients (paraplegic = 136, quadriplegic = 90) with SCI were included in the study. Overall, 203 patients had SCI because of trauma and 23 because of other miscellaneous causes, like transverse myelitis (n = 4), Potts spine (n = 7), tumors (n = 3), and contusion (n = 9). The majority of these patients were men (167; 73.9%) and the mean age was 28 years (range, 20–34 years). Among paraplegic patients, 124 had complete injury, whereas among the quadriplegic patients, 71 had complete injury. The average days of treatment in T1 was 73 days for quadriplegic patients and 62 days for paraplegic patients, and the average gap period was 122 days for quadriplegic patients and 136 days for paraplegic patients.
All the patients started intensive dosing and 50 patients were present in all the study periods. Overall, the patients who discontinued the study because of various reasons cited as personal status and financial reasons (39%), satisfaction with their progress and cure (32%), not satisfied with their progress (5%), and returned for treatment after a long gap (24%) (i.e., after 31 August 2012, these patients were not part of this analysis).
Efficacy evaluation
Change in ASIA impairment scale from admission to discharge
Change in ASIA impairment scale from admission to discharge at the end of each treatment period is presented in Table 1. Of 153 patients in ASIA scale A at the beginning of T1, a significantly higher number of patients (n = 80; 52.3%) moved to lower scales at the end of T1 (p = 0.02). At the beginning of T2, 32 patients were in ASIA scale A, of these, 20 patients remained in scale A and 12 (37.5%) moved to lower scales by the end of T2 (p = 0.01). Of 19 patients at the start of T3, 8 patients were in ASIA scale A. At the end of T3, 3 of these patients (37.5%) moved to scale B (p = 0.02). The improvement in scales at the end of T1 and T2 is shown in Figure 2.
Table 1.
Change in American Spinal Injury Association scales of patients (overall) from admission to discharge at the end of each treatment period
| End of the treatment | ||||||
|---|---|---|---|---|---|---|
| ASIA scale | ||||||
| Baseline characteristics | A; no. (%) | B; no. (%) | C; no. (%) | D; no. (%) | E; no. (%) | p value |
| T1 (n = 226) | 0.02 | |||||
| A (n = 153) | 73 (47.7) | 23 (15) | 57 (37.3) | – | – | |
| B (n = 32) | – | 18 (56.3) | 13 (40.6) | 1 (3.1) | – | |
| C (n = 36) | – | – | 31 (86.1) | 5 (13.9) | – | |
| D (n = 5) | – | – | – | 2 (40.0) | 3 (60.0) | |
| T2 (n = 58) | 0.01 | |||||
| A (n = 32) | 20 (62.5) | 12 (37.5) | – | – | – | |
| B (n = 9) | – | 7 (77.8) | 2 (22.2) | – | – | |
| C (n = 17) | – | – | 17 (100) | – | – | |
| T3 (n = 19) | 0.02 | |||||
| A (n = 8) | 5 (62.5) | 3 (37.5) | – | – | – | |
| B (n = 4) | – | 4 (100) | – | – | – | |
| C (n = 7) | – | – | 6 (85.7) | 1 (14.3) | – | |
ASIA, American Spinal Injury Association.
Figure 2.
