Abstract
The inactivation of bacterial endospores by hydrostatic pressure requires the combined application of heat and pressure. We have determined the resistance of spores of 14 food isolates and 5 laboratory strains of Bacillus subtilis, B. amyloliquefaciens, and B. licheniformis to treatments with pressure and temperature (200 to 800 MPa and 60 to 80°C) in mashed carrots. A large variation in the pressure resistance of spores was observed, and their reduction by treatments with 800 MPa and 70°C for 4 min ranged from more than 6 log units to no reduction. The sporulation conditions further influenced their pressure resistance. The loss of dipicolinic acid (DPA) from spores that varied in their pressure resistance was determined, and spore sublethal injury was assessed by determination of the detection times for individual spores. Treatment of spores with pressure and temperature resulted in DPA-free, phase-bright spores. These spores were sensitive to moderate heat and exhibited strongly increased detection times as judged by the time required for single spores to grow to visible turbidity of the growth medium. The role of DPA in heat and pressure resistance was further substantiated by the use of the DPA-deficient mutant strain B. subtilis CIP 76.26. Taken together, these results indicate that inactivation of spores by combined pressure and temperature processing is achieved by a two-stage mechanism that does not involve germination. At a pressure between 600 and 800 MPa and a temperature greater than 60°C, DPA is released predominantly by a physicochemical rather than a physiological process, and the DPA-free spores are inactivated by moderate heat independent of the pressure level. Relevant target organisms for pressure and temperature treatment of foods are proposed, namely, strains of B. amyloliquefaciens, which form highly pressure-resistant spores.
High-hydrostatic-pressure treatment of foods is a process for food preservation at moderate temperatures. Pressure in the range of 200 to 800 MPa is effective at eliminating vegetative bacteria in food (37, 39). Pressure treatment at ambient temperatures initiates germination of bacterial endospores (13), but they are not inactivated at 25°C and a pressure over 1,000 MPa (36, 37). Inactivation of bacterial endospores requires a combination of pressure and moderate heat (21), and the efficacy of pressure treatment is enhanced by the presence of nisin (33, 41), by low pH (41, 43), and by oscillatory compression procedures (10, 14). Currently available data indicate that endospores of Bacillus and Clostridium species are inactivated by treatments with pressures ranging from 500 to 800 MPa at temperatures ranging from 60 to 80°C.
Studies on the pressure effects on vegetative cells of bacteria have demonstrated that the resistance to pressure strongly varies within strains of one species (4, 12). Likewise, the heat resistance of endospores of various strains of one species may exhibit strong variations (38). The majority of studies on the pressure resistance of bacterial endospores were performed with a limited number of laboratory strains. Because the resistance of endospores to pressure does not correlate with their resistance to heat (24), target strains and species for food processing that have a high resistance to pressure remain to be identified. Furthermore, the sporulation conditions as well as the matrix in which the spores are suspended during pressurization affect the pressure resistance of spores of B. subtilis (1, 18).
Moderate pressures of 100 to 600 MPa induce the germination of endospores of B. subtilis (43, 44, 45). Therefore, pressure-induced germination of spores enables a subsequent inactivation of germinated spores by mild heat or pressure. At ambient pressure, the release of pydridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) from the spores results from activation of (nutrient) receptors and is one of the early steps in spore germination (27). Wuytack et al. (45) compared spores which were induced to germinate at 100 MPa or 600 MPa. Germination of spores induced by 100 MPa resulted in the loss of DPA from the spores, degradation of small acid-soluble proteins (SASPs), and rapid generation of ATP. DPA release was also observed in spores germinated under high-pressure conditions; however, the degradation of SASPs and ATP generation were not observed. Treatment with 550 MPa induces spore germination independent of nutrient receptors by opening channels that allow the loss of DPA and lead to later steps in spore germination (26, 44).
It was the aim of this study to determine the pressure resistance of spores of a large number of food isolates of the genus Bacillus. Mashed carrots were used as a food model system. The role of DPA release during pressure inactivation and germination of representative strains as a possible reason for the variation in pressure resistance was examined. The role of DPA in spore pressure resistance was further determined with a mutant strain of B. subtilis. The DPA content of spores of this strain can be controlled by addition of DPA to the sporulation medium.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and preparation of spore suspensions.
The bacterial strains used in this study and their origins are shown in Table 1. Strains were grown aerobically in ST1 broth (Merck, Darmstadt, Germany) at 30°C. Spore suspensions of the strains were prepared by plating 0.1 ml from fresh overnight cultures on ST1 agar additionally supplemented with 10 mg of MnSO4 · H2O liter−1 unless otherwise stated. Strain CIP 76.26 (2) was grown on ST1 or ST1 additionally containing DPA (50 μg ml−1) to control the DPA content of these spores. To investigate the effect of minerals on pressure resistance, 5 mM CaSO4 · 2H2O, MnSO4 · H2O, or ZnSO4 · 7 H2O was added to ST1 agar. The agar plates were incubated until 90 to 99% of the cells contained phase-bright spores, as determined by phase-contrast microscopy. Spores from wild-type strains were collected from the plates by flooding the surface of the plate with 10 ml of cold sterile distilled water. After harvesting, the spore suspensions were washed four times by centrifugation at 3,000 × g for 15 min at 5°C, resuspended in sterile distilled water, and stored at −80°C until used. Between the second and third wash cycles the spore suspensions were pasteurized at 80°C for 10 min to kill all the vegetative forms. Spores of strain CIP 76.26 were prepared after 7 days of growth, and the heating step was omitted. It was verified by microscopic observation that more than 99.9% of the cells had sporulated. Cell counts were determined on ST1 agar. Appropriate dilutions were plated with a spiral plater (IUL, Königswinter, Germany), and plates were incubated at 30°C for 36 h.
TABLE 1.
