Abstract
Phototherapy has been widely used in treating neonatal jaundice, but detailed metabonomic profiles of neonatal jaundice patients and response to phototherapy have not been characterized. Our aim was to depict the serum metabolic characteristics of neonatal jaundice patients relative to controls and changes in response to phototherapy. A 1H nuclear magnetic resonance (NMR)‐based metabonomic approach was employed to study the metabolic profiling of serum from healthy infants (n = 25) and from infants with neonatal jaundice (n = 30) pre‐ and postphototherapy. The acquired data were processed by multivariate principal component analysis (PCA) and orthogonal partial least‐squares‐discriminant analysis (OPLS‐DA). The PLS‐DA and OPLS‐DA model identified nine metabolites capable of distinguishing patients from controls. In addition, 28 metabolites such as β‐glucose, α‐glucose, valine, and pyruvate changed in response to phototherapy. This study offers useful information on metabolic disorders in neonatal jaundice patients and the effects of phototherapy on lipids, amino acid, and energy metabolism.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ A 1H nuclear magnetic resonance (NMR)‐based metabonomic approach was employed to study the metabolic profiling of serum from healthy infants and from infants with neonatal jaundice pre‐ and postphototherapy.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ The study exhibited the biochemical metabolites in the neonatal jaundice infants and illustrated the serum metabolic characteristics of neonatal jaundice in response to phototherapy.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE?
✓ The significant metabolites offer useful information to elucidate the detailed mechanism related to lipids, amino‐acid, and energy metabolism occurred during phototherapy.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
✓ Twenty‐eight metabolites such as β‐glucose, α‐glucose, valine, and pyruvate were found as biomarkers for neonatal jaundice in response to phototherapy, and further elucidated the detailed the mechanism of phototherapy.
Jaundice is the most common disease in newborn infants. It has been estimated that ∼60% of term babies and 80% of preterm babies develop jaundice in their first week of life.1, 2 Phototherapy is generally considered a safe and well‐tolerated therapy in neonatal jaundice. It can convert bilirubin into water‐soluble isomers that are easily eliminated through the gastrointestinal tract or lost in urine.3, 4 Bilirubin absorbs light most strongly in the blue region of the spectrum near 460 nm wavelength. Gallium nitride light‐emitting diodes (LEDs), which deliver high‐intensity light of a narrow wavelength spectrum, has been developed and is increasingly being used for neonatal jaundice.5, 6
Although phototherapy is an effective treatment for neonatal jaundice, few biomarkers are employed to evaluate the effectiveness of phototherapy. The guidelines for using phototherapy that were published in 2004 by the American Academy of Pediatrics are based primarily on the total serum bilirubin (TSB) levels.7 The commonly used criteria for the treatment of neonatal jaundice are based on measurements of TSB. The most widely used chemical methods for bilirubin measurement are those based on the coupling of bilirubin with a diazo compound or on the oxidation of bilirubin with bilirubin oxidase to biliverdin with molecular oxygen. They both suffer from hemolysis interference.8 We therefore evaluated the whole metabonomics in serum of neonatal jaundice before and after phototherapy to elucidate the dynamic effects of phototherapy.
METHODS
The study protocol was approved by the Ethics Committee of Guangdong Medical College. The Declaration of Helsinki on ethical principles for medical research involving human subjects was adhered to throughout the study.
Patients
Thirty neonatal jaundice patients (including 18 males and 12 females) and 25 healthy infants (including 14 males and 11 females) who served as controls participated in this study. All infants with gestation ages more than 35 weeks were enrolled from the Department of Newborn of Nanshan Affiliated Hospital of Guangdong Medical College, Shenzhen, Guangdong, P.R. China, from March 2011 to November 2012. Criteria for the intervention and starting phototherapy were based on the days of age and the level of TSB defined by the Society of Pediatrics of Chinese Medical Association.9 An overhead LED phototherapy system (Tende Electronics & Software, Ankara, Turkey; intensity: 30–120 μW/cm2/nm, spectrum 400–470 nm) was used. None of the infants in this study exhibited any other apparent clinical signs and did not receive other medical interventions. Blood samples were collected from the peripheral vein, separated by centrifugation at 3,000 rpm for 5 min, and stored at –80°C in the dark until analysis.