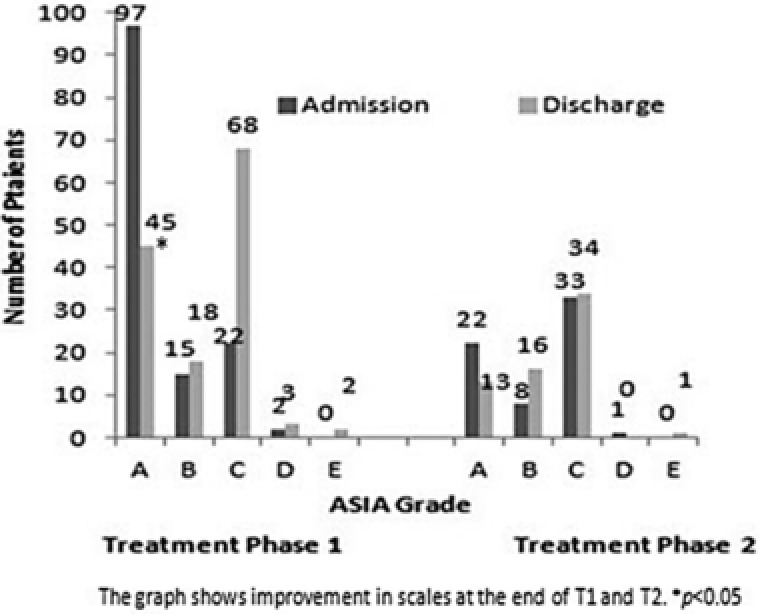
Overall change in American Spinal Injury Association (ASIA) scale at the end of treatment phase 1 and 2.
At the end of T1, 45% of the patients improved by at least one ASIA grade. At the end of T2, 58% of the patients improved by at least one ASIA grade, and at the end of T3, 70% of the patients improved by at least one ASIA grade (Table 2).
Table 2.
Change from baseline to last period in total American Spinal Injury Association scores by extent and level of injury
| ASIA grades | ||
|---|---|---|
| Study period | Results | No. of patients (%) |
| End of T1 (n = 226) | Improved by 1 ASIA scale | 102 (45) |
| Stationary | 124 (55) | |
| Not improved | – | |
| End of T2 (n = 58) | Improved by 1 ASIA scale | 62 (58) |
| Stationary | 44 (42) | |
| Not improved | – | |
| End of T3 (n = 19) | Improved by 1 ASIA scale | 35 (70) |
| Stationary | 15 (30) | |
| Not improved | – |
ASIA, American Spinal Injury Association.
We could do MRI scans for 65 of our patients and tractography for 25 patients before and after the therapy. The improvements were observed in the magnetic resonance tractography images of these patients taken before and after the therapy (Figure 3 a, b).
Figure 3.
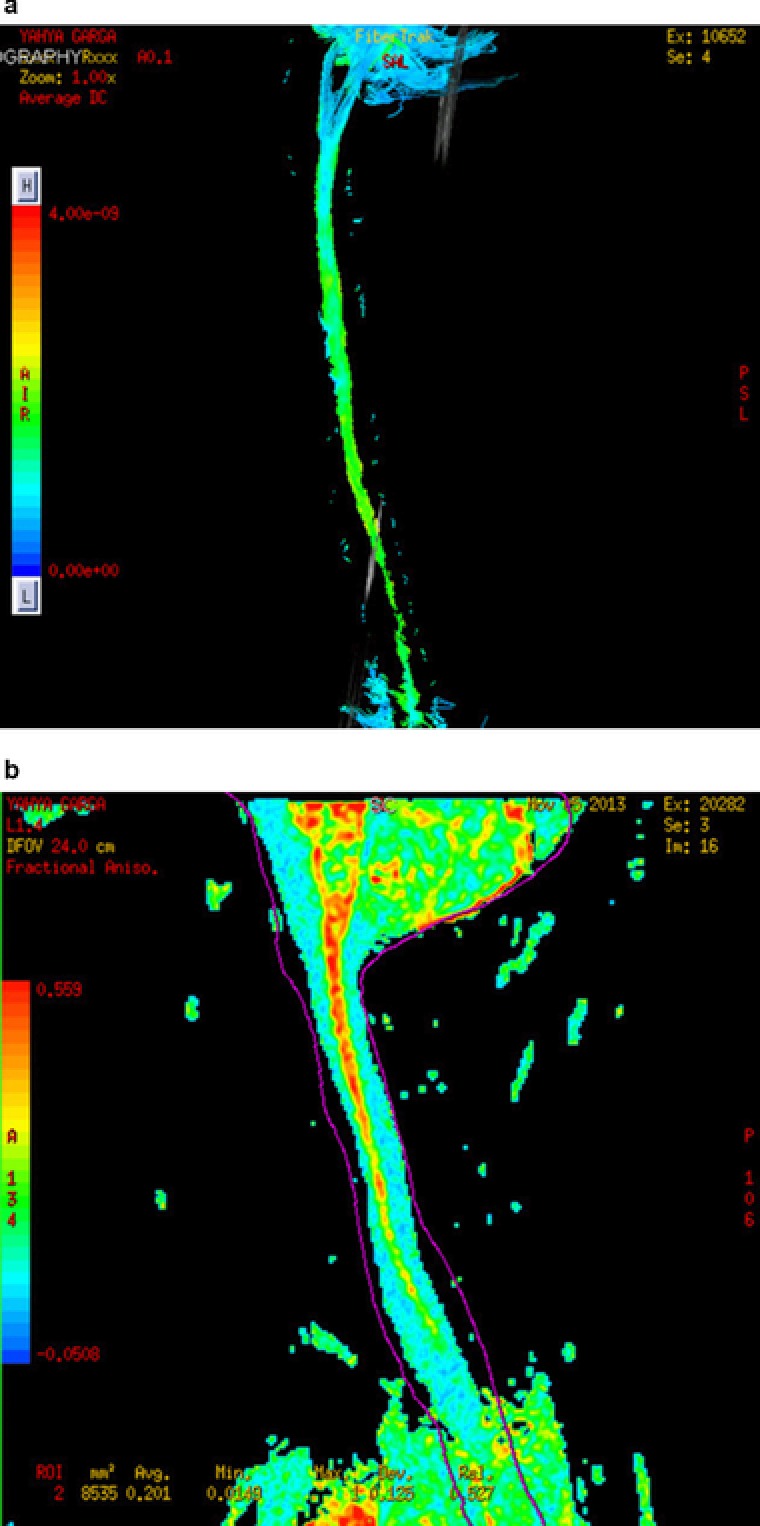
(a) Magnetic resonance (MR) tractography of a patient before receiving human embryonic stem cell (hESC) therapy. (b) MR tractography of a patient after receiving hESC therapy.
