Abstract
Current surface sampling methods for microbial contaminants are designed to sample small areas and utilize culture analysis. The total number of microbes recovered is low because a small area is sampled, making detection of a potential pathogen more difficult. Furthermore, sampling of small areas requires a greater number of samples to be collected, which delays the reporting of results, taxes laboratory resources and staffing, and increases analysis costs. A new biological surface sampling method, the Biological Sampling Kit (BiSKit), designed to sample large areas and to be compatible with testing with a variety of technologies, including PCR and immunoassay, was evaluated and compared to other surface sampling strategies. In experimental room trials, wood laminate and metal surfaces were contaminated by aerosolization of Bacillus atrophaeus spores, a simulant for Bacillus anthracis, into the room, followed by settling of the spores onto the test surfaces. The surfaces were sampled with the BiSKit, a cotton-based swab, and a foam-based swab. Samples were analyzed by culturing, quantitative PCR, and immunological assays. The results showed that the large surface area (1 m2) sampled with the BiSKit resulted in concentrations of B. atrophaeus in samples that were up to 10-fold higher than the concentrations obtained with the other methods tested. A comparison of wet and dry sampling with the BiSKit indicated that dry sampling was more efficient (efficiency, 18.4%) than wet sampling (efficiency, 11.3%). The sensitivities of detection of B. atrophaeus on metal surfaces were 42 ± 5.8 CFU/m2 for wet sampling and 100.5 ± 10.2 CFU/m2 for dry sampling. These results demonstrate that the use of a sampling device capable of sampling larger areas results in higher sensitivity than that obtained with currently available methods and has the advantage of sampling larger areas, thus requiring collection of fewer samples per site.
Biological warfare agents, such as the spores of Bacillus anthracis, can be efficiently dispersed in aerosols. Once released, the particles eventually settle onto surfaces, as occurred at the Hart Senate Office building in Washington, D.C., on 15 October 2001. Research shows that surface-associated biocontaminants can become reaerosolized, resulting in exposure of building occupants (6). Currently, locations where a pathogen may have been released are sampled to identify the presence of a contaminant, to map the extent of contamination, and to conduct forensic analysis for law enforcement. However, released microbial agents may not be detected by currently available surface sampling methodologies that sample small areas and utilize culture analysis for detection. When a small area is sampled, the total number of microorganisms recovered is low, making detection of a potential pathogen more difficult. Furthermore, small-area sampling requires a greater number of samples to be collected, delaying the reporting of results, taxing laboratory resources and staff, and increasing analysis costs. An alternative to the commonly used swab sampling method is a cellulose sponge-based Swipe kit (BIO-HAZ kit; EAI Corporation, Abingdon, Md.). Although the Swipe kit has been shown to be an effective system (2), it contains many components that can be difficult to handle in a potentially contaminated environment. Personal protective equipment, such as neoprene gloves, makes manipulation of tiny pieces, tearing of bags, and handling of vials difficult. Sponge kits designed as single-use kits were meant to be a cost-effective means to sample suspected bioterrorism laboratories and production plants, but they were never intended to sample settings such as construction sites or subways or to collect a number of samples in quick succession.
The Biological Sampling Kit (BiSKit) is designed to sample surfaces for bacteria, viruses, and toxins and to deliver a sample in a liquid with sufficient volume for extensive testing and archiving. This kit is designed to (i) minimize cross-contamination when multiple samples are taken, (ii) be transportable following sampling, and (iii) safely capture the liquid sample in a collection vial that can dispense fluid drop-wise for analysis. The unit should be particularly useful when multiple samples are taken due to the ease of use and because it minimizes the possibility of cross-contamination.
Validation is vital for any sampling kit that is used by inspection teams, first responders, and agencies tasked to collect samples for forensics. Therefore, the overall objectives of this project were to evaluate the BiSKit for sampling of surfaces for biological contaminants and to compare the results with the results of swab-based sampling. Standard operating procedures for use of the BiSKit were also developed.
MATERIALS AND METHODS
Test organisms and culture media.
Bacillus atrophaeus, a simulant for B. anthracis, was used in this study. Dry B. atrophaeus spores were obtained from the Critical Reagents Program antigen repository (U.S. Army Dugway Proving Ground, Dugway, Utah). B. atrophaeus was cultured on tryptic soy agar (TSA) (pH 7.0) (Difco Laboratories, Detroit, Mich.) and incubated at 28°C for 24 to 48 h.
