Abstract
Multiple myeloma (MM) is a plasma cell neoplasm and constitutes 10% of hematologic malignancies. Malignant myelomatous pleural effusions are very rare and occur in <1% of cases of MM. In this article, we report a rare case of a patient who initially presented with pleural effusion and was subsequently found to be secondary to MM with an underlying raised IgG paraprotein. The patient symptomatically improved and was in partial remission with palliative radiotherapy, VTD chemotherapy, and bisphosphonates.
KEY WORDS: IgG paraprotein, multiple myeloma, myelomatous pleural effusion
INTRODUCTION
Multiple myeloma (MM) is a plasma cell neoplasm. It constitutes 10% of all hematologic malignancies. The incidence of MM is 1.4% in Caucasians and 2.0% of African Americans.[1] Malignant myelomatous pleural effusions (MPEs) are very rare and occur in <1% of cases of MM.[2,3,4] Since this condition is associated with poor prognosis, an accurate diagnosis of this condition is necessary.[3,4]
In this article, we report a rare case of a patient who initially presented with pleural effusion, which was subsequently found to be secondary to MM.
CASE REPORT
A 63-year-old female presented to the outpatient department of our hospital in December 2014, with a complaint of feeling increasingly breathless and fatigued over a period of 2 months. She also complained of right sided chest pain and nonradiating pain in the lower back since a month. On evaluation, the chest X-ray revealed a homogenous opacity in the right mid and lower zones [Figure 1]. A diagnostic pleural aspiration revealed numerous plasma cells in a hemorrhagic background. Biochemical analysis of the pleural fluid confirmed that it was exudative in nature (protein 9.8 g/dL, glucose 4.9 mg/dL, lactate dehydrogenase 1020 IU/L, adenosine deaminase 69.6 U/L [ref: 33 U/L]). The pleural fluid sent for culture, and cytology showed no acid-fast bacilli. A computed tomography (CT) scan thorax was done and showed an ill-defined nodular heterogeneously enhancing lesion in right posterior hemithorax with right sided gross pleural effusion, collapse of right lung, and involvement of the right 4th rib [Figure 2]. Fine-needle aspiration cytology (FNAC) done from the right pleural lesion showed numerous plasma cells, binucleate forms, and few atypical plasma cells [Figure 3]. A cell block was prepared after centrifuging the pleural fluid and Immunohistochemistry done on the cell block showed tumor cells positive for CD20, CD138, and lambda and negative for leukocyte common antigen, CD3, cytokeratin, and kappa [Figure 4]. These features are suggestive of MPE. Further staging work up for MM was done. Serum electrophoresis showed an M-spike in beta region with elevated serum IgG levels (3586 mg/dL, ref range 700–1600 mg/dL). Serum free light chain assay was normal. Beta 2 microglobulin level was elevated (3.23 mg/L ref: 0.8–2.2 mg/L). Magnetic resonance imaging whole spine was done, and confirmed the presence of a lobulated enhancing soft tissue lesion in the right paravertebral region, measuring 6 cm × 8.6 cm, with involvement of the adjacent rib and also intraspinal extension. It also showed 2 more lytic lesions in T12 and L2 vertebra. CT-guided FNAC done from this paravertebral mass showed multiple plasma cells, contributing to the diagnosis of MM. Bone marrow aspiration and biopsy showed scattered and <2% infiltration with plasma cells. Other than this, the skeletal survey was normal. Therapeutic pleural aspiration was done. The diagnosis of MM with MPE was made in this patient. She received palliative radiotherapy of 30 Gray in 10 fractions to the T12–L2 spine and paravertebral mass. She was started on VTD regimen with bortezomib, thalidomide, and dexamethasone chemotherapy along with monthly bisphosphonates. After 6 cycles of chemotherapy, she was symptomatically better, and her chest X-ray showed resolution of the pleural effusion. She requires long-term follow-up and regular reassessment.
Figure 1.
