Abstract
It was hypothesized that the four‐factor prothrombin complex concentrate (4F‐PCC) Kcentra 25 unit/kg would reverse impairment of thrombin generation in healthy volunteers dosed with apixaban to steady state. In this randomized, two‐period crossover, assessor‐blinded trial, 12 healthy subjects received 5 mg apixaban every 12 h. Three h after the fifth dose, four‐factor prothrombin complex concentrate (4F‐PCC) 25 unit/kg or saline were infused. Serial blood samples were assessed for thrombin generation using PPP‐reagent and PPP‐reagent low, anti‐Xa, PT, and PTT assays. Geometric mean ratio was calculated at 30 min postinfusion, and at 24, 48, and 72 h. Peak thrombin generation was 76% higher at 30 min postinfusion with 4F‐PCC (p = 0.025). The difference declined to 24% at 24 h and resolved by 48 h. Other thrombin generation parameters were also partially normalized. There was no difference between 4F‐PCC and saline in anti‐Xa assessment at 30 min or later time points.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Apixaban is a commonly used anticoagulant without a reliable reversal agent in circumstances of acute bleeding. Factor prothrombin concentrate complex products are sometimes used off label in this clinical situation.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ The study sought to evaluate if a four‐factor prothrombin concentrate complex would improve thrombin generation in humans dosed to steady state with apixaban.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE?
✓ The four‐factor prothrombin concentrate complex Kcentra improves thrombin generation parameters compared with placebo.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
✓ This proof of concept finding provides evidence of improvement in hemostatic potential in patients with apixaban use and bleeding.
For more than 50 years, the only class of oral anticoagulants available had been the vitamin K antagonists (VKAs), such as warfarin.1, 2 The direct factor Xa inhibitors apixaban, edoxaban, and rivaroxaban are now clinical options.3, 4 These agents have been shown to be as effective or superior to VKAs in preventing thrombotic events and offer several benefits over the VKAs, which include more predictable pharmacokinetics and pharmacodynamics and fewer food and drug interactions.3, 4, 5 Unlike the VKAs, however, there are no established reversal agents in cases of emergent bleeding for Xa inhibitors.6
Preclinical trials have suggested that prothrombin complex concentrates can reverse the anticoagulant effects caused by factor Xa inhibition. Clinical trials evaluating this effect, however, are scarce. Eerenberg et al.7 demonstrated partial reversal of anticoagulant effects of the factor Xa inhibitor rivaroxaban by the nonactivated four‐factor prothrombin complex concentrate (4F‐PCC) Cofact. In an in vitro study, Escolar et al.8 showed that PCCs have the potential to restore fibrin components of hemostasis previously altered by apixaban. Cofact may be partially beneficial in the reversal of the anticoagulant effect of apixaban. Kcentra currently is the only nonactivated 4F‐PCC available in the United States, and could potentially have some activity against apixaban induced anticoagulation, but has not been systematically evaluated. We studied the effect of Kcentra at the dose of 25 unit/Kg in reversing the anticoagulant effects of therapeutic dose apixaban (5 mg twice daily) in 12 healthy subjects.
METHODS
Study design
This was a phase I, investigator‐initiated, placebo‐controlled, single site, open‐label, assessor blinded, crossover trial to evaluate the reversibility of the anticoagulant effects of apixaban by Kcentra. The study was approved by the Institutional Review Board of Thomas Jefferson University and registered with Clinicaltrials.gov (NCT 02270918). All enrolled subjects received 5 mg of oral apixaban every 12 h (Q12) for two and a half days. Subjects were randomized to receive either Kcentra 25 unit/kg or placebo in the form of normal saline solution intravenously 3 h after the last apixaban dose. Kcentra was infused at a rate of 0.12 mL/kg/min, with a similar duration for matched placebo saline. Blood was collected for analysis of thrombin generation, anti‐factor Xa (anti‐Xa) activity, prothrombin time (PT) and activated partial thromboplastin time (PTT). Blood collection took place at screening and before administration of the last dose of apixaban and at 1, 2, 3 (time of Kcentra/placebo infusion), 3.75 (30 min postinfusion), 4.25, 5, 6, 8, 10, 12, 24, and 72 h post‐apixaban administration. Subjects had a washout period of 10 days and then crossed over to the other treatment arm after another two and a half days of apixaban dosing (Figure 1). Blood for study outcome measures was frozen and stored at −70 C until processing. Staff performing all laboratory assessments were blinded to the treatment allocations. Thrombin generation was performed on a Thrombinoscope (Stago). Anti‐Xa analysis was performed using an ACL TOP 500 instrument (Instrumentation Laboratory).
