Abstract
16S rRNA gene-targeted group-specific primers were designed and validated for specific detection and quantification of the Clostridium leptum subgroup and the Atopobium cluster. To monitor the predominant bacteria in human feces by real-time PCR, we used these specific primers together with four sets of group-specific primers for the Clostridium coccoides group, the Bacteroides fragilis group, Bifidobacterium, and Prevotella developed in a previous study (T. Matsuki, K. Watanabe, J. Fujimoto, Y. Miyamoto, T. Takada, K. Matsumoto, H. Oyaizu, and R. Tanaka, Appl. Environ. Microbiol. 68:5445-5451, 2002). Examination of DNA extracted from the feces of 46 healthy adults showed that the C. coccoides group was present in the greatest numbers (log10 10.3 ± 0.3 cells per g [wet weight] [average ± standard deviation]), followed by the C. leptum subgroup (log10 9.9 ± 0.7 cells per g [wet weight]), the B. fragilis group (log10 9.9 ± 0.3 cells per g [wet weight]), Bifidobacterium (log10 9.4 ± 0.7 cells per g [wet weight]), and the Atopobium cluster (log10 9.3 ± 0.7 cells per g [wet weight]). These five bacterial groups were detected in all 46 volunteers. Prevotella was found in only 46% of the subjects at a level of log10 9.7 ± 0.8 cells per g (wet weight). Examination of changes in the population and the composition of the intestinal flora for six healthy adults over an 8-month period revealed that the composition of the flora of each volunteer remained stable throughout the test period.
The human intestinal tract harbors a large, active, and complex community of microbes. The intestinal microflora plays significant roles in the digestion of food, the metabolism of endogenous and exogenous compounds, immunopotentiation, and the prevention of colonization of the gastrointestinal tract by pathogens and hence is involved in maintaining human health (6, 31). To understand the relationship between the intestinal flora and human health, it is important to develop an accurate method to analyze the various microbial populations.
The microflora of the gut has been monitored in great detail by cultivation-based techniques (3, 20), but limitations inherent in these techniques and the development of more sensitive and accurate molecular detection methods have brought new insights to the field (35). In complex mixed populations, 16S rRNA-targeted oligonucleotide probes have been used with fluorescent in situ hybridization (FISH) as a culture-independent method (5, 7, 13). Techniques such as the clone library method and temperature gradient gel electrophoresis allow analysis of predominant bacteria that are difficult to culture (9, 10, 28, 36). Currently, PCR with 16S rRNA-based specific primers has been applied to flora analysis as the most sensitive and rapid method. So far, specific oligonucleotide primers have been designed for many bacterial species known to be present in the intestinal tract (12, 18, 19, 27, 33, 34). Although conventional PCR does not permit quantitative detection of target bacteria, real-time PCR with species-specific primers can provide precise quantification through measurement of SYBR Green I fluorescence to determine the amounts of PCR products in each cycle (11, 14, 16, 17, 24, 29).
For analysis of the human gut microflora, it is useful to prepare specific primers not only for species but also for major genera and groups due to the complexity of this ecosystem. To date, group-specific primers for the Clostridium coccoides group, the Bacteroides fragilis group, Bifidobacterium, and Prevotella have been developed and applied to the analysis of the human intestinal microflora (18).
In the present study, we developed new sets of primers to detect the Clostridium leptum subgroup and the Atopobium cluster. After validation of their specificity, these primers were used with previously developed group-specific primers to quantify populations in fecal samples from 46 healthy individuals by real-time PCR analysis. The changes in the intestinal floras of six healthy adults during an 8-month period were also examined.
MATERIALS AND METHODS
Development of 16S rRNA gene-targeted group-specific primers.
Through the use of 16S rRNA sequences obtained from the DDBJ/GenBank/EMBL database, multiple alignments of the target groups and reference organisms were constructed by using the program Clustal X (32). After comparison of sequences unique to groups with large numbers of reference strains, potential target sites for specific detection were identified (Table 1). The specificity of the primers shown in Table 2 was then checked with the database by submitting the sequences to the Check Probe program of the Ribosomal Database Project (www.cme.msu.edu/RDP) (15).
TABLE 1.
Partial 16S rRNA gene sequences of reference organisms with group-specific primers
| Organism or sitea | Forward primer
|
Reverse primer
|
||
|---|---|---|---|---|
| Designation | Sequenceb | Designation | Sequenceb | |
| C. leptum subgroup-specific primers | sg-Clept-F | 5′ GCACAAGCAGTGGAGT 3′ | sg-Clept-R3 | 3′ AACTGTTTTGCCTCCTTC 5′ |
| Target site | 5′ GCACAAGCAGTGGAGT 3′ | 5′ TTGACAAAACGGAGGAAG 3′ | ||
| Clostridium cellulosic | ................. | ....T............. | ||
| Clostridium leptumc | ................. | .................. | ||
| Clostridium orbiscindensc | ........G........ | .................. | ||
| Clostridium sporosphaeroidesc | ........N........ | .................. | ||
| Clostridium viridec | ........G........ | .................. | ||
| Eubacterium desmolansc | ................. | .................. | ||
| Eubacterium siraeumc | ................. | .................. | ||
| Faecalibacterium prausnitziic | ................. | .................. | ||
| Ruminococcus albusc | ................. | .................. | ||
| Ruminococcus bromiic | ................. | .................. | ||
| Ruminococcus callidusc | ................. | .................. | ||
| Ruminococcus flavefaciensc | ................. | .................. | ||
| Clostridium coccoides | ........G.......C | .GG..TN.CC........ | ||
| Bifidobacterium longum | ........G.C.....C | .GG.TT..CT........ | ||
| Collinsella aerofaciens | ..........C.....C | .CC.T...GG-.....G. | ||
| Bacteroides vulgatus | ........G.A.....C | ..C.TA.G.TGT...... | ||
| Atopobium cluster-specific primers | c-Atopo-F | 5′ GGGTTGAGAGACCGACC 3′ | c-Atopo-R | 3′ GGACGTCTTCTTCGRGGC 5′ |
| Target site | 5′ GGGTTGAGAGACCGACC 3′ | 5′ CCTGCAGAAGAAGCYCCG 3′ | ||
| Atopobium minutumc | ................. | .................. | ||
| Atopobium fossorc | ................. | .................. | ||
| Atopobium rimaec | ................. | .................. | ||
| Atopobium parvulumc | ................. | .................. | ||
| Collinsella aerofaciensc | ................. | .................. | ||
| Collinsella intestinalisc | ................. | .................. | ||
| Collinsella stercorisc | ................. | .................. | ||
| Coriobacterium sp. strain EKSO3c | ................. | .................. | ||
| Coriobacterium glomeransc | ................A | .................. | ||
| Eggerthella lentac | ................. | .................. | ||
| Cryptobacterium curtumc | ................. | .................. | ||
| Slackia heliotrin reducens | ...C......G.T...T | .................. | ||
| Denitrobacterium detoxificans | ..AC......GT....T | .................. | ||
| Bifidobacterium longum | ..CC......GG..... | ..C.TT...T....A... | ||
| Bacteroides vulgatus | .TTC......GAA..GT | .T.TAT...T...GAT.. | ||
| Clostridium coccoides | ..CC......GGT...A | ....ACTN.......... | ||
| Faecalibacterium prausnitzii | ..AC......GTT...A | .TCAACA.G....TGA.. | ||
The positions of the target sites for the primers are as follows (numbering based on the Escherichia coli 16S rRNA gene sequence): sg-Clept-F, nucleotides 933 to 948; sg-Clept-R3, nucleotides 1164 to 1181; c-Atopo-F, nucleotides 292 to 308; and g-Atopo-R, nucleotides 488 to 505.