Strains used and their origin
| Organism | Strain designation(s) and origin (reference or source) |
|---|---|
| B. licheniformis | TMW 2.492, pasteurized carrots (this study) |
| B. subtilis | TMW 2.485, pasteurized carrots (this study) |
| B. subtilis | CIP 76.26 (2) |
| B. subtilis | TMW 2.484, pasteurized carrots (this study) |
| B. subtilis | DSM 347 |
| B. subtilis | DSM 6405 |
| B. subtilis | TMW 2.469, chocolate cracker (this study) |
| B. subtilis | DSM 618 |
| B. subtilis | DSM 10T |
| B. subtilis | TMW 2.476, Fad 110, ropy bread (34) |
| B. subtilis | TMW 2.483, Fad 109, ropy bread (34) |
| Bacillus species | TMW 2.480, Fad 94, ropy bread (34)a |
| B. amyloliquefaciens | TMW 2.474, Fad 99, ropy bread (34)b |
| B. amyloliquefaciens | TMW 2.475, Fad We, ropy bread (34)b |
| B. amyloliquefaciens | TMW 2.477, Fad 108, ropy bread (34)b |
| B. amyloliquefaciens | TMW 2.478, Fad 77, ropy bread (34)b |
| B. amyloliquefaciens | TMW 2.479, Fad 82, ropy bread (34)b |
| B. amyloliquefaciens | TMW 2.481, Fad 97, ropy bread (34)b |
| B. amyloliquefaciens | TMW 2.482, Fad 11/2, ropy bread (34)b |
A partial sequence of the 16 S rRNA gene was determined; 1,500 and 1,499 of 1,506 bases were identical to B. subtilis DSM 4424 and B. vallismortis DSM 11031T, respectively.
Strain reclassified as B. amyloliquefaciens based on the sequence of the 16S rRNA and randomly amplified polymorphic DNA patterns (data not shown).
Pressure treatment.
Heat-sterilized, mashed carrots for use as pressurization medium were obtained in a local supermarket. Alternatively, Tris-His buffer (THB; 10 mM Tris-HCl, 20 mM histidine-HCl) adjusted to the same pH as the mashed carrots (pH 5.15) was used. The pressurization media were inoculated with spores to a spore count of 2.2 × 107 to 9.6 × 108 spores ml−1 and transferred to 2-ml Eppendorf reaction tubes; the tubes were sealed with silicon stoppers to avoid enclosure of air and stored on ice until treatment. The samples were pressurized at temperatures ranging from 60 to 80°C and pressures ranging from 0.1 to 800 MPa with FoodMicroLab equipment (Stansted Fluid Power Inc., Stansted, United Kingdom) having a 15-ml internal volume. Ethanol-ricinus oil (80:20, vol/vol) was the pressure transmission fluid. The compression and decompression rates were 2 MPa s−1. In all figures, the pressure-holding time, excluding the times required for compression and decompression, are indicated. The temperature of the pressure cell was maintained by a water bath (Haake GH), and the sample temperature was monitored by a thermocouple in the pressure vessel reaching into the sample container. The samples were placed in the pressure vessel about 4 min prior to compression to equilibrate the sample temperature. Upon compression, the temperature in the samples rose by approximately 20°C due to adiabatic heating, and the temperature profiles for treatments at 80°C and 600 or 800 MPa are shown in Tables 2 and 3. Comparable temperature profiles were obtained for treatments with 400 MPa (data not shown). After decompression, the sample tubes were stored on ice until determination of plate counts and measurement of DPA release and stored at −20°C until investigation of lag times. For each combination of pressure and temperature, inactivation kinetics with six to eight different pressure-holding times were determined. For each experiment, an untreated sample was used as a control to determine the initial number of spores. Data are presented as means of at least two independent experiments, and the standard deviations generally were 0.66 log units or less.
TABLE 2.
Pressure-temperature profile for treatment in mashed carrots at 600 MPa and 80°C
| Process time (min) | Pressure (MPa) | Temp (°C) |
|---|---|---|
| 0.0 | 0.1 | 79.9 |
| 2.5 | 208 | 93.9 |
| 4.5 | 439 | 98.9 |
| 5.3 | 604 | 101.7 |
| 6.0 | 597 | 99.8 |
| 7.0 | 592 | 94 |
| 8.1 | 589 | 89.3 |
| 19.1 | 588 | 86.3 |
| 11.1 | 588 | 83.3 |
| 13.0 | 587 | 82.1 |
| 16.0 | 588 | 80.1 |
TABLE 3.
Pressure-temperature profile for treatment in mashed carrots at 800 MPa and 80°C
| Process time (min) | Pressure (MPa) | Temp (°C) |
|---|---|---|
| 0.0 | 0.1 | 79.3 |
| 2.5 | 173 | 91.7 |
| 4.5 | 403 | 98.8 |
| 5.7 | 512 | 98.6 |
| 6.5 | 621 | 97.9 |
| 7.3 | 816 | 101 |
| 8.0 | 809 | 98.4 |
| 9.1 | 804 | 92.9 |
| 11.0 | 801 | 86.2 |
| 13.0 | 800 | 83.2 |
| 16.0 | 800 | 81.6 |
Measurement of DPA release.
The release of DPA from spores was determined by measurements of the DPA concentration in the supernatant of pressure-treated and untreated spore suspensions. The DPA concentration of samples was analyzed by high-performance liquid chromatography using a Polyspher OAKC column (Merck, Darmstadt, Germany). The flow rate was 0.4 ml min−1, the mobile phase was 5% acetonitrile in 5 mM H2SO4, and the temperature of the column was 70°C. A UV detector at 280 nm (Gynkotek, Germering, Germany) was used for detection. The total DPA content of the spores was determined by quantification of DPA from spore suspensions after heat treatment at 121°C for 20 min to fully release the DPA from the spores (19), and the release of DPA by pressure treatment was calculated relative to the total DPA content of the spores. Data are presented as means of at least two independent experiments, and the standard deviations were generally less than 5%.