Surface sampling methods.
In the BiSKit (QuickSilver Analytics, Abingdon, Md.) a foam material (Willsorb; Illbruck Custom Foam Fabrication, Minneapolis, Minn.) is integrated into a screw-on lid, enabling either wet or dry sampling (Fig. 1). Selection of the foam sampling matrix, performed by Veridian (Charlottesville, Va.), was based on the following criteria: ability to perform wet and dry biological sampling, compatibility with microbial culture and PCR analyses, ability to take up liquid, abrasion resistance, sterility, and cost. Buffer can be added to the unit prior to use to prewet the foam when sampling in a wet format is performed. The top of the unit screws onto the base to seal the fluid and the wetted foam until they are needed. The sampler can also be used dry when testing cannot occur in a timely fashion or when the sample must be stored for long periods of time before it is analyzed. For this application the sampler is hydrated and processed in the laboratory, which allows the user to dispense a liquid sample just prior to testing.
FIG. 1.
BiSKit. The components include a BiSKit sampling unit, a 30-ml sample collection bottle with buffer, a dropper attachment, and a rubber cap.
For wet sampling, the foam collection material was hydrated with 20 ml of sterile 0.01 M potassium phosphate buffer with 0.05% Tween 20 (PBT) (pH 7.0) (Sigma-Aldrich Co., St. Louis, Mo.). The top of the unit containing the wetted foam was then unscrewed from the base, a 1-m2 surface was sampled, and the top of the unit was then screwed back onto the casing. The sample was processed by screwing the top of the unit into the base to the locked position, which compressed the foam and caused the test fluid to flow through a grating; the fluid was collected in the sample dropper bottle. The top of the unit was then repeatedly unscrewed and screwed into the base until the procedure was performed a total of seven times. For dry sampling, surfaces were sampled with a dry BiSKit, and the sample was processed by hydrating the foam with 20 ml of PBT and then squeezing the liquid sample from the BiSKit by screwing and unscrewing the top of the unit into the base as described above. Experiments were also conducted to determine the concentration of microorganisms remaining in the foam collection material after the BiSKit was processed. For these experiments, the BiSKit foam collection material was removed and placed in a sterile bag with 20 ml of PBT. The foam was squeezed by hand repeatedly for 1 min, and the liquid sample was analyzed.
Cotton swab sampling kits (Critical Reagents Program) consisted of two cotton swabs and a vial containing phosphate-buffered saline with an organic antimicrobial agent, Kathon (Supelco Inc., State College, Pa.), and Triton X-100 (pH 7.4) inside a 50-ml conical centrifuge tube. The sampling protocol consisted of wetting a cotton swab with the kit buffer and swabbing from left to right over a 100-cm2 area (4 by 4 in.). The sampling area was then swabbed a second time by using the same swab but with a 90° change in the sampling orientation and turning the swab over to sample with the unused side of the swab. The stick of the swab was then broken off inside the buffer tube, and the tube was capped. Prior to analysis, the buffer tube was shaken for 30 s, and the swab was removed and discarded.
The Swab Sample Processing (SSP) kit (ASD, Ft. Lauderdale, Fla.) was utilized according to the manufacturer's protocol, which consisted of moistening a 317-cm2 area (7 by 7 in.) of a surface with 20 drops of the supplied buffer, followed by sampling with a foam swab. Sampling was conducted by swabbing the first half of the surface material area, turning the swab over to expose the unused side, and swabbing the second half of the surface material. The foam swab was placed in a collection bottle containing 55 drops of buffer, incubated for 15 s, and then hand mixed in the buffer for 1 min (manufacturer's protocol, fraction B). The swab was then pressed against the sides of the bottle to remove the liquid sample from the foam, and the swab was discarded. Laboratory studies indicated that the highest concentration of the target organism was present in fraction B, and further purification of the liquid sample, as described in the manufacturer's protocol, resulted in losses of B. atrophaeus. Therefore, fraction B was analyzed for all SSP kit samples.
Experimental room.