Only nucleotides that differ from nucleotides in the target sequences are shown.
Targeted organism.
TABLE 2.
16S rRNA gene-targeted group-specific primers used in this study
| Target bacterial group | Primer | Sequence | Size (bp)a | Annealing temp (°C) | Reference |
|---|---|---|---|---|---|
| Clostridium coccoides group | g-Ccoc-F | AAATGACGGTACCTGACTAA | 440 | 50 | 18 |
| g-Ccoc-R | CTTTGAGTTTCATTCTTGCGAA | ||||
| Clostridium leptum subgroup | sg-Clept-F | GCACAAGCAGTGGAGT | 239 | 50 | This study |
| sg-Clept-R3 | CTTCCTCCGTTTTGTCAA | ||||
| Bacteroides fragilis group | g-Bfra-F | ATAGCCTTTCGAAAGRAAGAT | 495 | 50 | 18 |
| g-Bfra-R | CCAGTATCAACTGCAATTTTA | ||||
| Bifidobacterium | g-Bifid-F | CTCCTGGAAACGGGTGG | 550 | 55 | 18 |
| g-Bifid-R | GGTGTTCTTCCCGATATCTACA | ||||
| Atopobium cluster | c-Atopo-F | GGGTTGAGAGACCGACC | 190 | 55 | This study |
| c-Atopo-R | CGGRGCTTCTTCTGCAGG | ||||
| Prevotella | g-Prevo-F | CACRGTAAACGATGGATGCC | 513 | 55 | 18 |
| g-Prevo-R | GGTCGGGTTGCAGACC | 513 | 55 | 18 |
DNAs extracted from Ruminococcus productus YIT 6141T, Faecalibacterium prausnitzii YIT 6174, Bacteroides vulgatus YIT 6159T, Bifidobacterium longum YIT 4021T, Collinsella aerofaciens ATCC 25986T, and Prevotella melaninogenica YIT 6039T were used as real-time PCR controls.
Reference strains and culture conditions.
Seven strains of the C. leptum subgroup, five strains of the Atopobium cluster, seven strains of Bacteroides, nine strains of Bifidobacterium, eight strains of the C. coccoides group, seven species of Prevotella, and 18 species of a disparate cluster were used in this study (Table 3). These organisms were obtained from the Culture Collection of the Yakult Central Institute (Tokyo, Japan) (YIT). The strains were cultured anaerobically in GAM broth (Nissui Seiyaku, Tokyo, Japan) supplemented with 1% glucose at 37°C for 12 to 48 h. The numbers of cells of the strains listed in Table 4 were counted microscopically by the DAPI (4′,6′-diamidino-2-phenylindole) staining method as described previously (17). Serial 10-fold dilutions of the cultures were also plated on nonselective GAM agar (Nissui Seiyaku). The plates were subsequently incubated at 37°C for 3 to 5 days in an anaerobic chamber (Coy Laboratory Products Inc., Grass Lake, Mich.), and cultural counts (in CFU) were determined in triplicate.
TABLE 3.
Specificity test of group-specific primers for the C. leptum subgroup and the Atopobium cluster
| Speciesa | Strain | Other designation | PCR resultsb
|
|
|---|---|---|---|---|
| sg-Clept | c-Atopo | |||
| Clostridium leptum | YIT 6169T | DSM 753T | + | − |
| Clostridium viride | YIT 10050T | DSM 6836T | + | − |
| Eubacterium siraeum | YIT 10049T | DSM 3996T | + | − |
| Faecalibacterium prausnitzii | YIT 6174 | ATCC 27766 | + | − |
| Ruminococcus albus | YIT 6083T | ATCC 27210T | + | − |
| Ruminococcus bromii | YIT 6078T | ATCC27255T | + | − |
| Ruminococcus callidus | YIT 6175T | ATCC 27760T | + | − |
| Atopobium parvulum | YIT 10055T | JCM 10300T | − | + |
| Collinsella aerofaciens | ATCC 25986T | ATCC 25986T | − | + |
| Collinsella intestinalis | YIT 10051T | JCM 10643T | − | + |
| Collinsella stercoris | YIT 10052T | JCM 10641T | − | + |
| Eggerthella lenta | YIT 6077T | ATCC 25559T | − | + |
In addition, negative PCR results with primers sg-Clept and g-Atopo were obtained for the following bacterial species: Bacteroides fragilis YIT 6158T (= ATCC 25285T), Bacteroides ovatus YIT 6161T (= JCM 5824T); Bacteroides thetaiotaomicron YIT 6163T (= JCM 5827T), Bacteroides uniformis YIT 6164T (= JCM 5828T), Bacteroides vulgatus YIT 6159T (= ATCC 8424T), Bacteroides caccae ATCC 43185T, Bacteroides eggerthii DSM 20697T, Clostridium clostridiiforme YIT 6051T (= JCM 1291T), Clostridium coccoides YIT 6035T (= JCM 1395T), Clostridium nexile YIT 6170T (= ATCC 27757T), Clostridium oroticum YIT 6037T (= JCM 1429T), Clostridium sphenoides YIT 6059T (= JCM 1415T), Eubacterium rectale YIT 6082T (= ATCC 33656T), Ruminococcus gnavus YIT 6176T (= ATCC 29149T), Ruminococcus productus YIT 6141T (= ATCC 27340T), Prevotella corporis YIT 6132T (= JCM 8529T), Prevotella denticola YIT 6131 (= JCM 8528), Prevotella melaninogenica YIT 6039T (= ATCC 25845T), Prevotella veroralis YIT 6126T (= JCM 6290T), Prevotella buccae DSM 20615, Prevotella intermedia YIT 6130T (= JCM 7365T), Prevotella oralis YIT 6127 (= JCM 6330), Bifidobacterium adolescentis YIT 4011T (= ATCC 15703T), Bifidobacterium angulatum YIT 4012T (= ATCC 27535T), Bifidobacterium bifidum YIT 4039T (= ATCC 29521T), Bifidobacterium breve YIT 4014T (= ATCC 15700T), Bifidobacterium catenulatum YIT 4016T (= ATCC 27539T), Bifidobacterium pseudocatenulatum YIT 4072T (= JCM 1200T), Bifidobacterium longum YIT 4021T (= ATCC 15707T), Bifidobacterium infantis YIT 4018T (= ATCC 15697T), Bifidobacterium dentium YIT 4017T (= ATCC 27534T), Clostridium baratii YIT 6052T (= JCM 1385T), Clostridium celatum, YIT 6056T (= JCM 1394T), Clostridium perfringens YIT 6050T (= JCM 1290T), Clostridium sporogenes YIT 6060T (= JCM 1416T), Clostridium limosum YIT 6061T (= JCM 1427T), Clostridium bifermentans YIT 6053T (= JCM 1386T), Clostridium glycolicum YIT 6058T (= JCM 1401T), Clostridium sordelii YIT 6065T (= JCM 3814T), Fusobacterium varium ATCC 8501T, Fusobacterium gonidiaformans YIT 6079T (= ATCC 25563T), Escherichia coli YIT 6044T (= JCM 1649T), Enterococcus faecalis YIT 2031T (= ATCC 19433T), Enterococcus faecium YIT 2032T (= ATCC 19434T), Porphyromonas gingivalis YIT 6030 (= JCM 8525), Porphyromonas asaccharolytica ATCC 25260T, Propionibacterium acnes YIT 6118 (= ATCC 6919T), Lactobacillus acidophilus YIT 0070T (= ATCC 4356T), and Peptostreptococcus prevotii YIT 10027T (= ATCC 9321T).