Detection of lag times.
The determination of lag times as a measure for the population heterogeneity in vegetative cells of bacteria was previously proposed (3) Pressure-treated or untreated spore suspensions with known cell counts were diluted in ST1 broth to obtain 5, 2.5, and 1.25 spores per ml. For each of these three dilutions, 12 200-μl cultures were transferred to microtiter plates and the growth kinetics were monitored by measuring absorption at 590 nm in a Spectraflour microtiter plate reader (Tecan, Grödig, Austria) at 30-min intervals for up to 120 h at 30°C. It was assumed that the 200-μl cultures were inoculated with a single spore when 2 or more of the 12 cultures remained sterile. The experiment was repeated until observations for 96 or more individual spores from a given sample were obtained. The detection times were calculated as the time in hours that elapsed until the culture grew to an optical density of 0.02.
RESULTS
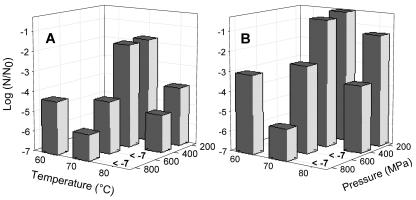
Effect of pressure and temperature on the inactivation of spores from Bacillus licheniformis and B. subtilis. To determine the inactivation of endospores in food, spores of the strains B. subtilis TMW 2.485 and B. licheniformis TMW 2.492 isolated from pasteurized carrots were subjected to treatments in mashed carrots with pressures ranging from 200 to 800 MPa and temperatures ranging from 60 to 80°C. For each parameter combination the inactivation kinetics was determined with eight different pressure-holding times. In Fig. 1 are shown the spore counts after a 16-min pressure-holding time. Spores of B. licheniformis TMW 2.492 showed a higher resistance to the pressure than those of B. subtilis TMW 2.485. An inactivation by less than 2 log units was observed for both strains at 200 or 400 MPa and 70°C, and a further increase in pressure or temperature resulted in an enhanced inactivation of the spores. Spores of both strains were completely inactivated at 80°C and 600 or 800 MPa after a 16-min pressure-holding time.
FIG. 1.
Log spore counts (N) of B. subtilis TMW 2.485 (A) and B. licheniformis TMW 2.492 (B) in mashed carrots after pressure and temperature treatment. Spore counts are depicted relative to the spore counts of untreated samples (N0). Data shown are means of at least two independent experiments, and the standard deviations were generally less than 0.66 log units. Spore counts below the detection limit (log [N/N0] = −7) are indicated.
Variation of pressure resistance among strains of B. subtilis and B. amyloliquefaciens.
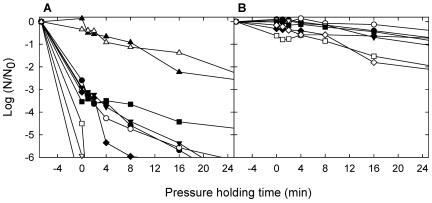
To determine the variability of pressure resistance within 18 strains of two species, 13 isolates from food and 5 strains from strain collections of the species B. subtilis and B. amyloliquefaciens were subjected to pressure treatment in mashed carrots. The inactivation kinetics of 10 strains of B. subtilis at 800 MPa and 70°C in carrots are displayed in Fig. 2A. High variations of pressure resistance were observed. Three laboratory strains formed spores that were highly pressure sensitive and that were reduced by more than 6 orders of magnitude within 1 min. Four food isolates and one laboratory strain formed more-pressure-resistant spores, which were reduced by more than 4 orders of magnitude after 16 min. Two strains isolated from ropy bread formed spores highly resistant to pressure, which were inactivated by less than 2 orders of magnitude after a 16-min pressure-holding time. These strains were more pressure resistant than strain B. licheniformis TMW 2.492 (compare Fig. 1B and 2A).
FIG. 2.
Log spore counts of B. subtilis and B. amyloliquefaciens spores in mashed carrots after treatment with 800 MPa and 70°C. (A) B. subtilis TMW 2.485 (•), TMW 2.484 (○), DSM 347 (▾), DSM 6405 (▿), Fad 94 (▪), DSM 618 (□), TMW 2.469 (♦), DSM 10T (⋄), Fad 110 (▴), and Fad 109 (▵). (B) B. amyloliquefaciens Fad 82 (•), Fad 11/2 (○), Fad 99 (▿), Fad 77 (▪), Fad 97 (□), Fad 108 (♦), and Fad We (⋄). Data shown are means of at least two independent experiments, and the standard deviations were generally less than 0.66 log units. Lines dropping below the x axis indicate spore counts below the detection limit (log [N/N0] = −6, where N and N0 are as defined for Fig. 1).
The inactivation of spores from seven strains of B. amyloliquefaciens is shown in Fig. 2B. All strains were previously isolated from the same source, ropy bread, and these strains essentially exhibited the same resistance to pressure and heat. After 16 min of pressure-holding time, 1 to 90% of the spores remained active. B. amyloliquefaciens formed the most-pressure-resistant spores among the strains in our study and tolerated 16 min at 800 MPa and 70°C (Fig. 2B) in mashed carrots without reduction of spore counts.
Effect of sporulation conditions on pressure resistance of endospores.
The sporulation temperature and the mineral content of spores influence the resistance of spores of B. subtilis ATCC 19659 to heat and pressure (18). We aimed to evaluate whether comparable effects govern the pressure resistance of spores of a food isolate of B. subtilis. The sporulation conditions strongly affected pressure resistance. For instance, after a 1-s pressure-holding time at 800 MPa and 70°C, spores obtained from cultures at 30, 44, and 48°C were inactivated by 2.5, 5, and 6 orders of magnitude, respectively. Spores obtained from cultures at 30°C in the presence of 5 mM CaSO4, ZnSO4, or MnSO4 were inactivated by 4 orders of magnitude, indicating that the presence of minerals decreased the spore resistance to high-pressure and high-temperature treatment.