Paraplegics and quadriplegics
Among 136 patients with paraplegia, 97 patients were in ASIA scale A at the beginning of T1. Of these, a significant number of patients (52; 53.6%) moved to lower scales by the end of T1 (p < 0.05). Of 64 patients with paraplegia at the beginning of T2, 22 patients were in ASIA scale A. At the end of T2, 9 patients (40.9%) moved to scale B. At the end of T3, of 5 patients in scale A, 2 (40%) patients moved to scale B.
Among quadriplegic patients, 28 patients (50%) showed an improvement in scales and shifted from scale A to lower scales at the end of T1 (p > 0.05). Of 10 patients in scale A at the beginning of T2, 3 patients (30%) improved and moved to lower scales. Of 3 patients in ASIA scale A before T3, 1 patient (33.3%) moved to scale B at the end of T3.
Gender wise analyses
Of 59 women in the study, 32 were in ASIA scale A at the beginning of the study. At the end of T1, 13 were in ASIA scale A and the rest moved to lower scales. Among 167 men, 121 were in ASIA scale A at baseline. At the end of T1, almost half (n = 60) of the patients remained in ASIA scale A and another half moved to lower scales. The improvement in scales at the end of T1, T2, and T3 is shown in Table 3. Gender was not a significant factor in the state of efficacy of the SCI cases.
Table 3.