1H NMR spectroscopic analysis of samples
Before nuclear magnetic resonance (NMR) experimentation, serum samples were thawed at room temperature for no longer than 20 min and 400 μL aliquots were combined with 100 μL of saline (0.9% NaCl in 10% D2O/90% H2O) and centrifuged at 12,000g for 5 min. A 400‐μL aliquot of this solution was put into a 5‐mm NMR tube for NMR analysis.
1H NMR analysis was performed on a Varian Unity INOVA 600 NMR spectrometer (Palo Alto, CA) operating at a frequency of 599.93 Hz. All experiments were run with 128 scans and a recycle time delay of 2.1 s to ensure a steady state of recovered magnetization and 64k time domain points. A Carr‐Purcell‐Meiboom‐Gill sequence was used to record the one‐dimensional spectra with a relaxation delay of 100 ms.
Multivariate analysis
All spectra were Fourier transformed and phased with TOPSPIN software (v. 3.0, Bruker Biospin, Germany). The data were analyzed using SIMCA‐P 11.0 software (Umetrics, Umea, Sweden) for multivariate statistics analysis. Analysis of metabolite signals in the 1H NMR serum profiles were first examined by unsupervised principal component analysis (PCA), which reduced the dimensionality of data and summarized the similarities and differences between multiple NMR spectra using score plots.
Subsequently, we obtained a more sophisticated orthogonal partial least‐squares‐discriminant analysis (OPLS‐DA) model to get the specific discriminant information between prephototherapy jaundice and controls, as well as pre‐ and postphototherapy changes.10, 11 The differences in the metabolites between groups were shown as coefficient of variation plots. In the correlation coefficients graph, the square root of each variable of the loading value and the value of standard deviation multiplied had a retrospective conversion. Then the data were compared with the corresponding correlation coefficient critical value table to obtain the significant metabolites between the two groups. Statistical analyses were performed using SPSS11.5 software (SPSS, Chicago, IL). The threshold P value was set at 0.05 throughout the study.
RESULTS
PCA
The clinical and laboratory data of neonatal jaundice infants and controls are shown in Table 1. Figure 1 displays the 2D PCA score plot of the three groups, pretherapy, posttherapy, and control groups. Although some overlap existed among samples from the three groups, this model explained more than half of the variability among the samples with an R2X of 0.824, an R2Y of 0.895, with a predictability value of Q2Y of 0.807.
Table 1.
Clinical and laboratory data of study groups
| Healthy controls (n = 25) | Neonatal jaundice infants (n = 30) | |
|---|---|---|
| BW (gr) | 3,152 ± 432 | 3,086 ± 398 |
| GA (week) | 38 (37–41) | 38 (37–40) |
| Sex (F/M) | 11/14 | 12/18 |
| Age at the beginning of therapy (h) | 145 (39–146) | 138 (37–134) |
| STB at the beginning of therapy (mg/dL) | 2.64 ± 0.26 | 18.23 ± 3.57* |
| TSB after 24 h therapy (mg/dL) | — | 3.07 ± 1.06** |
BW, birth weight, data presented as mean ± SD. GA, gestational age, data presented as median min–max. TSB, Total serum bilirubin, data presented as mean ± SD. Statistics: *P < 0.05, compared with healthy controls, **P < 0.05, compared with STB at the beginning of therapy.
Figure 1.
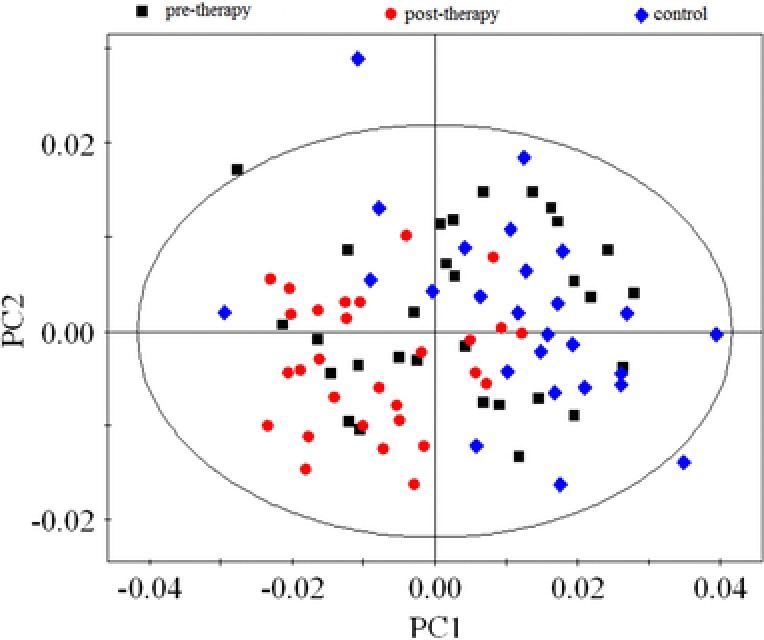
The PCA score plot based on the1H‐NMR spectra of serum samples. Black squares represent the pretherapy patients, red dots represent posttherapy patients, and blue diamonds represent the control group.