+, positive; −, negative.
TABLE 4.
Comparison of bacterial populations in GAM broth determined by real-time quantitative PCR, direct counting with DAPI staining, and the culture methoda
| Primer | Species | Strain | Log10 bacteria/ml as determined by:
|
||
|---|---|---|---|---|---|
| qPCRb | DAPI | Culture | |||
| g-Ccoc | Clostridium clostridiiforme | YIT 6051T | 9.5 ± 0.1 | 9.5 ± 0.1 | 9.1 ± 0.1 |
| Clostridium coccoides | YIT 6035T | 9.3 ± 0.2 | 9.4 ± 0.3 | 8.7 ± 0.1 | |
| Clostridium nexile | YIT 6170T | 8.9 ± 0.1 | 8.7 ± 0.1 | 7.4 ± 0.3 | |
| Clostridium oroticum | YIT 6037T | 8.6 ± 0.1 | 8.5 ± 0.1 | 8.3 ± 0.1 | |
| Clostridium sphenoides | YIT 6059T | 9.1 ± 0.2 | 9.2 ± 0.2 | 9.3 ± 0.1 | |
| Eubacterium rectale | YIT 6082T | 8.9 ± 0.1 | 8.9 ± 0.1 | <7.0 | |
| Ruminococcus gnavus | YIT 6176T | 8.7 ± 0.2 | 8.7 ± 0.2 | 8.4 ± 0.1 | |
| Ruminococcus productus | YIT 6141T | 9.5 ± 0.2 | 9.3 ± 0.2 | 9.1 ± 0.1 | |
| sg-Clept | Faecalibacterium prausnitzii | YIT 6174 | 8.9 ± 0.1 | 8.9 ± 0.2 | <7.0 |
| Eubacterium siraeum | YIT 10049T | 9.1 ± 0.1 | 8.9 ± 0.2 | <7.0 | |
| Ruminococcus bromii | YIT 6078T | 9.3 ± 0.1 | 9.2 ± 0.1 | <7.0 | |
| Ruminococcus callidus | YIT 6175T | 8.5 ± 0.1 | 8.8 ± 0.2 | <7.0 | |
| Ruminococcus albus | YIT 6083T | 8.3 ± 0.1 | 9.0 ± 0.1 | <7.0 | |
| Clostridium viride | YIT 10050T | 8.0 ± 0.1 | 8.9 ± 0.1 | <7.0 | |
| Clostridium leptum | YIT 6169T | 8.1 ± 0.2 | 9.1 ± 0.1 | <7.0 | |
| g-Bfra | Bacteroides caccae | ATCC 43185T | 9.4 ± 0.1 | 9.6 ± 0.1 | 8.8 ± 0.2 |
| Bacteroides eggerthii | DSM 20697T | 9.0 ± 0.1 | 8.8 ± 0.1 | 9.4 ± 0.1 | |
| Bacteroides fragilis | YIT 6158T | 9.4 ± 0.1 | 9.4 ± 0.2 | 8.3 ± 0.3 | |
| Bacteroides ovatus | YIT 6161T | 9.1 ± 0.1 | 9.4 ± 0.2 | 8.5 ± 0.1 | |
| Bacteroides thetaiotaomicron | YIT 6163T | 8.8 ± 0.1 | 9.0 ± 0.2 | 8.8 ± 0.2 | |
| Bacteroides uniformis | YIT 6164T | 8.8 ± 0.1 | 8.8 ± 0.1 | 8.6 ± 0.1 | |
| Bacteroides vulgatus | YIT 6159T | 8.7 ± 0.1 | 8.7 ± 0.1 | 8.6 ± 0.1 | |
| c-Atopo | Atopobium parvulum | YIT 10055T | 8.8 ± 0.2 | 8.7 ± 0.1 | 9.3 ± 0.1 |
| Collinsella aerofaciens | ATCC 25986T | 9.3 ± 0.1 | 9.1 ± 0.1 | 8.9 ± 0.2 | |
| Collinsella intestinalis | YIT 10051T | 9.2 ± 0.1 | 9.3 ± 0.1 | 10.0 ± 0.1 | |
| Collinsella stercoris | YIT 10052T | 8.8 ± 0.2 | 9.0 ± 0.2 | 8.7 ± 0.2 | |
| Eggerthella lenta | YIT 6077T | 8.1 ± 0.1 | 8.7 ± 0.2 | 8.2 ± 0.1 | |
| g-Prevo | Prevotella corporis | YIT 6132T | 9.0 ± 0.1 | 8.8 ± 0.2 | 8.7 ± 0.1 |
| Prevotella denticola | YIT 6131 | 8.8 ± 0.1 | 8.9 ± 0.3 | 9.4 ± 0.1 | |
| Prevotella melaninogenica | YIT 6039T | 9.4 ± 0.1 | 9.2 ± 0.2 | 9.5 ± 0.1 | |
| Prevotella veroralis | YIT 6126T | 8.8 ± 0.1 | 8.8 ± 0.1 | 8.9 ± 0.1 | |
A comparison of bifidobacterial species has been reported previously (17).
PCR, real-time quantitative PCR.
Collection and preparation of fecal samples.
Forty-six healthy volunteers from our institute staff (41 males and 5 females; ages, 25 to 59 years [average, 37 ± 9 years]) who subsisted primarily on a Japanese diet provided fresh fecal samples. Forty volunteers provided samples only once (subjects Y-1 to Y-40), while six volunteers provided samples once a month for an 8-month period (subjects A to F). No subject had received antibiotics, probiotics, or prebiotics during the 2 weeks prior to the sampling, although subject A had received antibiotics up until 2 weeks prior to the first sampling date. The samples were collected in sterile plastic bags, refrigerated under anaerobic conditions, and immediately taken to the laboratory.