Pressure-induced release of DPA from bacterial endospores.
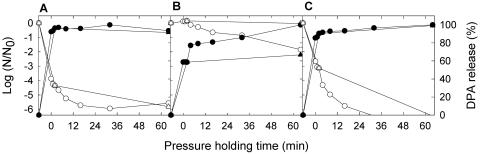
The high levels of DPA in bacterial endospores are an important factor in their resistance to chemical and physical stressors, and the pressure-induced release of DPA is considered a trigger for nutrient receptor-independent spore germination. To determine whether the variation in pressure resistance of bacterial endospores corresponds to the pressure-induced release of DPA from the respective spores, the release of DPA from spores with low, intermediate, and high pressure resistance, i.e., B. subtilis TMW 2.485, B. licheniformis TMW 2.492, and B. amyloliquefaciens Fad 82, was determined after pressure treatment at 800 MPa and 70°C. Experiments were performed in THB, because compounds from the carrots interfered with the quantification of DPA. The release of DPA from the spores is compared to the decrease of spore counts in Fig. 3. No correlation between the total DPA content and pressure resistance was found (Fig. 3). No significant differences were observed when the inactivation of spores in THB was compared to the inactivation in mashed carrots with the same pH. The DPA release of the spores took place at the same time or prior to inactivation. For example, after a 0-min pressure-holding time, spore counts of B. licheniformis TMW 2.494 were reduced by 2.6 log units and 85% of total DPA was released from the spores. Spores of B. subtilis and B. licheniformis with low and intermediate pressure resistance released 96 and 90% DPA, respectively, after a 2-min pressure-holding time. Spores that were pressure treated for 2 min and that lost essentially all of their DPA could be inactivated at 70°C and 0.1 MPa. Following this short pressure pulse for the release of DPA, the kinetics of inactivation at 70°C and 0.1 MPa was not different from the kinetics of inactivation at 70°C and 800 MPa. Thus, the generation of pressure-induced DPA-free spores was accompanied by the loss of their heat resistance, and pressure did not further influence spore inactivation once the spores had lost more than 90% of their DPA. In contrast, spores of the highly pressure-resistant B. amyloliquefaciens Fad 82 released only 58% of their DPA after 2 min at 800 MPa, and spores having lost 58% of their DPA were not heat sensitive.
FIG. 3.
Effect of continuous pressurization and pressure pulse treatment at 70°C in THB on counts of Bacillus spores and the release of DPA from the spores. (A) B. subtilis TMW 2.485; (B) B. amyloliquefaciens Fad 82; (C) B. licheniformis TMW 2.492. ○ and ▵, spore counts; • and ▴, DPA release relative to the initial DPA content of the spores. • and ○, continuous pressurization at 800 MPa; ▴ and ▵, pressure pulse treatment, 800 MPa for 2 min followed by 0.1 MPa. The DPA contents of B. subtilis, B. amyloliquefaciens, and B. licheniformis spores were 1.45 ± 0.15, 0.96 ± 0.13, and 0.39 ± 0.05 mM/109 spores, respectively. Data shown are means of at least two independent experiments. The standard deviations for the determination of spore counts and the DPA release were generally less than 0.66 log units and 5%, respectively. Lines dropping below the x axis indicate spore counts below the detection limit (log [N/N0] = −6.4, where N and N0 are as defined for Fig. 1).
Role of DPA in heat and pressure resistance of the DPA-deficient mutant B. subtilis CIP 76.26.
To elucidate the role of DPA in the pressure resistance of bacterial endospores in more detail, the inactivation of the DPA-deficient mutant strain B. subtilis CIP 76.26 by heat and/or pressure was determined. The level of DPA in spores from cultures of this strain grown in the absence of DPA is only 16% of the DPA levels in spores obtained from media with DPA (2). Results of Balassa et al. (2) were verified in this study (data not shown). DPA-free spores of CIP 76.26 were inactivated at 65°C and 0.1 MPa (Fig. 4). Leaving aside the spore inactivation in the first 3 min of the pressure treatments, during which the temperature exceeds 65°C due to adiabatic heating, inactivation with 65°C and 600 MPa was not substantially accelerated compared to the inactivation at ambient pressure. Spores with DPA were heat stable; however, the inactivation with 65°C and 600 MPa was comparable to the inactivation of DPA-free spores if the first 3 min of the pressure treatments, during which accelerated inactivation under pressure is attributable to the temperature rise due to adiabatic heating, are neglected (Fig. 4). Comparable to spores of the wild-type strains of B. subtilis, and B. licheniformis, DPA-containing spores of CIP 76.26 were inactivated at 65°C and 0.1 MPa after a treatment at 65°C and 600 MPa for 2 min. In short, DPA-containing spores were heat resistant and DPA-free spores were heat sensitive, independent of whether the DPA-free spores were obtained by cultivation on a DPA-free medium or by a pressure pulse to release DPA from the spores.
FIG. 4.
Effect of heat (65°C at 0.1 MPa), combined heat and pressure treatment (65°C and 600 MPa), or pressure pulse treatment (65°C and 600 and 0.1 MPa) on the inactivation of spores of the DPA-deficient B. subtilis mutant CIP 76.26. Spores were obtained from medium with external DPA (•, ▪, and ▴) or medium without external DPA (○, □, and ▵). • and ○, continuous pressurization at 600 MPa and 65°C; ▴ and ▵, pressure pulse, 600 MPa and 65°C for 2 min, followed by 0.1 MPa and 65°C; ▪ and □, 0.1 MPa and 65°C. Data shown are means of at least two independent experiments, and the standard deviations were generally less than 0.66 log units. Lines dropping below the x axis indicate spore counts below the detection limit (log [N/N0] = −6, where N and N0 are as defined for Fig. 1).
Distribution of detection times of pressure-treated spores: germination or sublethal injury?