OPLS‐DA
Obvious separation was observed between controls and neonatal jaundice patients (prephototherapy) in the OPLS‐DA score plot, with an R2X of 0.287, an R2Y of 0.843, and a Q2Y of 0.698 (Figure 2 a). The corresponding coefficient loading plots (Figure 2 b) revealed the metabolites that are mainly responsible for separating the controls from the prephototherapy patients. According to the cutoff value of the correlation coefficient, (i.e., |correlation coefficient| > 0.301), prephototherapy neonatal jaundice patients showed increased valine, myo‐inositol, lysine, leucine, lactate, isoleucine, glycine creatine, alanine, and decreased LDL(CH3‐(CH2)n), as shown in Table 2.
Figure 2.
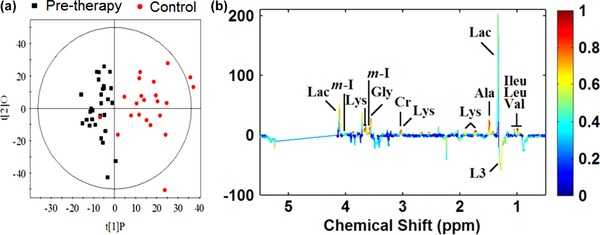
(a) OPLS‐DA score plots obtained from pretherapy neonatal jaundice patients (black squares) and control groups (red dots). (b) The corresponding coefficient loading plots for the discrimination of the OPLS‐DA model shown in (a).
Table 2.
OPLS‐DA coefficients derived from the NMR data of metabolites in serum obtained from different groups
| ra | |||
|---|---|---|---|
| Metabolites | δ1H | Postphototherapy vs. prephototherapy | prephototherapy vs. controls |
| β‐Glucose | 3.25, 3.41, 3.46, 3.49, 3.90, 4.65 | –0.666 | — |
| α‐Glucose | 3.42, 3.54, 3.71, 3.73, 3.84, 5.24 | –0.658 | — |
| Valine | 0.99, 1.04 | –0.809 | 0.811 |
| Tyrosin | 6.89, 7.19 | –0.387 | — |
| Trimethylamine | 2.83 | –0.402 | — |
| Pyruvate | 2.37 | –0.346 | — |
| Phosphocholine | 3.21 | –0.435 | — |
| myo‐Inositol | 3.64, 4.07 | –0.535 | 0.726 |
| Lysine | 1.72, 1.91, 3.02, 3.76 | –0.720 | 0.713 |
| Leucine | 0.96 | –0.805 | 0.533 |
| Lactate | 1.33, 4.11 | 0.599 | 0.592 |
| Lipid, =CH‐CH 2‐CH= | 2.78 | 0.408 | — |
| Lipid, ‐CH 2‐C=O | 2.25 | 0.433 | — |
| Lipid, ‐CH2‐CH=CH‐ | 2.02 | 0.519 | — |
| VLDL, CH3‐(CH 2)n‐ | 1.29 | 0.517 | — |
| LDL, CH3‐(CH 2)n‐ | 1.27 | 0.715 | –0.579 |
| VLDL, CH 3‐(CH2)n‐ | 0.89 | 0.664 | — |
| LDL, CH 3‐(CH2)n‐ | 0.87 | 0.565 | — |
| Isoleucine | 0.96, 1.01 | –0.797 | 0.575 |
| Glycerolphosphocholine | 3.22 | –0.354 | — |
| Glycine | 3.57 | 0.416 | 0.667 |
| Glutamine | 2.14, 2.45, 3.78 | 0.358 | — |
| Glutamate | 2.10, 2.35, 3.78 | –0.541 | — |
| Creatine | 3.04, 3.93 | — | 0.677 |
| Alanine | 1.48 | –0.620 | 0.719 |
| Acetone | 2.23 | 0.524 | — |
| Acetoacetate | 2.28 | 0.364 | — |
| 3‐Hydroxybutyrate | 1.20 | 0.541 | — |
| 1‐Methylhistidine | 7.05, 7.77 | –0.454 | — |
δ1H, chemical shift of 1H.