Change in American Spinal Injury Association scales of patients (gender wise) from admission to discharge at the end of each treatment period
| No. of patients | |||||||
|---|---|---|---|---|---|---|---|
| At the end of treatment phase | |||||||
| Gender | Treatment phase | Admission | A; no. (%) | B; no. (%) | C; no. (%) | D; no. (%) | E; no. (%) |
| Women | T1 (n = 59) | A (n = 32) | 13 (41) | 7 (22) | 12 (38) | – | – |
| B (n = 10) | – | 7 (70) | 2 (20) | 1 (10) | – | ||
| C (n = 15) | – | – | 14 (93) | 1 (7) | – | ||
| D (n = 2) | – | – | – | 1 (50) | 1 (50) | ||
| T2 (n = 23) | A (n = 4) | 2 (50) | 2 (50) | – | – | – | |
| B (n = 6) | – | 4 (67) | 2 (33) | – | – | ||
| C (n = 12) | – | – | 12 (100) | – | – | ||
| D (n = 1) | – | – | – | – | 1 (100) | ||
| T3 (n = 11) | A (n = 1) | 1 (100) | – | – | – | – | |
| B (n = 4) | – | 3 (75) | 1 (25) | – | – | ||
| C (n = 6) | – | – | 5 (83) | 1 (17) | – | ||
| Men | T1 (n = 167) | A (n = 121) | 60 (50) | 16 (13) | 45 (37) | – | – |
| B (n = 22) | – | 11 (50) | 11 (50) | – | – | ||
| C (n = 21) | – | – | 17 (81) | 4 (19) | – | ||
| D (n = 3) | – | – | – | 1 (33) | 2 (67) | ||
| T2 (n = 83) | A (n = 28) | 18 (64) | 10 (36) | – | – | – | |
| B (n = 16) | – | 16 (100) | – | – | – | ||
| C (n = 39) | – | – | 39 (100) | – | – | ||
| T3 (n = 39) | A (n = 7) | 4 (57) | 3 (43) | – | – | – | |
| B (n = 12) | – | 12 (100) | – | – | – | ||
| C (n = 20) | – | – | 20 (100) | – | – | ||
Safety evaluation
There was no death or serious adverse events (AEs) observed during the entire study period. No teratoma formation was observed in the patients during or after the study. The adverse events observed during each treatment period are tabulated in Table 4. Mild fever was the most frequent AE observed during the study that resolved without sequel.
Table 4.
Adverse events observed during each treatment period (safety population)
| AE parameter; no. | T1 (n = 226) | T2 (n = 106) | T3 (n = 50) |
|---|---|---|---|
| Total AEs; no. (%) | 57 (25.2) | 9 (8.5) | 6 (12) |
| Fever | 23 | 3 | 1 |
| Headache | 15 | 5 | 3 |
| Loose motions | 3 | – | – |
| Abdominal pain | 2 | – | 1 |
| Constipation | 2 | – | – |
| Itching | 2 | – | – |
| Pain | 2 | – | – |
| Fever and headache | 1 | – | – |
| Fever, anorexia, and hematuria | 1 | – | – |
| Headache and vomiting | 1 | – | 1 |
| Headache and vertigo | 1 | – | – |
| Weight loss | 1 | – | – |
| Nausea | 1 | 1 | – |
| Redness and itching | 1 | – | – |
| Acidity | 1 | – | – |
AE, adverse event.
DISCUSSION
During the past 2 decades, cell‐based therapies for SCI have been researched in several studies. Replacement of damaged neural tissues and reestablishing connections between the central and peripheral nervous system is vital for the treatment strategy for patients with SCI. Thus, the cells having a potential of self‐renewal and differentiating into multiple cell types would be best suited for patients with SCI. hESCs are able to replicate indefinitely, differentiate into all three primary germ layers cell lines, and are karyotypically stable.8, 9, 32
A few studies have reported use of stem cell therapy in human patients with SCI. Park et al.33 performed autologous bone marrow cell transplantation at the injury site in conjunction with the administration of granulocyte macrophage‐colony stimulating factor in five patients with complete SCI and followed up for 6–18 months. Overall, three patients improved from ASIA scale A to C, one improved from ASIA scale A to B, and one did not show any notable improvement. None of the patients showed any serious complications.33 Lima et al.34 transplanted olfactory mucosa autografts in seven patients (range, 18–32 years) with ASIA scale A SCI. They observed an improvement in ASIA scale in every patient and two of the patients moved to ASIA scale C at the end of the treatment. In another study, a 37‐year‐old female patient with SCI was transplanted with HLA‐matched human cord blood cells at the site of injury. The investigators observed an improved sensory perception and movement in the hips and thighs of the patient 41 days after the transplantation. Regeneration of the spinal cord at the injured site was observed in computed tomography and MRI scan.35
Although such smaller studies have been conducted in the past, no clinical trials have been conducted so far. The first and only phase I human clinical trial using hESC for patients with SCI, popularly referred to as the Geron trial was conducted in 2009 but was discontinued because of financial constraints.36 OPCs derived from Geron's protocol, GRNOPC1 were efficient in rat models of thoracic and cervical SCI. These cells contained animal components, such as B27 supplement or matrigel.36 Recently, the US Food and Drug Administration gave approval for a clinical trial to Asterias Biotechnology of Menlo Park for the use of hESC in SCI. This company had bought the rights of Geron to conduct trial with hESC in humans.37 Lukovic et al.36 recommended that the hESCs transplantation protocols should encourage the use of human material as animal components carry a risk of xenogeneic pathogen cross transfer.