Enumeration and identification of predominant bacteria by the culture method.
Fecal specimens were collected from six healthy volunteers (subjects A to F) at the eighth month, and cultural counts (in CFU) of total anaerobes were determined by using medium 10 agar as described previously (18). All colonies that appeared at the first- and second-highest dilutions were transferred with a sterile toothpick to 50 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA; pH 8.0). Each suspension was boiled for 15 min to lyse the cells that were to be used for template DNA, and each isolate was identified by using group-specific PCR primers as described previously (Table 1) (18). The number of CFU of each group was calculated from the number of colonies and the results of the identification procedure.
Enumeration of predominant bacteria in the fecal samples by FISH.
FISH analyses with 16S rRNA-targeted group-specific oligonucleotide probes were carried out by using the procedure described by Franks et al. (5). The following probes were used to enumerate the target bacterial groups in the fecal samples: Bact338 (5′-GCT GCC TCC CGT AGG AGT-3′) for Bacteria, ATO291 (5′-GGT CGG TCT CTC AAC CC-3′) for the Atopobium cluster (8), Bac281 (5′-CTA CCT ATC CCC AAG ACT C-3′) for the B. fragilis group and Bacteroides distasonis, Bif153 (5′-ACC ACC CGT TTC CAG GAG-3′) for Bifidobacterium (30), Clept1240 (5′-GTT TTR TCA ACG GCA GTC-3′) for the C. leptum subgroup (26), and Erec482 (5′-TCC ATG RAC TGA TTC TTC G-3′) for the C. coccoides group (5). Fecal samples were applied to glass slides and were hybridized with the probes or stained with DAPI. The fluorescent cells in the samples were counted with a Leica Q550FW system (Leica, Wetzlar, Germany) by using the Image-Pro Plus image analysis software (version 4; Media-Cybernetics, Silver Spring, Md.). Microscopic counts were determined from 10 images, and a minimum of 50 cells per image were counted.
DNA extraction from fecal samples.
Fecal samples (20 mg) were washed three times by suspending them in 1.0 ml of phosphate-buffered saline and centrifuging each preparation at 14,000 × g in order to remove possible PCR inhibitors. The fecal pellets were resuspended in a solution containing 450 μl of extraction buffer (100 mM Tris-HCl, 40 mM EDTA; pH 9.0) and 50 μl of 10% sodium dodecyl sulfate. Three hundred milligrams of glass beads (diameter, 0.1 mm) and 500 μl of buffer-saturated phenol were added to the suspension, and the mixture was vortexed vigorously for 30 s by using a FastPrep FP 120 (BIO 101, Vista, Calif.) at a power level of 5.0. After centrifugation at 14,000 × g for 5 min, 400 μl of the supernatant was collected. Subsequently, phenol-chloroform extractions were performed, and 250 μl of the supernatant was subjected to isopropanol precipitation. Finally, the DNA was suspended in 1 ml of Tris-EDTA buffer.
Real-time PCR.
PCR amplification and detection were performed with an ABI PRISM 7900HT sequence detection system (Applied Biosystems, Foster City, Calif.). Each reaction mixture (10 μl) was composed of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM, a 1:75,000 dilution of SYBR Green I (Molecular Probes, Eugene, Oreg.), 11 ng of TaqStart antibody (ClonTech, Palo Alto, Calif.) per μl, 0.05 U of Taq DNA polymerase (Takara, Tokyo, Japan) per μl, each of the specific primers at a concentration of 0.25 μM, and 1 μl of 1×-, 10×-, or 100×-diluted template DNA. The amplification program consisted of one cycle of 94°C for 5 min and then 40 cycles of 94°C for 20 s, 55 or 50°C for 20 s (Table 2), and 72°C for 50 s and finally one cycle of 94°C for 15 s. The fluorescent products were detected at the last step of each cycle. A melting curve analysis was done after amplification to distinguish the targeted PCR product from the nontargeted PCR product (23). The melting curves were obtained by slow heating at temperatures from 60 to 95°C at a rate of 0.2°C/s, with continuous fluorescence collection. DNAs extracted from Ruminococcus productus YIT 6141T, Faecalibacterium prausnitzii YIT 6174, Bacteroides vulgatus YIT 6159T, Bifidobacterium longum YIT 4021T, Collinsella aerofaciens ATCC 25986T, and Prevotella melaninogenica YIT 6039T were used as real-time PCR controls for the group-specific g-Ccoc, sg-Clept, g-Bfra, g-Bifid, c-Atopo, and g-Prevo primers, respectively.
RESULTS
Primer specificity.
To analyze the predominant bacteria in human feces, we designed and evaluated two group-specific primers. Primers sg-Clept-F and sg-Clept-R3 were developed to detect the C. leptum subgroup, including certain members of the genera Clostridium, Ruminococcus, Eubacterium, and Faecalibacterium, which belong to Clostridium cluster IV (2). Primers c-Atopo-F and c-Atopo-R are specific for the Atopobium cluster (8), including members of the genera Atopobium, Collinsella, Eggerthella, and Coriobacterium. The specificity of the group-specific primers was then tested by using DNA extracts from strains representing 61 different bacterial species (Table 3). Melting temperature analysis was used to evaluate the PCR results, as the shape and position of a melting curve can be used to differentiate target PCR products from nontarget products (data not shown) (23). Each specific primer gave positive PCR results for the corresponding target bacteria and negative PCR results for nontarget microorganisms.
Real-time PCR detection.
DNA extracted from a known amount of C. aerofaciens ATCC 25986T was added in serial dilutions from 106 to 0 cells to a series of PCR mixtures with Atopobium cluster-specific primers, and fluorescence was monitored throughout the reactions. As a result, the number of starting cells and the cycle number at which the product fluorescence surpassed a defined threshold were found to be linear over the range of DNA concentrations from 106 to 10 cells per PCR mixture (r2 = 0.99) (data not shown). This indicates that the linear range for the procedures used in this study is 1011 to 106 cells per g of feces. Virtually identical results were obtained for R. productus, F. prausnitzii, B. vulgatus, and P. melaninogenica with their group-specific primers (data not shown).
Quantification of the target bacteria in the culture medium.
The number of bacteria in GAM broth was quantified by real-time PCR by using group-specific primers for the C. coccoides group, the C. leptum subgroup, the B. fragilis group, the Atopobium cluster, and Prevotella. The results were compared with those obtained by using the DAPI staining method and the culture method (Table 4). Compared to the DAPI staining method, the quantitative PCR method gave similar counts for most members of the C. coccoides group, the B. fragilis group, and the Atopobium cluster except Eggerthella lenta, with a difference of less than log10 0.2 cell per g. However, quantitative PCR gave estimates for E. lenta and a member of the C. leptum subgroup that were lower than the corresponding counts obtained by the DAPI staining method. With culture methods, the levels of members of the C. leptum subgroup and the C. coccoides group were underestimated compared to the results obtained by real-time PCR and the DAPI counting method (P < 0.05, as determined by a paired t test). This may have been due to biases inherent in the media used.