Correlation coefficients, positive and negative signs indicate positive and negative correlation in the concentrations, respectively. The correlation coefficient of│r│> 0.301 or 0.330 was used as the cutoff value for the statistical significance based on the discrimination significance at the level of P = 0.05 and df (degrees of freedom) = 29 (prephototherapy vs. controls) or 24 (postphototherapy vs. prephototherapy), respectively. ‘‘—’’ means the correlation coefficient│r│ is less than the cutoff value.
OPLS‐DA modeling revealed a clear separation between the pre‐ and postphototherapy profile with an R2X of 0.238, an R2Y of 0.809 and a Q2Y of 0.753 (Figure 3 a). Postphototherapy metabolic profiles showed that the main metabolites changing were β‐glucose, α‐glucose, valine, tyrosine, phosphocholine, pyruvate, trimethylamine, etc. (Figure 3 b). According to the cutoff value of the correlation coefficient (i.e. |correlation coefficient| > 0.330), 28 metabolites were found to discriminate prephototherapy and postphototherapy patients (Table 2).
Figure 3.
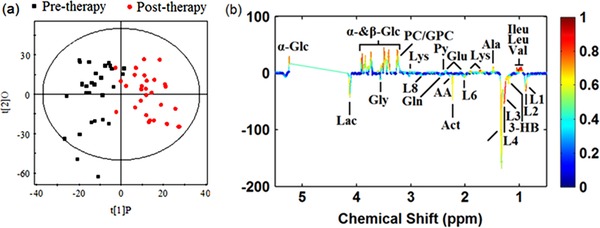
(a) OPLS‐DA score plots obtained from pretherapy neonatal jaundice patients (black squares) and posttherapy neonatal jaundice patients (red dots). (b) The corresponding coefficient loading plots for the discrimination of the OPLS‐DA model shown in (a).
DISCUSSION
The level of TSB is used as a criterion for the diagnosis of neonatal jaundice patients and evaluating the effectiveness of phototherapy.12 In the present study we applied NMR‐based metabolic profiling to examine the serum from neonatal jaundice patients who underwent phototherapy and identified some new low‐molecular‐weight biomarkers.
Comparison the metabolic profiling in neonatal jaundice and healthy controls
As shown in Table 2, compared with the healthy controls, neonatal jaundice patients showed increased valine, myo‐Inositol, lysine, leucine, lactate, isoleucine, alanine, creatine, and glycine, as well as decreased low‐density lipoprotein cholesterol (LDL). The elevated levels of valine, leucine, lysine, isoleucine, and alanine reflected the abnormal metabolism of amino acids in neonatal jaundice patients. It is well known that lactate is a product of glycolysis; more recently, scientists have reported that lactate changes were found in severe neonatal hyperbilirubinemia patients.13 Creatine is a nitrogen‐containing compound synthesized mainly in the liver, and it serves as an energy shuttle between the mitochondrial sites of adenosine triphosphate (ATP) production and the cytosol.14 We conjectured that the observed higher levels of creatine might result from hepatic cell dysfunction.
In addition, neonatal jaundice patients showed decreased LDL. Previous studies have showed that bilirubin has antiatherogenic properties and is negatively correlated with lipid level in serum.15 In brief, the findings of this pilot study showed that the mebabolites mentioned above may be used as potential biomarkers in the diagnostic assessment of neonatal jaundice.
Comparison the metabolic profiling in the prephototherapy and postphototherapy groups
In the present study, 28 metabolites were altered in postphototherapy neonatal serum compared with prephototherapy, 13 metabolites increased, notably lactate and lipid, and 15 metabolites decreased, notably glucose and some amino acids; most of these metabolites are mainly associated with lipid, amino acid, and glucose metabolism.