The present study is the first of its kind to demonstrate adequate efficacy of hESC in patients with SCI with a good tolerability profile. Our patients gained voluntary movement of the areas below the level of injury. We also observed an improvement in bladder sensation and control; bowel sensation and control as well as the gait and hand grip.38 The MRI scan and tractography images taken before and after the therapy of our patients also confirmed the improvements observed. We did not observe any difference in the response to therapy between men and women with SCI.
The hESCs used in our study have been derived and characterized using an in‐house patented technology. The hESCs are generated in a culture from a one‐time harvest made at the preblastocyst stage. Thus, the cell line developed is created from a single fertilized ovum 24–48 h after fertilization when the conceptus is assumed to have reached the 4–16 cell stage.39 All the media used in the culture are free from animal contaminants and cells of animal origin. The compositions of the present therapy are simple to prepare and cost‐effective. The ready to inject form is easily transportable, scalable, and has a good shelf life (about 6 months in a temperature‐controlled environment). The evidence for the use of hESCs at our facility has been gathered over a number of years and was accepted as written evidence to House of Lords, Regenerative Medicine, Science, and Technology Committee report.40
We used a validated treatment protocol for our patients that included treatment phases (T1, T2, and T3) separated by gap periods in between. The protocol was based on our prior experience with hESCs, as we observed that treatment period of >8 weeks for paraplegic patients and >12 weeks for quadriplegic patients did not yield any better results. During embryonic development in humans, all organs develop within 14–16 weeks of gestation.41 We included a gap phase of 4–6 months between out‐treatment periods to allow hESCs to grow, repair, and regenerate the affected area, keeping in view the time taken by embryonic cells in utero to develop to organs. Previous studies suggest that various factors, like chemokines, cytokines, and other growth factors, are released from the site of injury that act as attractants to the stem cells. The stem cells then migrate to the damage site because of upregulation of selectins and integrins on their surface. This homing action has also previously been described for mesenchymal stem cells and in experimental models of acute liver failure.42, 43, 44, 45 Thus, stem cells do not persist at the injection site but make their way to the injured site and help in recovery/regeneration of the injured tissue or help in “rescue” and “replacement” of injured cells. In our patients with SCI, the hESCs could have followed the same route from the site of injection to the site of action and helped in regeneration of the injured tissue. However, reestablishing the lost axonal connections after recovery takes more time and the new tissues have to gain their functionality. In the majority of the cases, the process is rarely complete (as the patients have a chronic condition) and it might take more than 2 years for a person with SCI to start walking with support after hESC therapy. In a recent 18 months study conducted on four dogs with SCI, authors transplanted mesenchymal stem cells obtained from the bone marrow of the dogs were injected at the site of injury. The authors could observe a continuous clinical recovery in these animals even up to 18 months after administration of mesenchymal stem cells. This indicated that the clinical improvements observed in their study could be due to the interaction between engrafted mesenchymal stem cells and endogenous spinal cord derived factors.46 The improvement in our patients was reflected in the MRI and tractography reports that showed regeneration of the lost axonal connections. Fear of teratomas and immune rejection hinder the use of hESC therapy. However, none of the patients in our study had teratoma or an immune response. We did not give steroids or immunosuppressants to our patients. The AEs observed in the present study were mild and resolved without any sequel. Headache and fever were the common AEs