Figure 1.
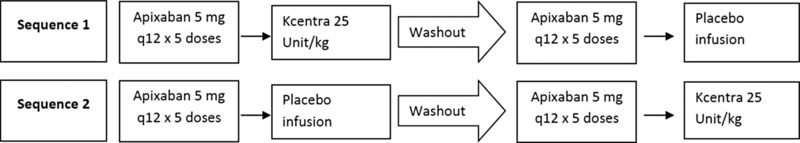
Study design schema.
Statistical methods
Study end points are presented in Table 1. It was anticipated that the administration of apixaban 5 mg Q12 h (estimated in vivo concentration of approximately 0.2 uM) would reduce peak thrombin generation from 250 nM to 50 nM at 30 min postinfusion.9 The sample size of 12 subjects was calculated to provide 80% power to detect the effect size of 0.89 (mean difference of 0.89 if the SD of the differences is 1, using paired t test with alpha 0.05). To investigate the carryover and period effects, the peak thrombin generation measures at 3 and 3.75 h after apixaban dosing (corresponding to before and 30 min postinfusion) in each period were modeled using linear mixed effects (LMEs) models with treatment group, period, and sequence as fixed effects. Statistical significance of the period effects and carryover (sequence effect) was tested using model‐based type III tests of the fixed effects with Kenward & Rodger10 estimated denominator degrees of freedom. The effectiveness of washout was evaluated by testing the difference between day ‐1 and day 13 (corresponding to the day before dosing with apixaban was initiated in each period) using LME model‐based paired t test with alpha 0.05. The residuals and best linear unbiased predictors from all fitted LME models were evaluated for adequacy of the normal distribution assumptions. The time trends in all repeated measures of anticoagulation from time 0 to 72 h were evaluated descriptively. The anticoagulation measured at later times was analyzed in separate LME models with the nominal fixed effect of treatment, time and their interaction. Based on these models, the treatment differences at 24, 48, and 72 h were evaluated. Multiple testing adjustments were not implemented because of the use of a single a priori primary end point and the congruency of methods and biologic mechanisms for the secondary end points.
Table 1.
Study end points
| Assay | Reagent | Time point | |
|---|---|---|---|
| Primary end point | Peak thrombin generation | PPP‐reagent low (Stago) | 30 min after Kcentra/placebo infusion |
| Secondary end points |
|
|
30 min after Kcentra/placebo infusion and 0–72 h |
| Chromogenic anti‐factor Xa | Biophen Heparin (Anaria) | ||
| PT | PT reagent (Instrumentation Laboratory) | ||
| PTT | APTT reagent (Instrumentation Laboratory) |
mVRI, mean velocity rate index; PT, prothrombin time; PTT, partial thromboplastin time.