The release of DPA from spores following pressurization at 550 MPa or greater was interpreted as a consequence of spore germination by some authors (45), whereas other authors suggested that pressure-induced, unphysiological release of DPA acts as a trigger for germination after pressure release (27). To discriminate between these two hypotheses, we determined detection times of individual spores before and after pressure treatment. In all samples a pronounced distribution of spore germination and outgrowth was detected, indicating large differences in the physiological states of individual spores within a sample. A small fraction of spores germinated faster than the average, whereas another small fraction exhibited prolonged detection times (Fig. 5). Figure 5A shows the detection times of single, untreated spores of B. subtilis TMW 2.485 and of single spores treated at 200 MPa and 70°C for 16 min. Pressure-treated spores exhibited a distribution of detection times, shifted to longer detection times compared to untreated spores, indicating pressure-induced sublethal injury rather than pressure-induced germination. A comparable result was obtained with spores of B. licheniformis TMW 2.492 (Fig. 5B), both untreated and treated at 800 MPa and 70°C for 4 min. The shortest detection time observed was 12 h, and the detection times for 90% of untreated spores were less than 31 h. When spores of strain TMW 2.492 were treated with 70°C and 0.1 MPa for 10 min, 90% of the spores had a detection time of 23 h or less (Fig. 5B), indicating activation of spore germination by heat treatment. In comparison, germination and outgrowth of 90% of the spores in the pressure-treated (800 MPa and 70°C) sample were observed only after 81 h. None of the spores exhibited detection times of less than 20 h, indicating that all spores of the population were injured by the treatment. Treatment of strain TMW 2.492 with 100 MPa and 20°C for 30 min, followed by storage at −20°C, did not shift the distribution of detection times.
FIG. 5.
Detection times of spores of B. subtilis TMW 2.485 (A), B. licheniformis TMW 2.492 (B), and the DPA-deficient B. subtilis mutant CIP 76.26 (C). (A) Untreated spores (•; n = 97) and spores treated with 200 MPa and 70°C for 16 min (○; n = 97). (B) Untreated spores (•; n = 282) and spores treated with 800 MPa and 70°C for 4 min (○; n = 110), 100 MPa and 20°C for 30 min (▿; n = 183), or 0.1 MPa and 70°C for 10 min (▵; n = 177). (C) DPA-containing spores (•, ○; n = 96), DPA-free spores (▾, ▿; n = 184), untreated spores (•, ▾), and spores treated with 600 MPa and 65°C for 8 min (○, ▿).
To investigate the influence of DPA on spore germination, the detection times for untreated spores of the DPA-deficient B. subtilis CIP 76.26 with and without DPA were determined, and compared to the distribution of detection times after pressure treatment (Fig. 5C). Spores containing DPA exhibited much shorter detection times than DPA-free spores, indicating that the lack of DPA in the absence of any other physical stressors delays spore germination. After pressure treatment at 600 MPa and 65°C for 8 min, no difference between the distributions of detection times for DPA-free and DPA-containing spores was observed.
DISCUSSION
The inactivation of bacterial endospores by pressure is generally considered to rely on pressure-induced spore germination, followed by inactivation of germinated spores. In this study the pressure resistance of food isolates and reference strains of Bacillus was determined. The role of DPA release for representative strains as a possible reason for the variation in pressure resistances was examined. Furthermore, we aimed to elucidate whether the altered properties of pressure-treated spores of highly pressure-resistant food isolates—loss of DPA and heat resistance—are attributable to pressure-induced germination or sublethal injury.
Variability of pressure resistance in spores of Bacillus species.
In this study the levels of resistance of 14 food isolates and 5 laboratory strains of Bacillus species to combined pressure and temperature treatments were compared. In agreement with literature data, appreciable inactivation of spores of B. subtilis TMW 2.485 and B. licheniformis TMW 2.492 was observed when the pressure exceeded 400 MPa and the temperature exceeded 60°C and both an increase of pressure and an increase in temperature enhanced spore inactivation (15, 20, 30, 32, 35). To date, kinetics data for the inactivation of spores of Bacillus species are available for a few laboratory strains only. We observed a large variability of pressure resistance in food isolates of the closely related species B. subtilis, B. licheniformis, and B. amyloliquefaciens. Using two strains of Clostridium botulinum type E, Reddy et al. (31) also observed differences in pressure resistance within one species. Remarkably, the strain B. licheniformis TMW 2.492, used in this study, exhibited a intermediate pressure resistance compared to other food isolates of B. subtilis and B. amyloliquefaciens but a higher resistance than other strains of B. subtilis for which literature data are available (10, 11, 16, 18, 23, 41). This finding highlights the need for studies with food isolates to establish pressure processes in food preservation.
The highest resistance to pressure was observed in strains of B. subtilis and B. amyloliquefaciens previously isolated from ropy bread. The spores of rope-forming bacilli are more heat resistant than other strains of B. subtilis and B. amyloliquefaciens because these spores survive the baking process, i.e., heat treatment at 100°C for 45 to 60 min (34). This finding may imply a correlation between heat resistance and pressure resistance. However, for strains of rope-forming bacilli heat resistance (34) does not correlate with pressure resistance. Furthermore, spores of B. amyloliquefaciens are considerably more pressure resistant than spores of Geobacillus stearothermophilus, which exhibit a higher resistance to wet heat (1). Likewise, the pressure resistance of spores of six Bacillus strains did not correlate with their heat resistance (24). Therefore, those target organisms used to determine suitable process conditions for the thermal treatments of foods are not suitable target organisms for pressure processes. On the basis of published data, spores of B. amyloliquefaciens Fad 11/2, Fad 77, Fad 82, Fad 99, and Fad 108 are more pressure resistant than spores of Bacillus, Geobacillus, Alicyclobacillus, or Clostridium species, including strains of C. botulinum type A and type E (1, 5, 9, 10, 11, 16, 18, 20, 23, 29, 30, 31, 32, 41, 45). Therefore, we currently consider them relevant target organisms for the pressure sterilization of foods.