observed during the study. It has been reported previously that inadvertent dural puncture can lead to the post dural puncture headache.47 We started using hESCs for treatment first in the year 2002 and our first patient received only four doses of hESCs in that year. No AEs was observed in this patient until 2004 and the patient benefitted remarkably.29 The therapy was made available for other patients with SCI and other terminal and life‐threatening conditions only after adequate safety and efficacy observations. The early access to medicine scheme encourages products for treatment of life‐threatening conditions or diseases for which therapy is not available to improve access on an unlicensed or off‐label basis.48 The results of the present study have given a new ray of hope to patients with SCI. However, apart from the shortcomings of a retrospective compilation, the findings of the study have certain interpreting challenges. We considered all the patients for analyses, irrespective of the level and extent of injury. Further, the number of patients decreased during the subsequent treatment phases and thus limited the study to three treatment cycles to allow for a substantial number of patients. Nevertheless, given the improvement shown by our patients, we propose that the transplantation of hESC in patients with SCI presents a unique opportunity to address this huge unmet medical need.
CONCLUSION
SCI causes permanent disability and largely affects the everyday working of the affected patients. Even small clinical improvements in such patients could help them live a better life. In our patients with SCI, hESC transplantation was safe and effective and helped improve their clinical condition. hESC therapy may present a significant advance in the treatment and rehabilitation of patients with SCI. Further, prospective controlled studies with control groups would give a better clarity on the use of hESC in patients with SCI.
Author Contributions
G.S. wrote the manuscript. G.S. designed the research. G.S. performed the research. G.S. analyzed the data.
Conflict of Interest
The author declared no conflict of interest.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supporting Information
Supporting Information
Acknowledgments
The author sincerely thanks the patients who participated in this study. The author acknowledges Nayan Sonowal, Avinash Mishra, J. K. Barthakur, the Rehabilitation Department, and the team at Nutech Mediworld. The author also acknowledges Knowledge Isotopes Pvt. Ltd (www.knowledgeisotopes.com) for the medical writing support provided.
References
- 1. Hartkopp, P.A. , Brønnum‐Hansen, H. , Seidenschnur, A.M. & Biering‐Sørensen, F . Survival and cause of death after traumatic spinal cord injury. A long‐term epidemiological study [in Danish]. Ugeskr. Laeger 160, 6207–6210 (1998). [PubMed] [Google Scholar]
- 2. Lukovic, D. , Moreno Manzano, V. , Stojkovic, M. , Bhattacharya, S.S. & Erceg, S . Concise review: human pluripotent stem cells in the treatment of spinal cord injury. Stem Cells 30, 1787–1792 (2012). [DOI] [PubMed] [Google Scholar]
- 3. Rahimi‐Movaghar, V. et al Epidemiology of traumatic spinal cord injury in developing countries: a systematic review. Neuroepidemiology 41, 65–85 (2013). [DOI] [PubMed] [Google Scholar]
- 4. Gupta, N. , Solomon, J.M. & Raja, K . Demographic characteristics of individuals with paraplegia in India – a survey. Indian J. Physiother. Occup. Ther. 2 (2008). [Google Scholar]
- 5. Kirshblum, S. , Millis, S. , McKinley, W. & Tulsky, D . Late neurologic recovery after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1811–1817 (2004). [DOI] [PubMed] [Google Scholar]
- 6. Marino, R.J. , Ditunno, J.F. Jr , Donovan, W.H. & Maynard, F. Jr. Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch. Phys. Med. Rehabil. 80, 1391–1396 (1999). [DOI] [PubMed] [Google Scholar]
- 7. Ronsyn, M.W. , Berneman, Z.N. , Van Tendeloo, V.F. , Jorens, P.G. & Ponsaerts, P . Can cell therapy heal a spinal cord injury? Spinal Cord 46, 532–539 (2008). [DOI] [PubMed] [Google Scholar]