Quantification of the six predominant bacterial groups in fecal samples.
Real-time PCR analyses were performed to quantify individual bacterial groups in fecal samples collected from six subjects. The results were then compared to the results obtained by the FISH and culture methods (Table 5). Overall, the populations of the bacterial groups determined by real-time PCR and FISH methods were similar. However, the real-time PCR method gave higher values than the FISH method for certain samples (e.g., the C. leptum subgroup in subjects B, C, and E; the B. fragilis group in subject F; and the Atopobium cluster in subjects C and E). The populations of Bifidobacterium determined by the culture method were smaller than the populations determined by the real-time PCR (P < 0.05, as determined by a paired t test). In addition, culture-based methods tended to give lower values than real-time PCR for the C. leptum subgroup and the C. coccoides group. Detection of Prevotella by FISH was not performed, since a group-specific probe for this genus has not been established.
TABLE 5.
Comparison of quantitative PCR, FISH, and the culture method for detection and quantification of predominant bacteria in fecal samplesa
| Bacteria | Log10 cells/g (wet wt) fora:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject A
|
Subject B
|
Subject C
|
Subject D
|
Subject E
|
Subject F
|
|||||||||||||
| qPCRb | FISH | Culture | qPCR | FISH | Culture | qPCR | FISH | Culture | qPCR | FISH | Culture | qPCR | FISH | Culture | qPCR | FISH | Culture | |
| C. coccoides group | 10.4 | 10.4 | 10.4 | 10.7 | 10.4 | 10.3 | 10.4 | 10.2 | 9.9 | 10.5 | 10.5 | 10.3 | 10.3 | 10.3 | 10.2 | 9.5 | 9.4 | NDc |
| C. leptum subgroup | 10.1 | 10.3 | 10.1 | 10.7 | 10.2 | 9.9 | 10.7 | 10.0 | 10.4 | 10.8 | 10.4 | 10.2 | 10.5 | 9.8 | 10.1 | 6.5 | ND | ND |
| B. fragilis group | 10.5 | 10.3 | 10.7 | 10.1 | 10.4 | 10.3 | 10.1 | 10.3 | 10.3 | 9.4 | 9.7 | 9.4 | 10.0 | 10.0 | 10.2 | 9.7 | 8.5 | 9.7 |
| Bifidobacterium | 9.9 | 9.7 | 9.2 | 10.3 | 10.3 | 9.9 | 10.3 | 9.9 | 10.0 | 9.7 | 9.3 | 9.4 | 9.8 | 9.4 | 9.4 | 6.5 | 7.8 | ND |
| Atopobium cluster | 9.6 | 9.7 | 9.9 | 9.6 | 9.9 | 9.9 | 10.3 | 9.6 | 10.2 | 9.9 | 9.7 | 10.1 | 9.9 | 9.1 | 9.8 | 6.8 | ND | ND |
| Prevotella | ND | NTd | ND | ND | NT | ND | 10.2 | NT | 9.8 | 10.7 | NT | 10.4 | ND | NT | ND | ND | NT | ND |
| Sum of six groups | 10.9 | 10.9 | 11.0 | 11.1 | 11.0 | 10.8 | 11.2 | 10.8 | 10.9 | 11.2 | 10.8 | 10.9 | 10.9 | 10.6 | 10.7 | 9.9 | 9.5 | 9.7 |
| Total bacteria | NT | 11.0e | 11.1f | NT | 11.1 | 10.9 | NT | 10.9 | 11.0 | NT | 10.9 | 11.0 | NT | 10.8 | 10.7 | NT | 9.7 | 9.9 |
| Total cells (DAPI) | 11.2 | 11.3 | 11.2 | 11.1 | 11.1 | 10.3 | ||||||||||||
Fecal samples collected in the eighth month were used for comparison.
qPCR, quantitative PCR.
ND, not detected.
NT, not tested.
Total number of bacteria determined by hybridization with probe Bact338. In the present study, 66, 63, 46, 60, 53, and 28% (average±standard deviation, 53% ± 14%) of the total cells were detected with the Bact338 probe in samples from subjects A, B, C, D, E, and F, respectively.
Total cultivated bacteria with medium 10.
Distribution of the six bacterial groups in intestinal flora.
Table 6 shows the distribution of bacterial groups in the intestinal tracts of 46 healthy adult volunteers. The total bacterial count as determined by DAPI staining was log10 10.9 ± 0.2 cells per g (wet weight). We found larger populations of the C. coccoides group (log10 10.3 ± 0.3 cells per g or 29% ± 12% of the total cell count) than of the other five groups of bacteria (P < 0.01, as determined by a paired t test). The C. leptum subgroup (log10 9.9 ± 0.7 cells per g or 15% ± 10% of the total cell count) and the B. fragilis group (log10 9.9 ± 0.3 cells per g or 11% ± 7.8% of the total cell count) were present at nearly equal levels. These two groups were present at higher levels than Bifidobacterium (log10 9.4 ± 0.8 cells per g or 6.0% ± 6.4% of the total cell count) and the Atopobium cluster (log10 9.3 ± 0.7 cells per g or 4.9% ± 4.2% of the total cell count) (P < 0.01, as determined by a paired t test). The variations in the populations of the C. coccoides group and the B. fragilis group (SD values, log10 0.3 cell per g) were less marked than those of the C. leptum subgroup, Bifidobacterium (SD values, log10 0.7 cell per g), or the Atopobium cluster (standard deviation, log10 0.7 cells per g) (Table 7). Except for Prevotella, the five bacterial groups were detected in all volunteers. Prevotella was found in only 21 of 46 subjects (46%) at a level of log10 9.7 ± 0.8 cells per g (4.4% ± 4.9% of the total cell count). The total population of these six bacterial groups was log10 10.8 ± 0.3 cells per g or 71% ± 22% of the total cell count.
TABLE 6.