Effect of sporulation conditions on pressure resistance.
We observed a large variation in the pressure resistance of spores of B. subtilis TMW 2.485 depending on the sporulation conditions. The observed decrease in pressure resistance with increasing sporulation temperature is consistent with results from Igura et al. (18). We could further show that addition of minerals to the sporulation medium reduced the pressure resistance of spores. The effect of sporulation temperature and spore mineralization on pressure resistance was opposite to the effect on heat resistance (18).
Detection times as a measure of physiological heterogeneity and sublethal injury.
The determination of detection times of individual vegetative bacterial cells was proposed as a suitable measure for the physiological heterogeneity of a population (3) and has been used to determine sublethal injury in heat-stressed cells of Lactobacillus plantarum (40). In this study, the method was applied to determine population heterogeneity in untreated and pressure-treated spores of Bacillus species. Physiological heterogeneity within an isogenic bacterial culture occurs because of chemical and physical gradients in the culture vessel and because of statistical events in gene expression (8). A knowledge of the physiological heterogeneity of bacterial cultures is a prerequisite for the mathematical modeling of bacterial growth and inactivation (16, 22). As reported for vegetative cells of L. plantarum, we observed a strong increase of the detection times after application of sublethal stress. Moreover, upon pressure treatment, a broad distribution of detection times was noted, and spores from a given sample required 24 to 96 h for germination and growth. This results in a systematic error in the determination of spore counts by surface plating, as shown here and in most other studies dealing with inactivation of bacterial endospores by pressure. Incubation of the agar plates for more than 96 h is required to achieve outgrowth of more than 99% of the surviving spores, and shorter incubation times underestimate the spore counts.
However, with the rope-forming Bacillus isolates used in this work, longer incubation times also result in systematic errors. Those spores that germinate in less than 24 h rapidly cover the entire agar plate, thus make enumeration of those spores that germinate later impossible.
Pressure-induced loss of DPA and heat resistance: germination or sublethal injury?
Spore germination induced by moderate pressures up to 250 MPa at ambient temperature is similar to nutrient-induced germination (6, 13, 17, 45). Pressure germination at moderate pressures results in a release of DPA from the spores, and phase-dark spores which exhibit sensitivity to heat and pressure comparable to that of vegetative cells are obtained. Germination at pressure exceeding 500 MPa and ambient temperature (25 to 40°C) is induced by a mechanism different from that operating at low pressures (44, 45). Pressure application causes the unphysiological loss of DPA from the spore and allows spore germination independent of the presence of nutrient receptors after decompression (27, 28, 44).
In this work, the loss of DPA and an enhanced heat sensitivity of spores of Bacillus species were observed after treatments with 800 MPa and 70°C, which may be interpreted either as a consequence of a physiological process, germination, or a result of the physicochemical loss of DPA from the spores. Spores of B. subtilis TMW 2.485 remained phase bright after lethal pressure applications (Fig. 1 and data not shown), arguing against pressure-induced germination. To further differentiate between pressure-induced germination and pressure-induced sublethal injury, we have determined the distribution of detection times of single spores. Induction of spore germination with heat reduced detection times, indicating that our experimental setup is suitable to detect spore activation. Treatment of spores with moderate pressure (100 MPa and 20°C) did not affect the detection times; however, an activation of spore germination by pressure (44) may have been reversed by frozen storage following pressure treatment (7). Treatment of spores with 200 MPa and 70°C or 800 MPa and 70°C increased the detection times by a factor of two to four, indicating that combined application of heat and pressure did not induce germination but rather inflicted sublethal injury. Experiments with the DPA-deficient mutant B. subtilis CIP 76.26 demonstrated that the enhanced detection times could be partially explained by the lack of DPA in pressure-treated spores. Other injuries inflicted by pressure may include the inactivation of cortex lytic enzymes. Therefore, the loss of DPA during combined application of heat and pressure must be considered a result of a physicochemical process. In contrast to what was found for treatments at high pressure and low temperature, the loss of DPA after high-pressure and high-temperature treatment does not lead to initiation of spore germination after decompression. The release of DPA may be caused by an increased permeability of the plasma membrane, the cortex, or the outer membranes of the spores. It is well established that pressure application increases the permeability of bacterial membranes and compromises the function of integral membrane proteins (25, 42).
The release of DPA from the spores was accompanied by an increased heat sensitivity of the spores. The comparison of the DPA releases and the heat sensitivities of pressure-treated spores of B. subtilis, B. licheniformis, and B. amyloliquefaciens indicates that a complete loss of DPA (>90% for untreated spores) is required to obtain heat-sensitive spores. These DPA-free, phase-bright spores are less heat resistant than dormant spores but are much more heat and pressure resistant than vegetative cells of bacilli (16). The combined application of pressure and heat was required to obtain DPA-free, heat-sensitive spores. However, once more than 90% of the DPA was released from the cells, the inactivation of spores was not further influenced by pressure. Following a pressure pulse with 800 MPa and 70°C for 2 min to fully release DPA from the spores, further treatments with 800 MPa and 70°C or 0.1 MPa and 70°C had equivalent effects on the spores of B. subtilis and B. licheniformis. Comparable effects were obtained with the DPA-deficient mutant B. subtilis CIP 76.26. In B. amyloliquefaciens, treatment with 2 min at 800 MPa and 70°C released only 58% of the DPA from the spores and the spores remained heat resistant. Therefore, the inactivation of spores of bacilli may be considered a two-stage process. First, as a result of combined application of pressure and heat, sublethally injured, DPA-free spores that are heat sensitive are generated. Second, these spores are heat inactivated independent of the pressure level. This proposed mechanism of inactivation of spores by heat and pressure may provide an explanation why, in some cases, a correlation between the heat and pressure resistance of spores is found, whereas such a correlation is absent in other cases.