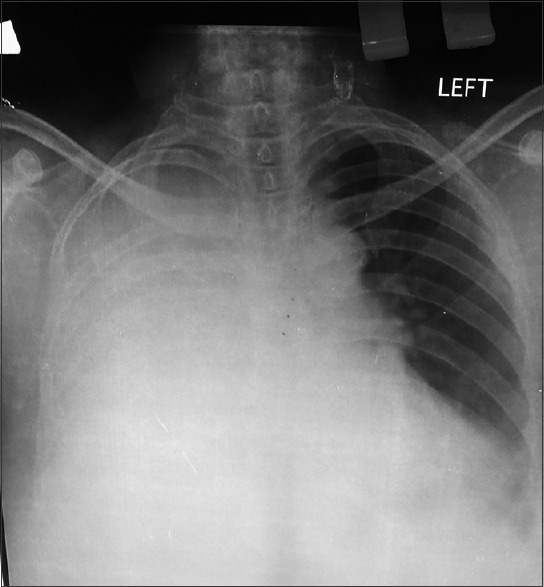
Chest X-ray
Figure 2.
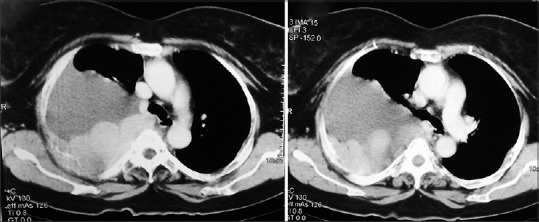
Computed tomography image
Figure 3.
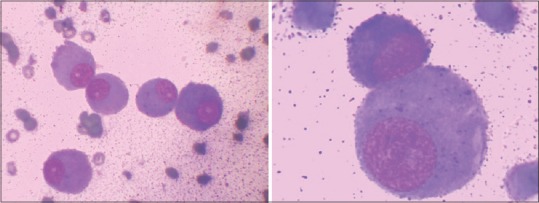
Plasma cells on cytology low- and high-power view
Figure 4.
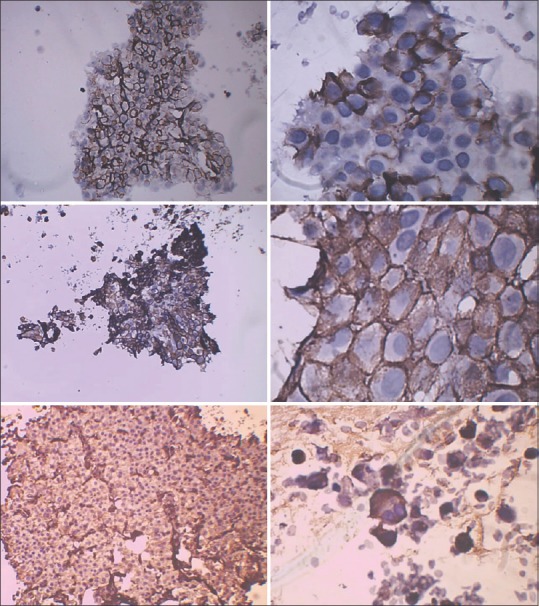
CD20 (top – low- and high-power), CD138 (middle- low- and high-power), lambda (bottom – low- and high-power)
DISCUSSION
In history, MM was first described in Egyptian mummies. The word MM was first coined by Rustizky in 1873. The median age of presentation is 69 years and is more common in men, and increases with advancing age. Patients with MM may be asymptomatic or can usually present with hematologic manifestations such as anemia, bleeding disorders, or bone related problems, infections and other end organ damage like renal failure.[1]
In MM, the development of pleural effusion is unusual.[2,3,4,5] Pleural effusion occurs in around 6% of patients with MM during the course of the disease, while MPE is even rarer presenting in <1% of patients as reported by Kintzer et al.[4] MPE is usually a late complication of the disease.[4] It is associated with poor prognosis, and there are previous studies with the survival of <4 months.[6,7]
MPEs may arise from either extension of plasmacytomas of the chest wall, invasion from adjacent skeletal lesions, direct pleural involvement by myeloma (pleural plasmacytoma) or also following lymphatic obstruction secondary to lymph node infiltration. Pleural effusions are rarely a direct consequence of the myeloma itself and usually occur due to the concurrent disease process or coexisting illness such as cardiac failure, amyloidosis, pulmonary embolism, pneumonia, or a second malignancy.[4,5] The first possibility is usually heart failure. Kintzer et al. reported that about 50% of MM patients accompanied with pleural effusion were due to congestive heart failure.[4] Heart failure may be caused by cardiac amyloidosis secondary to MM. The other possible mechanisms are a pulmonary embolism, chronic renal failure accompanying MM, and second malignancy.[5,6,7] Occasionally, pleural effusion can be secondary to a myelomatous ascites.[8,9,10]
MPEs are most commonly associated with the presence of an IgA paraprotein (in up to 80% of cases).[5,7] Sasser et al. reported 56 cases of MM with the involvement of the serous cavities. The sites of involvement were mostly the pleural cavity (thirty cases) and among them, 50% of the cases with cavitary involvement were of the IgA type.[9,11]
In this case, histological analysis demonstrated an immature population of plasma cells. This is an important contributory factor for the development of MPEs and explains the apparent aggressive nature of myelomatous disease. This case is distinctive in regards to the direct involvement of the pleural cavity by malignant plasma cells, which was confirmed with the FNAC and IHC. The case reported here is unusual as she had an underlying raised IgG paraprotein. Pleural involvement in MM is very unusual.[12] In a review of English literature, only 11 cases have been described previously, including the present case (to the best of our knowledge).[12,13,14,15,16,17,18,19,20,21,22,23]