Mean counts of six major bacterial groups in fecal samples collected from 46 health Japanese volunteers as determined by real-time PCR
| Subject | Log10 cells/g (wet wt)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| With genus- and species-specific primersa
|
Sum of six groups | Total cells (DAPI count) | ||||||
| g-Ccoc | sg-Clept | g-Bfra | g-Bifid | c-Atopo | g-Prevo | |||
| A | 10.4 | 10.1 | 10.5 | 10.0 | 9.6 | ND | 10.9 | 11.2 |
| B | 10.7 | 10.7 | 10.1 | 10.3 | 9.6 | ND | 11.1 | 11.3 |
| C | 10.4 | 10.7 | 10.1 | 10.6 | 10.3 | 10.2 | 11.2 | 11.2 |
| D | 10.5 | 10.8 | 9.4 | 10.4 | 9.9 | 10.7 | 11.3 | 11.1 |
| E | 10.3 | 10.5 | 10.0 | 9.3 | 9.9 | ND | 10.8 | 11.1 |
| F | 9.5 | 6.5 | 9.7 | 6.9 | 6.8 | ND | 9.9 | 10.3 |
| Y-1 | 10.3 | 9.7 | 9.7 | 9.6 | 9.5 | ND | 10.5 | 10.9 |
| Y-2 | 10.1 | 9.8 | 9.9 | 10.1 | 10.0 | ND | 10.7 | 11.0 |
| Y-3 | 10.5 | 10.2 | 9.7 | 9.2 | 9.7 | 9.9 | 10.8 | 11.0 |
| Y-4 | 10.1 | 10.0 | 9.7 | 8.2 | 7.7 | ND | 10.4 | 10.6 |
| Y-5 | 10.2 | 10.2 | 9.2 | 8.4 | 9.6 | 10.3 | 10.7 | 10.9 |
| Y-6 | 10.4 | 9.9 | 10.0 | 9.5 | 9.7 | ND | 10.7 | 10.9 |
| Y-7 | 10.3 | 9.8 | 9.6 | 9.1 | 8.8 | ND | 10.5 | 10.6 |
| Y-8 | 10.4 | 10.3 | 9.8 | 9.6 | 9.8 | 10.4 | 10.9 | 10.8 |
| Y-9 | 10.6 | 10.5 | 10.1 | 9.4 | 8.6 | 10.0 | 11.0 | 11.1 |
| Y-10 | 10.6 | 10.5 | 10.0 | 9.6 | 9.2 | ND | 10.9 | 11.0 |
| Y-11 | 11.0 | 10.6 | 9.9 | 10.2 | 9.9 | 10.2 | 11.3 | 11.3 |
| Y-12 | 10.4 | 9.9 | 9.6 | 9.4 | 9.9 | ND | 10.7 | 10.6 |
| Y-13 | 10.0 | 9.7 | 9.5 | 10.0 | 9.7 | ND | 10.5 | 11.1 |
| Y-14 | 10.4 | 10.2 | 9.6 | 10.1 | 10.1 | 10.4 | 11.0 | 11.0 |
| Y-15 | 10.0 | 9.9 | 9.4 | 8.9 | 9.6 | 10.1 | 10.6 | 10.7 |
| Y-16 | 10.6 | 10.2 | 10.3 | 8.4 | 9.1 | ND | 10.9 | 11.0 |
| Y-17 | 10.8 | 10.4 | 10.3 | 9.0 | 9.8 | 9.6 | 11.1 | 11.1 |
| Y-18 | 10.5 | 10.0 | 10.2 | 9.7 | 9.5 | ND | 10.8 | 11.0 |
| Y-19 | 10.4 | 10.3 | 9.9 | 9.6 | 10.1 | 9.9 | 10.9 | 11.0 |
| Y-20 | 10.2 | 9.6 | 9.8 | 9.3 | 9.0 | ND | 10.5 | 10.9 |
| Y-21 | 10.5 | 10.5 | 10.2 | 9.3 | 9.3 | 9.9 | 11.0 | 11.1 |
| Y-22 | 10.3 | 9.5 | 9.7 | 10.1 | 9.9 | 8.4 | 10.7 | 10.8 |
| Y-23 | 10.5 | 10.1 | 10.3 | 7.5 | 9.6 | 9.5 | 10.8 | 11.0 |
| Y-24 | 10.3 | 10.3 | 10.3 | 9.6 | 9.4 | ND | 10.8 | 10.9 |
| Y-25 | 10.7 | 10.5 | 10.2 | 10.1 | 9.9 | ND | 11.1 | 11.1 |
| Y-26 | 9.9 | 8.8 | 9.4 | 9.0 | 9.1 | 9.9 | 10.3 | 10.6 |
| Y-27 | 10.4 | 9.9 | 9.5 | 9.1 | 9.5 | ND | 10.6 | 10.9 |
| Y-28 | 10.6 | 10.4 | 10.0 | 8.9 | 7.3 | 8.3 | 10.8 | 11.0 |
| Y-29 | 10.4 | 9.8 | 10.1 | 9.7 | 8.2 | ND | 10.7 | 10.7 |
| Y-30 | 10.6 | 10.1 | 10.2 | 9.4 | 9.6 | ND | 10.8 | 11.0 |
| Y-31 | 10.0 | 8.4 | 10.2 | 10.0 | 8.1 | 9.4 | 10.6 | 10.9 |
| Y-32 | 10.4 | 10.3 | 10.0 | 9.2 | 8.8 | 10.1 | 10.8 | 11.0 |
| Y-33 | 10.5 | 10.1 | 9.5 | 9.4 | 10.0 | 9.5 | 10.8 | 11.1 |
| Y-34 | 9.7 | 9.2 | 10.0 | 9.5 | 9.7 | ND | 10.4 | 10.8 |
| Y-35 | 10.4 | 9.8 | 9.9 | 10.1 | 10.0 | ND | 10.8 | 11.0 |
| Y-36 | 10.7 | 10.1 | 10.0 | 9.8 | 9.8 | 9.0 | 11.0 | 11.3 |
| Y-37 | 9.7 | 9.1 | 10.0 | 10.2 | 8.9 | ND | 10.5 | 10.6 |
| Y-38 | 10.2 | 9.9 | 9.9 | 8.6 | 9.3 | ND | 10.5 | 10.8 |
| Y-39 | 10.3 | 9.7 | 9.3 | 9.6 | 9.6 | 7.5 | 10.6 | 10.7 |
| Y-40 | 9.9 | 8.5 | 10.0 | 9.6 | 8.9 | ND | 10.3 | 10.5 |
| Mean ± SD | 10.3 ± 0.3 | 9.9 ± 0.7 | 9.9 ± 0.3 | 9.4 ± 0.7 | 9.3 ± 0.7 | 9.7 ± 0.8 | 10.8 ± 0.3 | 10.9 ± 0.2 |
Primers g-Ccoc, g-Bfra, sg-Clept, g-Bifid, and c-Atopo are specific for the C. coccoides group, the B. fragilis group, the C. leptum subgroup, Bifidobacterium, and the Atopobium cluster, respectively.
ND, not detected (<log10 6 cells/g).
TABLE 7.
Variations in fecal flora populations between volunteers (SDinter) and within each volunteer over time (SDlong)
| Organism or group | Variationa
|
|||
|---|---|---|---|---|
| Mean for total | SD for total | SDinterb | SDlongc | |
| C. coccoides group | 10.25 | 0.44 | 0.45 | 0.22 |
| B. fragilis group | 9.99 | 0.40 | 0.39 | 0.22 |
| C. leptum subgroup | 9.83 | 1.34 | 1.43 | 0.30 |
| Bifidobacterium | 9.21 | 1.48 | 1.55 | 0.65 |
| Atopobium cluster | 9.38 | 1.23 | 1.29 | 0.30 |
| Prevotellad | 9.18 | 1.72 | 1.50 | 1.11 |
Means and standard deviations were calculated by using all 48 fecal samples collected from subjects A to F over an 8-month period.
SDinter represents the difference in bacterial count between volunteers.
SDlong represents change in bacterial counts within individual volunteers over time.