In conclusion, we have observed a strong variability of the resistance to pressure and temperature treatments within bacilli. Relevant target organisms for pressure and temperature treatment of foods are proposed, i.e., the five strains of B. amyloliquefaciens (Fad 11/2, Fad 82, Fad 77, Fad 99, and Fad 108) which form highly pressure-resistant spores. Our data indicate a two-stage mechanisms of spore inactivation in the pressure and temperature ranges used in this study, i.e., temperatures of >60°C and pressures of >600 MPa. First, pressure and temperature act to generate sublethally injured DPA-free and phase-bright spores. Second, these spores are inactivated by moderate heat independent of the pressure. Therefore, the resistance of spores to combined pressure and temperature treatments depends on their ability to retain DPA, and on the heat resistance of DPA-free spores. This mechanism may explain why some of the spore properties with importance for wet heat resistance of spores are also relevant for pressure resistance, whereas others are not. Furthermore, it may enable pressure pulse treatments of foods to safely inactivate bacterial endospores with a minimal treatment intensity.
Acknowledgments
We thank Annegret Beck, Margot Maier, and Matthias Werner for their excellent assistance. We thank Walter P. Hammes for providing the ropy-bread isolates.
The Deutsche Bundesstiftung Umwelt is acknowledged for financial support (grant 13053/20).
REFERENCES
- 1.Ananta, E., V. Heinz, O. Schlüter, and D. Knorr. 2001. Kinetic studies on high-pressure inactivation of Bacillus stearothermophilus spores suspended in food matrices. Innov. Food Sci. Emerg. Technol. 2:261-272. [Google Scholar]
- 2.Balassa, G., P. Milhaud, E. Raulet, M. T. Silva, and J. C. F. Sousa. 1979. A Bacillus subtilis mutant requiring dipicolinic acid for the development of heat-resistant spores. J. Gen. Microbiol. 110:365-379. [DOI] [PubMed] [Google Scholar]
- 3.Baranyi, J., and C. Pin. 1999. Estimating bacterial growth parameters by means of detection times. Appl. Environ. Microbiol. 65:732-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benito, A., G. Ventoura, M. Casadei, T. Robinson, and B. Backey. 1999. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl. Environ. Microbiol. 65:1564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cléry-Barraud, C., A. Gaubert, P. Masson, and D. Vidal. 2004. Combined effects of high hydrostatic pressure and temperature for inactivation of Bacillus anthracis spores. Appl. Environ. Microbiol. 70:635-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clouston, J. G., and P. A. Wills. 1969. Initiation of germination and inactivation of Bacillus pumilus spores by hydrostatic pressure. J. Bacteriol. 97:684-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collado, J., A. Fernández, M. Rodrigo, J. Camats, and A. Martínez Lopez. 2003. Kinetics of deactivation of Bacillus cereus spores. Food Microbiol. 20:545-548. [Google Scholar]
- 8.Elowitz, R. B., A. J. Levine, E. D. Siggia, and P. S. Swain. 2002. Stochastic gene expression in a single cell. Science 297:1183-1187. [DOI] [PubMed] [Google Scholar]
- 9.Fujii, K., A. Ohtani, J. Watanabe, H. Ohgoshi, T. Fujii, and K. Honma. 2002. High-pressure inactivation of B. cereus spores in the presence of argon. Int. J. Food Microbiol. 72:239-242. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa, S., M. Shimoda, and I. Hayakawa. 2003. Mechanism of the inactivation of bacterial spores by reciprocal pressurization treatment. J. Appl. Microbiol. 94:836-841. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa, S., S. Noma, S. Yoshikawa, H. Furuya, M. Shimoda, and I. Hayakawa. 2001. Effect of filtration of bacterial suspensions on the inactivation ratio in hydrostatic pressure treatment. J. Food Eng. 50:59-61. [Google Scholar]
- 12.Garcia-Graells, C., B. Masschalck, I. van Opstal, E. Wuytack, and C. Michiels. 2002. Mild non-thermal food preservation, abstr. S9. Abstr. Front. Microb. Ferment. Preserv., Wageningen, The Netherlands, January 2002.
- 13.Gould, G. W., and J. H. Sale. 1970. Initiation of germination of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:335-346. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa, I., T. Kanno, K. Yoshiyama, and Y. Fujio. 1994. Oscillatory compared with continuous high pressure sterilization on Bacillus stearothermophilus spores. J. Food Sci. 59:164-167. [Google Scholar]
- 15.Hayakawa, I., T. Kanno, M. Tomita, and Y. Fujio. 1994. Application of high pressure for spore inactivation and protein denaturation. J. Food Sci. 59:159-163. [Google Scholar]
- 16.Heinz, V., and D. Knorr. 1996. High pressure inactivation kinetics of Bacillus subtilis cells by a three-state-model considering distributed resistance mechanisms. Food Biotechnol. 10:149-161. [Google Scholar]
- 17.Heinz, V., and D. Knorr. 1998. High pressure germination and inactivation kinetics of bacterial spores, p. 435. In N. S. Isaacs (ed.), High pressure food science, bioscience and chemistry. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 18.Igura, N., Y. Kamimura, M. S. Islam, M. Shimoda, and I. Hayakawa. 2003. Effects of minerals on resistance of Bacillus subtilis spores to heat and hydrostatic pressure. Appl. Environ. Microbiol. 69:6307-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen, F. W., A. J. Lund, and L. E. Anderson. 1958. Colorimetric assay for dipicolinic acid in bacterial spores. Science 127:26-27. [DOI] [PubMed] [Google Scholar]