Chemotherapy with various newer drugs such as bortezomib, thalidomide, lenalidomide along with dexamethasone or 2nd generation proteasome inhibitors like carfilzomib remains the first-line treatment for MM. In practice, pleural involvement with myeloma cells is associated with an aggressive course which is poorly responsive to first or second-line therapies used in conventional myeloma treatment.[1,24,25] In this case, the patient was doing better with VTD chemotherapy and palliative radiotherapy. This patient was started on treatment early and had IgG paraprotein MPE probably indicating better response in these cases.
CONCLUSION
The occurrence of MPEs in MM is rare. It indicates a poor prognosis because of an aggressive natural course. Early consideration of MPEs helps in rapid diagnosis and early initiation of treatment which may help in improving prognosis as seen in the present case.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Munshi NC, Anderson KC. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2015. Plasma cell neoplasms; pp. 1682–720. [Google Scholar]
- 2.Barlogie B, Shaughnessy J, Munshi N, Epstein J. Beutler E, Lichtman MA, Coller BS, Kipps TJ, Seligsohn U. Williams Hematology. 6th ed. New York: McGraw Hill; 2011. Plasma cell myeloma; pp. 1279–304. [Google Scholar]
- 3.Deshpande AH, Munshi MM. Pleural effusion as an initial manifestation of multiple myeloma. Acta Cytol. 2000;44:103–4. [PubMed] [Google Scholar]
- 4.Kintzer JS, Jr, Rosenow EC, 3rd, Kyle RA. Thoracic and pulmonary abnormalities in multiple myeloma. A review of cases. Arch Intern Med. 1978;138:727–30. [PubMed] [Google Scholar]
- 5.Rodríguez JN, Pereira A, Martínez JC, Conde J, Pujol E. Pleural effusion in multiple myeloma. Chest. 1994;105:622–4. doi: 10.1378/chest.105.2.622. [DOI] [PubMed] [Google Scholar]
- 6.Kamble R, Wilson CS, Fassas A, Desikan R, Siegel DS, Tricot G, et al. Malignant pleural effusion of multiple myeloma: Prognostic factors and outcome. Leuk Lymphoma. 2005;46:1137–42. doi: 10.1080/10428190500102845. [DOI] [PubMed] [Google Scholar]
- 7.Dhingra KK, Singhal N, Nigam S, Jain S. Unsuspected multiples myeloma presenting as bilateral pleural effusion – A cytological diagnosis. Cytojournal. 2007;4:17. doi: 10.1186/1742-6413-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gogia A, Agarwal PK, Jain S, Jain KP. Myelomatous pleural effusion. J Assoc Physicians India. 2005;53:734–6. [PubMed] [Google Scholar]
- 9.Yokoyama T, Tanaka A, Kato S, Aizawa H. Multiple myeloma presenting initially with pleural effusion and a unique paraspinal tumor in the thorax. Intern Med. 2008;47:1917–20. doi: 10.2169/internalmedicine.47.1296. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y, Chua K, McClure RF, Jimenez MC, Gocke CD, Badros AZ, et al. Multiple myeloma presenting initially as a solitary pleural effusion later complicated by malignant plasmacytic ascites. Leuk Res. 2005;29:715–8. doi: 10.1016/j.leukres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Sasser RL, Yam LT, Li CY. Myeloma with involvement of the serous cavities. Cytologic and immunochemical diagnosis and literature review. Acta Cytol. 1990;34:479–85. [PubMed] [Google Scholar]