The values were calculated from serial samples C and D.
Temporal fluctuations in the six bacterial groups.
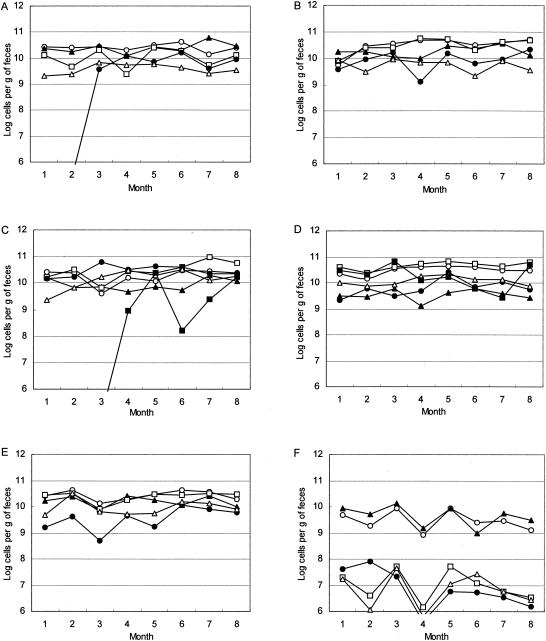
Fecal samples were collected monthly from six healthy adults over an 8-month period to assess changes in the level of each bacterial group (Fig. 1). Table 7 provides a summary of the longitudinal variations (SDlong) and the interindividual variations (SDinter) of these bacteria and shows that there were smaller longitudinal temporal variations than interindividual variations for all bacterial groups. The interindividual and longitudinal variations of the C. coccoides group and the B. fragilis groups were less than those of the other bacterial groups (Table 7). Although the SDinter values of the C. leptum subgroup and the Atopobium cluster are relatively large, the longitudinal shifts were moderate. On the other hand, bifidobacteria showed significant variations in SDinter and SDlong values.
FIG. 1.
Populations of predominant bacteria in fecal samples collected from six human subjects over an 8-month period. (A) Subject A; (B)
(A) subject B; (C) subject C; (D) subject D; (E) subject D; (F) subject F. Symbols: ○, C. coccoides group; □, C. leptum subgroup; ▴, B. fragilis group; •, Bifidobacterium; ▵, Atopobium cluster; ▪, Prevotella.
DISCUSSION
In order to clarify the population structure of the predominant phylogenetic groups in the human intestinal flora, newly developed group-specific primers for the C. leptum subgroup and the Atopobium cluster were used in a real-time PCR analysis with primers for the C. coccoides group, the B. fragilis group, Bifidobacterium, and Prevotella. Since the quantitative PCR method targets extracted DNAs, the number of 16S rRNA genes in the genome, differences in DNA extraction efficiency, and point mutations in the target region of a primer may influence the measurements. However, the cell counts determined by the quantitative PCR method were similar to those determined by the DAPI counting method for members of the C. coccoides group, the B. fragilis group, and the Atopobium cluster (Table 4). This indicates that the sensitivity of quantitative PCR assays may not vary greatly, depending on the species or strains. On the other hand, the real-time quantitative PCR method gave lower values for certain members of the C. leptum subgroup, such as Ruminococcus albus, Clostridium viride, and C. leptum, compared to DAPI counting methods (Table 4). This suggests that the population of the C. leptum subgroup may have been underestimated when these species were the predominant species of the C. leptum subgroup. Therefore, real-time PCR detection of the C. leptum subgroup from fecal DNA should be improved further.
Although the populations of each bacterial group in six healthy volunteers enumerated by the three methods were similar, the real-time PCR method sometimes gave higher values than the FISH method (Table 5). This variation may be explained in terms of the different targets of the methods used. Since real-time PCR targets extracted DNA, PCR methods can detect all bacterial cells present in a specimen. While the FISH method targets rRNA, it is difficult to detect bacterial cells that have little rRNA but are present in feces. Probe permeation of the gram-positive cell wall has been reported to be problematic in FISH experiments (5). In this study, 34, 37, 54, 40, 47, and 72% of DAPI-counted cells were not detected by FISH with the Bact338 probe in subjects A, B, C, D, E, and F, respectively (Table 5). Such undetected bacteria have been reported to account for approximately 30 to 40% of the total cell counts (7, 28). Therefore, the presence of these bacteria may explain the differences in the results of the real-time PCR and FISH methods observed in this study. Extensive efforts have been made in the past to cultivate the bacteria found in human feces, with the result that the human intestinal flora is one of the most successfully studied natural communities of bacteria (1, 3, 4, 20, 21). However, the culture method tended to give lower population values than the quantitative PCR method, probably due to the presence of bacterial cells lacking colony-forming capacity, cell aggregation, and the selection bias of the medium.
When the results for 46 healthy adults were compared to current knowledge, the total bacterial count determined by the DAPI staining method (log10 10.9 ± 0.2 cells per g [Table 6]) was in good agreement with values obtained by other investigators (7). The populations of the C. leptum subgroup, the B. fragilis group, Bifidobacterium, and the Atopobium cluster determined in this study were in general agreement with results obtained by molecule-based methods, such as FISH (5, 7, 8), quantitative hybridization (26), and the cloning library method (9, 10, 28). On the other hand, the populations and proportions of the C. coccoides group obtained by real-time PCR tended to be greater than the populations and proportions obtained by the FISH method (7). However, the proportions for this group were in good agreement with those obtained by the cloning library method (9, 10, 28). The considerable variation may be explained in terms of the different targets of the methods used (rRNA gene versus rRNA molecule), as discussed above.
Although Prevotella has been reported to be one of the major components of the oral flora (25), there have been few reports of detection of this bacterial group in the human intestinal flora (18, 28). Bacteroides and Prevotella have not been distinguished in the previous studies by the FISH method (7). Therefore, the present quantitative PCR analysis likely represents the first report of a comprehensive analysis of the distribution of the genus Prevotella in the intestinal flora. As shown in Table 6, Prevotella was detected in only 21 of 46 subjects (46%), but the mean count in those individuals was log10 9.7 ± 0.8 cells per g. This indicates that Prevotella was one of the predominant bacterial groups in these subjects. The count for Prevotella in the remaining 25 subjects did not exceed 106 cells per g of feces, suggesting that there were considerable differences in this bacterial population among individuals. Since most Prevotella species are reportedly sensitive to bile acids (22), it should be of interest to investigate the species of Prevotella in the intestine, their resistance to bile acids, and factors in the intestinal environment that may influence colonization by this bacterial group.
In the present 8-month analysis, changes in bacterial counts over time within individual volunteers (SDlong) were compared to differences in bacterial counts between volunteers (SDinter) (Table 7). The SDlong values were found to be smaller than the SDinter values, suggesting that the variations in the flora over time within one individual tended to be less marked than the differences among volunteers. Although considerable changes were observed in the population of Bifidobacterium in subject A, it should be noted that subject A had received antibiotics 2 weeks prior to the first sampling date, treatment that may have resulted in significant changes in the Bifidobacterium population (17). We also found interesting the significant change in the Prevotella population observed in serial sample C (Fig. 1). The cause of the considerable change requires further investigation, involving, for example, a change in bile acid secretion caused by dietary variations.