- 20.Lee, S. Y., R. H. Dougherty, and D. H. Kang. 2002. Inhibitory effects of high pressure and heat on Alicyclobacillus acidoterrestris spores in apple juice. Appl. Environ. Microbiol. 68:4158-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallidis, C. G., and D. Drizou. 1991. Effect on simultaneous application of heat and pressure on the survival of bacterial spores. J. Appl. Bacteriol. 71:285-288. [DOI] [PubMed] [Google Scholar]
- 22.McKellar, R. C., X. Lu, and K. P. Knight. 2002. Growth pH does not affect the initial physiological state parameter (p0) of Listeria monocytogenes. Int. J. Food Microbiol. 73:137-144. [DOI] [PubMed] [Google Scholar]
- 23.Moerman, F., B. Mertens, L. Demey, and A. Huyghebaert. 2001. Reduction of Bacillus subtilis, Bacillus stearothermophilus and Streptococcus faecalis in meat batters by temperature-high hydrostatic pressure pasteurization. Meat Sci. 59:115-125. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama, A., Y. Yutaka, S. Kobayashi, M. Ishikawa, and K. Sakai. 1996. Comparison of pressure resistances of spores of six Bacillus strains with their heat resistances. Appl. Environ. Microbiol. 62:3897-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagán, R., and B. Mackey. 2000. Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential- and stationary-phase cells and variation among strains. Appl. Environ. Microbiol. 66:2829-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat, M., B. Setlow, A. Drinks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paidhungat, M., B. Setlow, W. B. Daniels, D. Hoover, E. Papafragkou, and P. Setlow. 2002. Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Appl. Environ. Microbiol. 68:3172-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raso, J., M. M. Góngora-Nieto, G. V. Barbosa-Cánovas, and B. G. Swanson. 1998. Influence of several environmental factors on the initiation of germination and inactivation of Bacillus cereus by high hydrostatic pressure. Int. J. Food Microbiol. 44:125-132. [DOI] [PubMed] [Google Scholar]
- 30.Raso, J., G. Barbosa-Cánovas, and B. G. Swanson. 1998. Sporulation temperature affects initiation of germination and inactivation by high hydrostatic pressure of Bacillus cereus. J. Appl. Microbiol. 85:17-24. [DOI] [PubMed] [Google Scholar]
- 31.Reddy, N. R., H. M. Solomon, G. A. Fingerhut, E. J. Rhodehamel, V. M. Balasubramaniam, and S. Palaniappan. 1999. Inactivation of Clostridium botulinum type E spores by high pressure processing. J. Food Saf. 19:277-288. [Google Scholar]
- 32.Reddy, N. R., H. M. Solomon, R. C. Tetzloff, E. J. Rhodehamel, V. M. Balasubramaniam, and S. Palaniappan. 2003. Inactivation of Clostridium botulinum type A spores by high-pressure processing at elevated temperatures. J. Food Prot. 66:1402-1407. [DOI] [PubMed] [Google Scholar]
- 33.Roberts, C. M., and D. G. Hoover. 1996. Sensitivity of Bacillus coagulans spores to combinations of high hydrostatic pressure, heat, acidity and nisin. J. Appl. Bacteriol. 91:1582-1588. [Google Scholar]
- 34.Röcken, W., and G. Spicher. 1993. Fadenziehende Bakterien—Vorkommen, Bedeutung Gegenmaßnahmen. Getreide Mehl Brot 47:30-35. [Google Scholar]
- 35.Rovere, P., S. Gola, A. Maggi, N. Sacamuzza, and L. Miglioli. 1998. Studies on bacterial spores by combined high pressure-heat treatments: possibility to sterilize low acid foods, p. 354. In N. S. Isaacs (ed.), High pressure food science, bioscience and chemistry. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 36.Sale, J. H., G. W. Gould, and W. A. Hamilton. 1970. Inactivation of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:323-334. [DOI] [PubMed] [Google Scholar]
- 37.San Martín, M. F., G. V. Barbosa-Cánovas, and B. G. Swanson. 2002. Food processing by high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 42:627-645. [DOI] [PubMed] [Google Scholar]
- 38.Sarker, M. R., R. P. Shivers, S. G. Sparks, V. K. Juneja, and B. A. McClane. 2000. Comparative experiments to examine the effects of heating on vegetative cells and spores of Clostridium perfringens isolates carrying plasmid genes versus chromosomal enterotoxin genes. Appl. Environ. Microbiol. 66:3234-3240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Smelt, J. P. P. M., J. C. Hellemons, P. C. Wouters, and S. J. C. van Gerwen. 2002. Physiological and mathematical aspects in setting criteria for decontamination of foods by physical means. Int. J. Food Microbiol. 78:57-77. [DOI] [PubMed] [Google Scholar]
- 40.Smelt, J. P. P. M., G. D. Otten, and A. P. Bos. 2002. Modeling the effect of sublethal injury on the distribution of the lag times of individual cells of Lactobacillus plantarum. Int. J. Food Microbiol. 73:207-212. [DOI] [PubMed] [Google Scholar]
- 41.Stewart, C. M., C. P. Dunne, A. Sikes, and D. G. Hoover. 2000. Sensitivity of spores of Bacillus subtilis and Clostridium sporogenes PA3679 to combinations of high hydrostatic pressure and other processing parameters. Innov. Food Sci. Emerg. Technol. 1:49-56. [Google Scholar]
- 42.Ulmer, H. M., M. G. Gänzle, and R. F. Vogel. 2000. Effects of high pressure on survival and metabolic activity of Lactobacillus plantarum TMW 1.460. Appl. Environ. Microbiol. 66:3966-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wuytack, E. Y., and C. W. Michiels. 2001. A study of the effects of high pressure and heat on Bacillus subtilis spores at low pH. Int. J. Food Microbiol. 64:333-341. [DOI] [PubMed] [Google Scholar]
- 44.Wuytack, E. Y., J. Soons, F. Poschert, and C. W. Michiels. 2000. Comparative study of pressure- and nutrient-induced germination of Bacillus subtilis spores. Appl. Environ. Microbiol. 66:257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wuytack, E. Y., S. Boven, and C. W. Michiels. 1998. Comparative study of pressure-induced germination of spores at low and high pressures. Appl. Environ. Microbiol. 64:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]