- 12.Safa AM, Van Ordstrand HS. Pleural effusion due to multiple myeloma. Chest. 1973;64:246–8. doi: 10.1378/chest.64.2.246. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera A, Klein JS. Bilateral pleural masses and shortness of breath associated with multiple myeloma. Chest. 1997;111:1750–3. doi: 10.1378/chest.111.6.1750. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto A, Nagata K, Hamaguchi H, Taki K. Solitary bone plasmacytoma terminally developed myeloma with multiple extramedullary lesions and myelomatous pleural effusion and ascites. Int J Hematol. 1993;59:59–65. [PubMed] [Google Scholar]
- 15.Colonna A, Gualco G, Bacchi CE, Leite MA, Rocco M, DeMaglio G, et al. Plasma cell myeloma presenting with diffuse pleural involvement: A hitherto unreported pattern of a new mesothelioma mimicker. Ann Diagn Pathol. 2010;14:30–5. doi: 10.1016/j.anndiagpath.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Kushwaha RA, Verma SK, Mehra S, Prasad R. Pulmonary and nodal multiple myeloma with a pleural effusion mimicking bronchogenic carcinoma. J Cancer Res Ther. 2009;5:297–9. doi: 10.4103/0973-1482.59915. [DOI] [PubMed] [Google Scholar]
- 17.Galano AR. Unusual features of multiple myeloma. Radium Ther Nucl Med. 1955;74:304–14. [PubMed] [Google Scholar]
- 18.Favis EA, Kerman HD, Schildecker W. Multiple myeloma manifested as a problem in the diagnosis of pulmonary disease. Am J Med. 1960;28:323–7. doi: 10.1016/0002-9343(60)90193-5. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel S. Multiple myeloma presenting as pulmonary infiltration. Report of a case. Dis Chest. 1965;47:123–6. doi: 10.1378/chest.47.1.123. [DOI] [PubMed] [Google Scholar]
- 20.Edwards GA, Zawadzki ZA. Extraosseous lesions in plasma cell myeloma. A report of six cases. Am J Med. 1967;43:194–205. doi: 10.1016/0002-9343(67)90164-7. [DOI] [PubMed] [Google Scholar]
- 21.Nakazato T, Suzuki K, Mihara A, Sanada Y, Kakimoto T. Refractory plasmablastic type myeloma with multiple extramedullary plasmacytomas and massive myelomatous effusion: Remarkable response with a combination of thalidomide and dexamethasone. Intern Med. 2009;48:1827–32. doi: 10.2169/internalmedicine.48.2142. [DOI] [PubMed] [Google Scholar]
- 22.Miller J, Alton PA. Myelomatous pleural effusion – A case report. Respir Med Case Rep. 2012;5:59–61. doi: 10.1016/j.rmedc.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nayak MD, Suvarna N, Belurkar SV, Srilatha PS, Khanna R. Myelomatous pleural effusion: A case report. [Last accessed on 2016 May 06];Int J Sci Res Publ. 2013 3:1–2. Available from: http://www.ijsrp.org/research-paper-0213/ijsrp-p14111.pdf . [Google Scholar]
- 24.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–71. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 25.Rosiñol L, Cibeira MT, Bladé J, Esteve J, Aymerich M, Rozman M, et al. Extramedullary multiple myeloma escapes the effect of thalidomide. Haematologica. 2004;89:832–6. [PubMed] [Google Scholar]