Conclusions.
In this study, we developed a quantitative PCR detection method to investigate the composition of human intestinal flora by using DNA extracted from fecal samples. The advantages of this method are higher sensitivity, easy sample handling, and simple procedures. Therefore, the group-specific PCR method shows promise as an effective tool for investigating the effects of probiotics or prebiotics, the side effects of antibiotics, and the relationship between the microflora and digestive diseases, such as inflammatory bowel disease, infectious diseases, and colon cancer. The next challenge for real-time PCR is to develop group- and species-specific primers to cover most of human intestinal bacteria. The wide-ranging PCR primers should make the real-time PCR technique a more advanced method for the study of the human intestinal microflora.
Acknowledgments
We express our gratitude to K. Nomoto for his valuable advice. We also thank M. Sasaki for her assistance in this research.
REFERENCES
- 1.Benno, Y., K. Endo, T. Mizutani, Y. Namba, T. Komori, and T. Mitsuoka. 1989. Comparison of fecal microflora of elderly persons in rural and urban areas of Japan. Appl. Environ. Microbiol. 55:1100-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins, M. D., P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, and J. A. Farrow. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812-826. [DOI] [PubMed] [Google Scholar]
- 3.Finegold, S. M., H. R. Attebery, and V. L. Sutter. 1974. Effect of diet on human fecal flora: comparison of Japanese and American diets. Am. J. Clin. Nutr. 27:1456-1469. [DOI] [PubMed] [Google Scholar]
- 4.Finegold, S. M., V. S. Sutter, and G. E. Mathisen. 1983. Normal indigenous intestinal flora, p. 3-31. In D. J. Hentges (ed.), Human intestinal microflora in health and disease. Academic Press, New York, N.Y.
- 5.Franks, A. H., H. J. Harmsen, G. C. Raangs, G. J. Jansen, F. Schut, and G. W. Welling. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuller, R. 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66:365-378. [PubMed] [Google Scholar]
- 7.Harmsen, H. J., G. C. Raangs, T. He, J. E. Degener, and G. W. Welling. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmsen, H. J., A. C. Wildeboer-Veloo, J. Grijpstra, J. Knol, J. E. Degener, and G. W. Welling. 2000. Development of 16S rRNA-based probes for the Coriobacterium group and the Atopobium cluster and their application for enumeration of Coriobacteriaceae in human feces from volunteers of different age groups. Appl. Environ. Microbiol. 66:4523-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi, H., M. Sakamoto, and Y. Benno. 2002. Fecal microbial diversity in a strict vegetarian as determined by molecular analysis and cultivation. Microbiol. Immunol. 46:819-831. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi, H., M. Sakamoto, and Y. Benno. 2002. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol. Immunol. 46:535-548. [DOI] [PubMed] [Google Scholar]
- 11.Kageyama, A., M. Sakamoto, and Y. Benno. 2000. Rapid identification and quantification of Collinsella aerofaciens using PCR. FEMS Microbiol. Lett. 183:43-47. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann, P., A. Pfefferkorn, M. Teuber, and L. Meile. 1997. Identification and quantification of Bifidobacterium species isolated from food with genus-specific 16S rRNA-targeted probes by colony hybridization and PCR. Appl. Environ. Microbiol. 63:1268-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyons, S. R., A. L. Griffen, and E. J. Leys. 2000. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J. Clin. Microbiol. 38:2362-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maidak, B. L., J. R. Cole, C. T. Parker, Jr., G. M. Garrity, N. Larsen, B. Li, T. G. Lilburn, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malinen, E., A. Kassinen, T. Rinttila, and A. Palva. 2003. Comparison of real-time PCR with SYBR Green I or 5′-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology 149:269-277. [DOI] [PubMed] [Google Scholar]
- 17.Matsuki, T., K. Watanabe, J. Fujimoto, Y. Kado, T. Takada, K. Matsumoto, and R. Tanaka. 2004. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 70:167-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuki, T., K. Watanabe, J. Fujimoto, Y. Miyamoto, T. Takada, K. Matsumoto, H. Oyaizu, and R. Tanaka. 2002. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 68:5445-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuki, T., K. Watanabe, R. Tanaka, M. Fukuda, and H. Oyaizu. 1999. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 65:4506-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore, W. E. C., and L. V. Holdeman. 1974. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl. Microbiol. 27:961-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore, W. E. C., and L. H. Moore. 1995. Intestinal floras of populations that have a high risk of colon cancer. Appl. Environ. Microbiol. 61:3202-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paster, B. J., F. E. Dewhirst, I. Olsen, and G. J. Fraser. 1994. Phylogeny of Bacteroides, Prevotella, and Porphyromonas spp. and related bacteria. J. Bacteriol. 176:725-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ririe, K. M., R. P. Rasmussen, and C. T. Wittwer. 1997. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal. Biochem. 245:154-160. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto, M., Y. Takeuchi, M. Umeda, I. Ishikawa, and Y. Benno. 2001. Rapid detection and quantification of five periodontopathic bacteria by real-time PCR. Microbiol. Immunol. 45:39-44. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto, M., M. Umeda, I. Ishikawa, and Y. Benno. 2000. Comparison of the oral bacterial flora in saliva from a healthy subject and two periodontitis patients by sequence analysis of 16S rDNA libraries. Microbiol. Immunol. 44:643-652. [DOI] [PubMed] [Google Scholar]
- 26.Sghir, A., G. Gramet, A. Suau, V. Rochet, P. Pochart, and J. Dore. 2000. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 66:2263-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song, Y., N. Kato, C. Liu, Y. Matsumiya, H. Kato, and K. Watanabe. 2000. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S-23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 187:167-173. [DOI] [PubMed] [Google Scholar]
- 28.Suau, A., R. Bonnet, M. Sutren, J. J. Godon, G. R. Gibson, M. D. Collins, and J. Dore. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tajima, K., R. I. Aminov, T. Nagamine, H. Matsui, M. Nakamura, and Y. Benno. 2001. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl. Environ. Microbiol. 67:2766-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takada, T., K. Matsumoto, and K. Nomoto. 2004. Development of multi-color FISH method for analysis of seven Bifidobacterium species in human feces. J. Microbiol. Methods 58:413-421. [DOI] [PubMed] [Google Scholar]
- 31.Tannock, G. W. 1995. Normal microflora: an introduction to microbes inhabiting the human body. Chapman & Hall, London, United Kingdom.
- 32.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter, J., G. W. Tannock, A. Tilsala-Timisjarvi, S. Rodtong, D. M. Loach, K. Munro, and T. Alatossava. 2000. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl. Environ. Microbiol. 66:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, R. F., W. W. Cao, and C. E. Cerniglia. 1996. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl. Environ. Microbiol. 62:1242-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoetendal, E. G., A. D. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]