The levels of β‐glucose and α‐glucose were obviously decreased (r values –0.666 and –0.658, respectively) in the serum of neonatal jaundice patients after phototherapy, indicating that phototherapy directly affects neonatal energy metabolism. It should be noted that these findings are exploratory. Metabolic changes in healthy infants were not evaluated. Therefore, metabolic changes in the phototherapy group may be the result of postnatal maturation or feeding.
An earlier study found that phototherapy can result in an increase in peripheral blood flow, which can consume energy and lower the glucose levels.16 This may explain the decreased β‐glucose and α‐glucose levels in the postphototherapy group. Pyruvate levels were reduced and lactate levels were increased. Lactate (r value 0.599) is the end product of glycolysis and pyruvate is an intermediate product of glycolysis produced before lactate. Pyruvate (r value –0.346) may produce more ATP through the glycolytic pathway, which results in the increased lactate when the body has an extreme demand for energy. On the other hand, in the infant phase, the body has an important ability to maintain glucose homeostasis.17, 18 The aforementioned metabolites showed that phototherapy resulted in decreased β‐glucose and α‐glucose levels, which may cause the neonatal body to increase gluconeogenesis to resist the reduced blood glucose. In our study, the level of glucogenic amino acids including valine, lysine, leucine, isoleucine, and alanine were reduced and we speculate that these amino acids were converted to glucose to maintain the glucose homeostasis. In addition, as an isomer of glucose widely distributed in tissues, myo‐inositol maintained a lower level. Therefore, we think phototherapy can not only accelerate the metabolism of bilirubin, but also lead to altered glucose metabolism.
Our study also showed that the serum levels of lipids, such as VLDL (r values 0.517, 0.664) and LDL (r values 0.715, 0.565), markedly increased in postphototherapy compared with prephototherapy. The results in the postphototherapy group agreed with the studies of Yoshino et al.,19 which showed that TSB was inversely associate with LDL and VLDL. In our study, the markedly decreased levels of lipids such as VLDL and LDL in neonatal jaundice patients postphototherapy may be caused by photodynamic stress, which can induce lipid peroxidation. However, Bhuiyan et al.20 observed that the bilirubin level correlated positively with the HDL‐C level, and we did not observe an increase in HDL in the postphototherapy group; this result may be due to the small numbers of participants in our study.
CONCLUSION
Bilirubin can be converted into water‐soluble photoisomerization when exposed to light. However, the metabolism of the photoisomers is not known in detail. In our research, we applied an NMR‐based metabonomic approach to study the metabolic profiling of serum from infants with neonatal jaundice; the significant metabolite differences may provide some potential clues to elucidate the detailed mechanisms of action of the effect of phototherapy and serve to inform future studies of additional complementary biomarkers to diagnose and monitor treatment. More research on follow‐up measurement in the healthy infants is needed.
Author Contributions
A.C. wrote the article; Y.D. and A.C. designed the research; Z.S., H.S, Y.Y, and W.C. performed the research; Y.D., A.C., S.Q., and Z.S. analyzed the data; Y.D. and S.Q. contributed new reagents/analytical tools.
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgments
This work is supported by Natural Science Foundation of China (No. 81401750) and Science and technology project of Shenzhen city (No.CYJ20140415093052190).