RESULTS
Twelve nonsmoking healthy male (n = 11) or female (n = 1) subjects were enrolled. The mean age was 46 years (range, 36–51 years), weight 83 kg (range, 60–97 kg), and body mass index was 27 kg/m2 (range, 23–32 kg/m2). All subjects were in good health and had normal platelet counts and baseline coagulation parameters. No subjects used nonsteroidal anti‐inflammatory drugs or other antiplatelet agents, herbal products, or other prescription or over the counter medications. There were no serious adverse effects and all treatments were well tolerated. Thrombin generation geometric mean ratio end points 30 min after infusion of Kcentra or placebo are presented in Table 2. Kcentra infusion increased peak thrombin generation by 76% at 30 min postinfusion with both PPP‐reagent low and PPP‐reagent. PPP‐reagent is a mixture of phospholipids and tissue factor. PPP‐reagent low is a mixture of phospholipids with a low level of tissue factor. Partial reversal of apixaban effect was also demonstrated by increased endogenous thrombin potential, as well as decreased lag time and time to peak thrombin generation. Mean velocity rate index was increased, but there was not a statistically significant difference between treatments. At 30 min post‐Kcentra or placebo infusion, there were no differences in anti‐Xa or PTT values (Table 3). There was a statistically significant shortening of the PT, although all values were within the normal limits. By 24 h, there was largely no difference between the groups (Table 4). With the exception of peak thrombin generation and endogenous thrombin potential, thrombin generation parameters were generally similar between groups by 24 h (Table 5), and began to approach baseline (prestudy) values.
Table 2.
Thrombin generation geometric mean ratio end points 30 min after infusion of Kcentra or placebo
| Reagent | Measure | Kcentra/ placebo GMR | Lower 95% CI | Upper 95% CI | p value |
|---|---|---|---|---|---|
| PPP low | Peak thrombin generation | 1.76 | 1.09 | 2.83 | 0.025 |
| PPP | Peak thrombin generation | 1.76 | 1.19 | 2.60 | 0.009 |
| PPP low | Endogenous thrombin potential | 1.53 | 0.92 | 2.54 | 0.091 |
| PPP | Endogenous thrombin potential | 1.49 | 1.02 | 2.20 | 0.042 |
| PPP low | Lag time | 0.73 | 0.61 | 0.86 | 0.002 |
| PPP | Lag time | 0.72 | 0.62 | 0.85 | 0.001 |
| PPP low | Time to peak thrombin generation | 0.80 | 0.64 | 1.00 | 0.050 |
| PPP | Time to peak thrombin generation | 0.89 | 0.70 | 1.13 | 0.305 |
| PPP low | mVRI | 1.78 | 0.83 | 3.80 | 0.124 |
| PPP | mVRI | 1.62 | 0.91 | 2.89 | 0.094 |
CI, confidence interval; GMR, geometric mean ratio; mVRI, mean velocity rate index.
Table 3.
Anti‐Xa, PT, PTT 30 min after infusion of Kcentra or placebo
| Measure | Kcentra/ placebo GMR | Lower 95% CI | Upper 95% CI | p value |
|---|---|---|---|---|
| Anti‐Xa | 0.98 | 0.82 | 1.16 | 0.761 |
| PT | 0.85 | 0.80 | 0.91 | <0.001 |
| PTT | 1.01 | 0.91 | 1.11 | 0.880 |
CI, confidence interval; GMR, geometric mean ratio; PT, prothrombin time; PTT, partial thromboplastin time.
Table 4.
Anti‐Xa, PT, and PTT at 24, 48, and 72 h
| Measure | Time point | Kcentra/placebo GMR | Lower 95% CI | Upper 95% CI | p value |
|---|---|---|---|---|---|
| Anti‐Xa | 24 | 0.94 | 0.75 | 1.19 | 0.615 |
| Anti‐Xa | 48 | 0.89 | 0.71 | 1.12 | 0.315 |
| Anti‐Xa | 72 | 0.90 | 0.71 | 1.13 | 0.348 |
| PTT | 24 | 1.01 | 0.96 | 1.06 | 0.632 |
| PTT | 48 | 1.04 | 0.99 | 1.09 | 0.100 |
| PTT | 72 | 1.03 | 0.98 | 1.09 | 0.179 |
| PT | 24 | 0.97 | 0.95 | 0.99 | 0.019 |
| PT | 48 | 1.00 | 0.98 | 1.03 | 0.782 |
| PT | 72 | 1.00 | 0.97 | 1.02 | 0.859 |
CI, confidence interval; GMR, geometric mean ratio; PT, prothrombin time; PTT, partial thromboplastin time.