- 8. Erceg, S. et al Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS One 3, e2122 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keirstead, H.S. et al Human embryonic stem cell‐derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 25, 4694–4705 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee, H. et al Directed differentiation and transplantation of human embryonic stem cell‐derived motoneurons. Stem Cells 25, 1931–1939 (2007). [DOI] [PubMed] [Google Scholar]
- 11. Erceg, S. et al Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells 28, 1541–1549 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kakinohana, O. et al Survival and differentiation of human embryonic stem cell‐derived neural precursors grafted spinally in spinal ischemia‐injured rats or in naive immunosuppressed minipigs: a qualitative and quantitative study. Cell Transplant. 21, 2603–2619 (2012). [DOI] [PubMed] [Google Scholar]
- 13. Sharp, J. , Frame, J. , Siegenthaler, M. , Nistor, G. & Keirstead, H.S . Human embryonic stem cell‐derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 28, 152–163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cui, Y.F. , Xu, J.C. , Hargus, G. , Jakovcevski, I. , Schachner, M. & Bernreuther, C . Embryonic stem cell‐derived L1 overexpressing neural aggregates enhance recovery after spinal cord injury in mice. PLoS One 6, e17126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cloutier, F. , Siegenthaler, M.M. , Nistor, G. & Keirstead, H.S . Transplantation of human embryonic stem cell‐derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harm. Regen. Med. 1, 469–479 (2006). [DOI] [PubMed] [Google Scholar]
- 16. Perrotta, S. et al EPO receptor gain‐of‐function causes hereditary polycythemia, alters CD34 cell differentiation and increases circulating endothelial precursors. PLoS One 5, e12015 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. McDonald, J.W. et al Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 5, 1410–1412 (1999). [DOI] [PubMed] [Google Scholar]
- 18. Kerr, D.A. et al Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J. Neurosci. 23, 5131–5140 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roy, N.S. et al Telomerase immortalization of neuronally restricted progenitor cells derived from the human fetal spinal cord. Nat. Biotechnol. 22, 297–305 (2004). [DOI] [PubMed] [Google Scholar]
- 20. Hori, J. , Ng, T.F. , Shatos, M. , Klassen, H. , Streilein, J.W. & Young, M.J . Neural progenitor cells lack immunogenicity and resist destruction as allografts. Ocul. Immunol. Inflamm. 15, 261–273 (2007). [DOI] [PubMed] [Google Scholar]
- 21. Harper, J.M. et al Axonal growth of embryonic stem cell‐derived motoneurons in vitro and in motoneuron‐injured adult rats. Proc. Natl. Acad. Sci. USA 101, 7123–7128 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shroff, G. , Agarwal, P. , Mishra, A. & Sonowal, N . Human embryonic stem cells in treatment of spinal cord injury: a prospective study. J. Neurol. Res. 5, 213–220 (2015). [Google Scholar]
- 23. Shroff, G. , Gupta, A. & Barthakur, J.K . Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J. Transl. Med. 12, 318 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shroff, G. & Das, L . Human embryonic stem cell therapy in cerebral palsy children with cortical visual impairment: a case series of 40 patients. J. Cell Sci. Ther. 5, 1–7 (2014). [Google Scholar]
- 25. Shroff, G . A novel approach of human embryonic stem cells therapy in treatment of Friedrich's ataxia. Int. J. Case Rep. Imag. 6, 261–266 (2015). [Google Scholar]
- 26. Shroff, G . Human embryonic stem cells in the treatment of spinocerebellar ataxia: a case series. J. Clin. Case Rep. 5, 1–5 (2015). [Google Scholar]
- 27. Shroff, G . Human embryonic stem cells in the treatment of patients with Duchenne muscular dystrophy: a case series. J. Neurol. Res. 5, 186–191 (2015). [Google Scholar]
- 28. Shroff, G . Treatment of Lyme disease with human embryonic stem cells: a case series. J. Neuroinfect. Dis. 6, 167–172 (2015). [Google Scholar]
- 29. Shroff, G. & Barthakur, J.K . Safety of human embryonic stem cells in patients with terminal/incurable conditions– a retrospective analysis. Ann. Neurosci. 22, 132–138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Spinal Injury Association/International Medical Society of Paraplegia. International Standards for Neurologic and Function Classification of Spinal Cord Injury. Chicago, IL. Annual meeting (1996).