References
- 1. Sun, H.L. , Lue KHKu MS:Neonatal jaundice is a risk factor for childhood allergic rhinitis: a retrospective cohort study. Am. J. Rhinol. Allergy 27, 192–196 (2013). [DOI] [PubMed] [Google Scholar]
- 2. Riskin, A. , Cohen, K. , Kugelman, A. , Abend‐Weinger, M. & Hemo MBader, D. Influence of changes in the evaluation of neonatal jaundice. Am. J. Perinatol. 31, 203–208 (2014). [DOI] [PubMed] [Google Scholar]
- 3. Wickremasinghe, A.C. , Karon, B.S. , Saenger, A.K. & Cook, W.J. Effect of universal neonatal transcutaneous bilirubin screening on blood draws for bilirubin analysis and phototherapy usage. J. Perinatol. 32, 851–855 (2012). [DOI] [PubMed] [Google Scholar]
- 4. Stokowski, L.A. Fundamentals of phototherapy for neonatal jaundice. Adv. Neonatal Care 11, S10–S21 (2011). [DOI] [PubMed] [Google Scholar]
- 5. Kale, Y. et al Effects of phototherapy using different light sources on oxidant and antioxidant status of neonates with jaundice. Early Hum. Dev. 89, 957–960 (2013). [DOI] [PubMed] [Google Scholar]
- 6. Sivanandan, S. , Chawla, D. , Misra, S. , Agarwal, R. & Deorari, A.K. Effect of sling application on efficacy of phototherapy in healthy term neonates with nonhemolytic jaundice: a randomized conrolled trial. Indian Pediatr. 46, 23–28 (2009). [PubMed] [Google Scholar]
- 7. American Academy of Pediatrics Subcommittee On H Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297–316 (2004). [DOI] [PubMed] [Google Scholar]
- 8. Romagnoli, C. et al Task Force for Hyperbilirubinaemia of the Italian Society Of N Italian guidelines for management and treatment of hyperbilirubinaemia of newborn infants >/= 35 weeks' gestational age. Ital. J. Pediatr. 40, 11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Editorial Board of Chinese Journal of Pediatrics and the Subspecialty Group of Neonatology and the Society of Pediatrics CMA Expert consensus on the principles of diagnosis and treatment of neonatal jaundice. Chin. J. Pediatr. 48, 685–686 (2010). [PubMed] [Google Scholar]
- 10. Ni, Y. et al Metabolic profiling reveals disorder of amino acid metabolism in four brain regions from a rat model of chronic unpredictable mild stress. FEBS Lett. 582, 2627–2636 (2008). [DOI] [PubMed] [Google Scholar]
- 11. Ayshamgul, H. , Batur MIlyar, S. [1H‐MRS metabonomic analysis of plasma samples of esophageal cancer patients based on different pattern recognition]. Zhonghua Zhong Liu Za Zhi 32, 681–684 (2010). [PubMed] [Google Scholar]
- 12. Ichinomiya, K. , Inoue, F. , Koizumi, A. , Inoue, T. & Fujiu TMaruyama, K. Problems with using total serum bilirubin as a criterion for phototherapy in extremely low‐birthweight infants. Pediatr. Int. (2014). [DOI] [PubMed] [Google Scholar]
- 13. Franchini, M. , Targher, G. & Lippi, G. Serum bilirubin levels and cardiovascular disease risk: a Janus Bifrons? Adv. Clin. Chem. 50, 47–63 (2010). [DOI] [PubMed] [Google Scholar]
- 14. Pazourek, J. Fast separation and determination of free myo‐inositol by hydrophilic liquid chromatography. Carbohydr. Res. 391, 55–60 (2014). [DOI] [PubMed] [Google Scholar]
- 15. Franchini, M. , Targher, G. & Lippi, G. Serum bilirubin levels and cardiovascular disease risk: a Janus Bifrons? Adv. Clin. Chem. 50, 47–63 (2010). [DOI] [PubMed] [Google Scholar]
- 16. Maayan‐Metzger, A. , Yosipovitch, G. , Hadad, E. & Sirota, L. Transepidermal water loss and skin hydration in preterm infants during phototherapy. Am. J. Perinatol. 18, 393–396 (2001). [DOI] [PubMed] [Google Scholar]
- 17. Adamkin, D.H. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J. Perinatol. 29 (Suppl 2), S12–S17 (2009). [DOI] [PubMed] [Google Scholar]
- 18. Kalhan, S.C. et al Estimation of gluconeogenesis in newborn infants. Am. J. Physiol. Endocrinol. Metab. 281, E991–E997 (2001). [DOI] [PubMed] [Google Scholar]
- 19. Yoshino, S. et al Characterization of the effect of serum bilirubin concentrations on coronary endothelial function via measurement of high‐sensitivity C‐reactive protein and high‐density lipoprotein cholesterol. Heart Vessels 28, 157–165 (2013). [DOI] [PubMed] [Google Scholar]
- 20. Bhuiyan, A.R. , Srinivasan, S.R. , Chen, W. , Sultana, A. & Berenson, G.S. Association of serum bilirubin with pulsatile arterial function in asymptomatic young adults: the Bogalusa Heart Study. Metabolism 57, 612–616 (2008). [DOI] [PubMed] [Google Scholar]