Table 5.
Thrombin generation parameters at 24, 48, and 72 h
| Reagent | Measure | Hour | Kcentra/ Placebo GMR | Lower 95% CI | Upper 95% CI | p‐value |
|---|---|---|---|---|---|---|
| PPP low | Peak thrombin generation | 24 | 1.24 | 0.87 | 1.77 | 0.239 |
| PPP low | Peak thrombin generation | 48 | 0.88 | 0.62 | 1.26 | 0.476 |
| PPP low | Peak thrombin generation | 72 | 0.71 | 0.50 | 1.02 | 0.065 |
| PPP | Peak thrombin generation | 24 | 1.24 | 1.08 | 1.42 | 0.003 |
| PPP | Peak thrombin generation | 48 | 1.09 | 0.95 | 1.25 | 0.210 |
| PPP | Peak thrombin generation | 72 | 1.03 | 0.90 | 1.18 | 0.677 |
| PPP low | ETP | 24 | 1.28 | 1.01 | 1.62 | 0.041 |
| PPP low | ETP | 48 | 0.96 | 0.76 | 1.22 | 0.740 |
| PPP low | ETP | 72 | 0.81 | 0.64 | 1.03 | 0.080 |
| PPP | ETP | 24 | 1.32 | 1.23 | 1.41 | <0.001 |
| PPP | ETP | 48 | 1.20 | 1.12 | 1.28 | <0.001 |
| PPP | ETP | 72 | 1.11 | 1.04 | 1.18 | 0.003 |
| PPP low | Lag time | 24 | 1.08 | 0.97 | 1.21 | 0.168 |
| PPP low | Lag time | 48 | 1.11 | 0.99 | 1.24 | 0.070 |
| PPP low | Lag time | 72 | 1.07 | 0.96 | 1.20 | 0.232 |
| PPP | Lag time | 24 | 1.02 | 0.93 | 1.11 | 0.725 |
| PPP | Lag time | 48 | 1.06 | 0.98 | 1.16 | 0.150 |
| PPP | Lag time | 72 | 1.01 | 0.93 | 1.11 | 0.733 |
| PPP low | Time to peak thrombin generation | 24 | 1.06 | 0.98 | 1.15 | 0.117 |
| PPP low | Time to peak thrombin generation | 48 | 1.11 | 1.03 | 1.20 | 0.008 |
| PPP low | Time to peak thrombin generation | 72 | 1.09 | 1.01 | 1.18 | 0.030 |
| PPP | Time to peak thrombin generation | 24 | 1.04 | 0.98 | 1.10 | 0.184 |
| PPP | Time to peak thrombin generation | 48 | 1.07 | 1.01 | 1.13 | 0.018 |
| PPP | Time to peak thrombin generation | 72 | 1.02 | 0.97 | 1.08 | 0.389 |
| PPP low | mVRI | 24 | 1.19 | 0.75 | 1.89 | 0.451 |
| PPP low | mVRI | 48 | 0.80 | 0.50 | 1.27 | 0.335 |
| PPP low | mVRI | 72 | 0.64 | 0.41 | 1.02 | 0.062 |
| PPP | mVRI | 24 | 1.17 | 0.95 | 1.42 | 0.131 |
| PPP | mVRI | 48 | 1.03 | 0.84 | 1.25 | 0.790 |
| PPP | mVRI | 72 | 1.00 | 0.82 | 1.22 | 0.999 |
CI, confidence interval; ETP, endogenous thrombin potential; GMR, geometric mean ratio; mVRI, mean velocity rate index.