- 31. Paralysis, Paraplegia, and Quadriplegia . MD guidelines. http://www.mdguidelines.com/paralysis‐paraplegia‐and‐quadriplegia. Accessed 24 December 2015. [Google Scholar]
- 32. Ware, C.B. et al Derivation of naive human embryonic stem cells. Proc. Natl. Acad. Sci. USA 111, 4484–4489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park, H.C. et al Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte‐macrophage colony stimulating factor. Tissue Eng. 11, 913–922 (2005). [DOI] [PubMed] [Google Scholar]
- 34. Lima, C. et al Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil. Neural. Repair 24, 10–22 (2010). [DOI] [PubMed] [Google Scholar]
- 35. Kang, K.S. et al A 37‐year‐old spinal cord‐injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy 7, 368–373 (2005). [DOI] [PubMed] [Google Scholar]
- 36. Lukovic, D. , Stojkovic, M. , Moreno‐Manzano, V. , Bhattacharya, S.S. & Erceg, S . Perspectives and future directions of human pluripotent stem cell‐based therapies: lessons from Geron's clinical trial for spinal cord injury. Stem Cells Dev. 23, 1–4 (2014). [DOI] [PubMed] [Google Scholar]
- 37. Leuty, R. Stem cell trial for spinal cord injuries cleared by FDA. http://www.bizjournals.com/sanfrancisco/blog/biotech/2014/08/embryonic‐stem‐cells‐asterias‐geron‐spinal‐cord.html. Accessed 17 September 2014.
- 38. Shroff, G. & Barthakur, K.J. Bowel/bladder sensation and control in patients with spinal cord injury treated with human embryonic stem cell therapy. EC Neurol. 2.1, 47–54 (2015). [Google Scholar]
- 39. Shroff, G . Establishment and characterization of a neuronal cell line derived from a 2‐cell stage human embryo: clinically tested cell‐based therapy for neurological disorders. Int. J. Recent Sci. Res. 6, 3730–3738 (2015). [Google Scholar]
- 40. House of Lords SATSC . Regenerative Medicine. http://www.parliament.uk/documents/lords‐committees/science‐technology/RegenerativeMedicine/RegenMed.pdf. Accessed 7 May 2014. [Google Scholar]
- 41. Maahs, D.M. et al Urinary collagen fragments are significantly altered in diabetes: a link to pathophysiology. PLoS One 6, e13051 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang, S.K. , Shin, I.S. , Ko, M.S. , Jo, J.Y. & Ra, J.C . Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012, 342968 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borlongan, C.V. , Glover, L.E. , Tajiri, N. , Kaneko, Y. & Freeman, T.B . The great migration of bone marrow‐derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog. Neurobiol. 95, 213–228 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ezzat, T. , Dhar, D.K. , Malago, M. & Olde Damink, S.W . Dynamic tracking of stem cells in an acute liver failure model. World J. Gastroenterol. 18, 507–516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sohni, A. & Verfaillie, C.M . Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013, 130763 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Penha, E.M. et al Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014, 437521 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crawford, J.S. Experiences with epidural blood patch. Anaesthesia 35, 513–515 (1980). [DOI] [PubMed] [Google Scholar]
- 48. Safety and Effectiveness of Cord Blood Stem Cell Infusion for the Treatment of Cerebral Palsy in Children . https://clinicaltrials.gov/ct2/show/NCT01072370?term=stem+cells+and+cerebral+palsy&rank=5&submit_fld_opt=. Accessed 27 August 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supporting Information
Supporting Information