DISCUSSION
Results demonstrate that 25 unit/kg Kcentra reversed the anticoagulant effect of apixaban 5 mg twice daily in healthy human volunteers. The primary end point of the study, peak thrombin generation, increased significantly after treatment with Kcentra compared with placebo. This finding was consistent in both standard (PPP‐reagent) and low tissue factor (PPP‐reagent low) conditions. There was also immediate improvement in the other thrombin generation parameters. Although the optimal level of thrombin generation potential to prevent clinically impactful bleeding is not well defined, our study suggests that Kcentra may be an effective treatment option for a patient on apixaban with moderate to severe and life‐threatening bleeding.
Although Kcentra caused a statistically significant decrease in PT compared with placebo, the pre‐Kcentra treatment samples had a normal PT value in the presence of apixaban. There were no statistically significant differences in the PTT and anti‐Xa levels. These results demonstrate the lack of usefulness of these tests in discerning the amount of drugs in vivo. Newer anti‐Xa assays (Biophen DiXAL) have recently become commercially available that are designed specifically for measurement of the activity of oral anti‐Xa inhibitors with relative insensitivity to heparin concentrations up to 2 unit/mL, and may be more reliable than the anti‐Xa assay (Biophen Heparin) available at the time of this study. The thrombin generation assay may not be readily available in all hospitals. These findings stress the importance of not relying on the use of PT, PTT, or anti‐Xa assays for the decision‐making process as to whether or not to use prothrombin complex concentrates to treat apixaban‐associated bleeding.
Cheung et al.11 reported another use of an unactivated 4F‐PCC (Cofact administered at doses of 25 unit/kg and 37.5 unit/kg) for reversal of anticoagulant effects of apixaban. This study used endogenous thrombin potential (ETP) as their primary end point and demonstrated that Cofact was able to partially reverse the anticoagulant effects of a supratherapeutic dosage regimen of apixaban 10 mg twice daily. We are the first to study the effect of Kcentra, the only commercially available 4F‐PCC in the United States, on reversing the anticoagulant effects of apixaban. Although there are some minor differences between the various available 4F‐PCCs, their efficacy is most likely similar for treatment purposes.12 Our study had 12 subjects compared with the Cofact study, which had six subjects and our primary end point was peak thrombin generation instead of ETP. Patients on standard doses of apixaban would likely have steady‐state drug concentrations similar to the subjects in our study; therefore, our results have clinical relevance for apixaban‐associated bleeding. A 76% increase in immediate peak thrombin generation with 25 unit/kg of Kcentra may be adequate for most moderate to severe bleeding events, without increasing the risk of thrombosis. It is important to emphasize that patients who are taking apixaban may be at increased risk for thrombosis due to their underlying thrombotic condition and excess administration of a PCC can tip the balance toward the thrombotic spectrum and result in adverse events.
Our study is similar to the other studies in this field in terms of lack of assessment of clinical bleeding outcomes in patients. Animal studies demonstrated the benefit of 4F‐PCC in apixaban‐induced bleeding and in vitro studies with apixaban spiked human plasma demonstrated the efficacy of 4F‐PCC in increasing thrombin generation.8, 13, 14, 15 In a recent retrospective study, a 4F‐PCC demonstrated some clinical benefit in reducing intracranial bleeding in patients on direct Xa inhibitors (rivaroxaban and apixaban) without any PCC‐related adverse effects.16 Our study strengthens the observations made by Cheung et al.11 by demonstrating the benefit of PCC administration in human subjects on apixaban. All of the above studies suggest that using a 4F‐PCC in reversing the anticoagulant effects of apixaban in a bleeding patient may be a reasonable option. Based on our study results, we would recommend starting with a dose of 25 unit/kg of Kcentra to treat a moderate to severe life‐threatening bleeding event in a patient on an apixaban regimen of 5 mg or 2.5 mg twice daily with close monitoring to determine clinical response.
Source of Funding
This trial was supported by a grant from Bristol Myers Squibb. Kcentra was supplied by CSL Behring Clinicaltrials.gov number: NCT02270918.
Author Contributions
W.K.K. and Y.O. wrote the manuscript. W.K.K., S.N., and L.T. designed the research. W.K.K., L.T., Y.O., and B.B. performed the research. W.K.K., S.N., and I.C. analyzed the data. B.B. contributed new reagents/analytical tools.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to acknowledge efforts of the staff and study subjects of the Jefferson Clinical Research Unit and Cardeza Foundation. We thank Marie Stuart for use of the Thrombin Generation instrument.
References
- 1. Hirsh, J. et al Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 119 (1 Suppl), 8S–21S (2001). [DOI] [PubMed] [Google Scholar]
- 2. Holbrook, A. et al Evidence‐based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 141 (2 Suppl), e152S–e184S (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumann Kreuziger, L.M. , Morton, C.T. & Dries, D.J. New anticoagulants: a concise review. J. Trauma Acute Care Surg. 73, 983–992 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tahir, F. et al The new oral anti‐coagulants and the phase 3 clinical trials – a systematic review of the literature. Thromb. J. 11, 18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopes, R.D. et al Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am. Heart J. 159, 331–339 (2010). [DOI] [PubMed] [Google Scholar]
- 6. Siegal, D.M. & Cuker, A. Reversal of novel oral anticoagulants in patients with major bleeding. J. Thromb. Thrombolysis 35, 391–398 (2013). [DOI] [PubMed] [Google Scholar]
- 7. Eerenberg, E.S. , Kamphuisen, P.W. , Sijpkens, M.K. , Meijers, J.C. , Buller, H.R. & Levi, M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation 124, 1573–1579 (2011). [DOI] [PubMed] [Google Scholar]
- 8. Escolar, G. et al Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates: significance of studies in vitro with circulating human blood. PLoS One 8, e78696 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong, P.C. , White, A. & Luettgen, J. Inhibitory effect of apixaban compared with rivaroxaban and dabigatran on thrombin generation assay. Hosp. Pract. (1995) 41, 19–25 (2013). [DOI] [PubMed] [Google Scholar]
- 10. Kenward, M.G. & Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53, 983–997 (1997). [PubMed] [Google Scholar]
- 11. Cheung, Y.W. , Barco, S. , Hutten, B.A. , Meijers, J.C. , Middeldorp, S. & Coppens, M. In vivo increase of thrombin generation by four‐factor prothrombin complex concentrate in apixaban‐treated healthy volunteers. J. Thromb. Haemost. 13, 1799–1805 (2015). [DOI] [PubMed] [Google Scholar]
- 12. Dzik, W.H. Reversal of oral factor Xa inhibitors by prothrombin complex concentrates: a re‐appraisal. J. Thromb. Haemost. 13, (Suppl 1), S187–S194 (2015). [DOI] [PubMed] [Google Scholar]
- 13. Herzog, E. et al Correlation of coagulation markers and 4F‐PCC‐mediated reversal of rivaroxaban in a rabbit model of acute bleeding. Thromb. Res. 135, 554–560 (2015). [DOI] [PubMed] [Google Scholar]
- 14. Martin, A.C. et al Multimodal assessment of non‐specific hemostatic agents for apixaban reversal. J. Thromb. Haemost. 13, 426–436 (2015). [DOI] [PubMed] [Google Scholar]
- 15. Martin, A.C. et al Evaluation of recombinant activated factor VII, prothrombin complex concentrate, and fibrinogen concentrate to reverse apixaban in a rabbit model of bleeding and thrombosis. Int. J. Cardiol. 168, 4228–4233 (2013). [DOI] [PubMed] [Google Scholar]
- 16. Grandhi, R. et al Administration of 4‐factor prothrombin complex concentrate as an antidote for intracranial bleeding in patients taking direct factor Xa inhibitors. World Neurosurg. 84, 1956–1961 (2015). [DOI] [PubMed] [Google Scholar]