We used an experimental room that was designed to resemble a residential indoor environment. As previously described (1), the room is 4.0 by 4.0 m by 2.2 m high and has a sheet vinyl tile floor. The interior walls, exterior walls, and ceiling are covered with gypsum wallboard and coated with interior latex paint. The room is equipped with a heating, ventilation, and air conditioning (HVAC) system whose size simulates the size of a residential system with rectangular bare metal ductwork. During aerosolization experiments, the HVAC system was operated with an airflow of 4.2 m3/min, resulting in approximately seven room air volume exchanges per hour. The room was maintained with a positive static pressure (0.02 in. of water) during operation to minimize contamination from the surrounding area. An anteroom equipped with a HEPA-filtered air shower attached to the room entrance reduced mixing of air resulting from entering and exiting from the room during experiments. The temperature was monitored by 20 type T thermocouples (Thermo Electric Co., Saddle Brook, N.J.), and the relative humidity was monitored by five relative humidity probes (Hy-Cal Engineering, El Monte, Calif.) located within the room. During all activities in the experimental room, technicians wore full-face respirators and nonwoven protective clothing. Upon completion of each series of experimental room trials, contaminated surface materials were removed and the interior surfaces of the room were disinfected with a 10% household bleach solution.
B. atrophaeus spore aerosolization.
Dry B. atrophaeus spores were aerosolized into the experimental room by acoustic vibration by using a Pitt 3 dry aerosol generator as previously described (2). Particle concentrations in the room were measured by using a model 3320 aerodynamic particle sizer (TSI Inc., St. Paul, Minn.). Following aerosolization of B. atrophaeus spores, the room HVAC system was turned off to permit deposition of spores onto surfaces in the room. After settling of the B. atrophaeus aerosol, surface samples were collected and analyzed as described below.
Culture analysis.
Culture analysis was performed with liquid samples containing B. atrophaeus by inoculation onto TSA and incubation at 28°C for 24 to 48 h. Samples were either concentrated by filtration of 1 ml through 47-mm-diameter, 0.45-μm-pore-size mixed cellulose-ester membranes (Pall Corp., Ann Arbor, Mich.) or serially diluted in 0.01 M potassium phosphate buffer (pH 7.0) prior to inoculation onto TSA. The samples were incubated, and the number of CFU per milliliter and the number of CFU per sample were determined.
DNA extraction and purification.
A previously developed DNA extraction and concentration protocol was used for preparation of quantitation standards and all surface samples (2). Before extraction, BiSKit and cotton swab liquid samples were concentrated by filtration through a 0.65-μm-pore-size HA filter membrane (Millipore Corp., Bedford, Mass.), and the filter was resuspended in 0.5 ml of PBT. For BiSKit and cotton swab samples, the entire sample volume remaining after culture analysis was concentrated. Some samples required prefiltration through a 0.80-μm-pore-size membrane, and both membranes were resuspended in PBT. Due to the small sample volume obtained with the SSP kit, the samples were not filter concentrated, and a 0.5-ml aliquot was reserved for DNA extraction.
Each concentrated or reserved sample was pretreated with sodium dodecyl sulfate (final concentration, 0.5%) and proteinase K (final concentration, 20 μg/ml), incubated at 50°C for 5 min, and then boiled for 15 min. The sample was chilled on ice for 2 min, and bovine serum albumin (final concentration, 0.05%) was added; this was followed by incubation for 5 min at 37°C in a rotary shaker at 230 rpm. The membrane was removed from the BiSKit and cotton swab samples, and the DNA from all samples was purified by using the Pellet Paint protocol (Novagen, Madison, Wis.) and resuspended in 50 μl of Tris-EDTA buffer (pH 8.0). PCR quantitation standards were prepared from a purified B. atrophaeus spore suspension that was enumerated electronically with a Coulter Multisizer II (Beckman Coulter, Inc., Hialeah, Fla.) by using the same DNA extraction and purification methods used to process samples.
Quantitative PCR (QPCR).
The ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, Calif.) was used for PCR analysis. A segment of the B. atrophaeus recA gene was amplified by using primer and probe sequences obtained from the Naval Medical Research Center (Silver Spring, Md.), which produced a 131-bp amplicon. The sequences of the primers were as follows: ACCAGACAATGCTCGACGTT (forward) and CCCTCTTGAAATTCCCGAAT (reverse). The TaqMan probe sequence was 6-carboxyfluorescein-5′-ACTGAACAGCTGATCGAGACAGCTGCA-3′-6-carboxytetramethyl rhodamine. Primers were obtained from QIAGEN Operon (Alameda, Calif.), and the probe was obtained from Applied Biosystems. The amplification conditions specified by the Naval Medical Research Center for use with Applied Biosystems reagents were as follows (total reaction volume, 50 μl): B. atrophaeus DNA template, 1× TaqMan buffer A, 5 mM MgCl2, 0.1 mM dATP, 0.1 mM dCTP, 0.1 mM dGTP, 0.2 mM dUTP, 2.5 U of AmpliTaq Gold, 0.5 U of AmpErase uracyl N-glycosylase, each primer at a concentration of 0.2 μM, and 0.2 μM probe. The TaqMan cycling conditions were as follows: 2 min at 50°C; 10 min at 95°C; and 40 cycles of 15 s at 95°C followed by 1 min at 60°C. An internal positive control (IPC) (IPC-VIC probe; Applied Biosystems) was incorporated into selected PCR amplification mixtures to determine whether the samples contained PCR inhibitors. The IPC kit consisted of control DNA, primers, and a specific probe. The fluorescent probe was labeled with a dye that was different from the target DNA probe dye to allow differentiation of fluorescent signals generated during amplification. A known amount of IPC DNA was amplified with the sample, and inhibition was observed by a change in amplification of control DNA.
Quantitation was achieved by amplification of standards containing B. atrophaeus DNA extracted from spore suspensions having known concentrations (100 to 105 templates per reaction mixture). Extraction of standards by the same method that was used for samples provided absolute quantitation of B. atrophaeus templates and corrected for the occurrence of external B. atrophaeus DNA present in the samples, assuming that the amount of external DNA present in B. atrophaeus spore populations had a normal distribution. Standards were amplified in duplicate at the same time and under the same conditions as the replicate unknown samples. Once amplification was completed, the data were analyzed by using the software provided with the ABI Prism 7700 sequence detection system. By using the concentrations assigned to each standard, the software constructed a standard curve of Ct value versus concentration; Ct refers to the PCR cycle at which fluorescence (i.e., the amplification product) is first detected, and it is inversely proportional to the initial DNA template concentration. Concentration values for the unknown samples were extrapolated from the standard curve by the software and are reported as the means of two replicates. The mean values for templates for each reaction were converted to the number of templates per milliliter and the number of templates per sample.
HHA.
Hand-held assays (HHAs) (Critical Reagents Program) were used for analysis of selected samples. The B. atrophaeus HHA consists of an individually wrapped nitrocellulose immunochromatographic strip containing immobilized antibodies specific for the target organism, B. atrophaeus. Application of a liquid sample initiates a color change reaction when B. atrophaeus is present at a level of ≥105 spores. The HHA has a control well and a test well. If a pink or red line develops in both the control and test wells, the test is considered positive. If a pink or red line develops only in the control well, the test is considered negative. The HHAs were stored at 4°C until they were used. One hundred microliters of each surface sample was dispensed into the HHA sample well and incubated at room temperature for 15 min.
Experimental design.
The BiSKit was compared with the SSP kit and cotton swab sampling methods in experimental room trials. Wood laminate or metal surfaces were contaminated by aerosolization of dry B. atrophaeus spores into the room. The surface sample areas were 1 m2 for the BiSKit, 317 cm2 for the SSP kit, and 100 cm2 for the cotton swabs. A total of eight samples were collected by each method from wood laminate surfaces, and four samples were collected by each method from metal surfaces. Samples were processed and analyzed by culture and by QPCR.
The sampling efficiency of the BiSKit was determined for wet and dry sampling formats. One-square-meter areas of metal sheets were inoculated with a liquid suspension containing 105 B. atrophaeus spores and allowed to air dry. Samples were collected with either the wet BiSKit (n = 4) or the dry BiSKit (n = 8), processed as described above, and analyzed by culturing. The foam was removed from the lid of the BiSKit and processed a second time by hand mixing in buffer to determine the amount of culturable B. atrophaeus remaining in the foam. The sampling efficiencies were estimated by dividing the number of CFU per sample recovered by the number of CFU applied.
The effectiveness of the wet and dry sampling formats was also compared in experimental room aerosolization trials. Dry aerosols of B. atrophaeus spores were released into the room and allowed to settle onto 1-m2 metal and wood laminate surfaces. Samples were collected with either the wet BiSKit (n = 4) or the dry BiSKit (n = 4), processed as described above, and analyzed by culturing and PCR. The foam was removed and processed a second time by hand mixing in buffer to determine the amount of culturable B. atrophaeus remaining in the foam.
The sensitivity of detection of B. atrophaeus for metal surfaces in the experimental room was measured. Metal surfaces in the experimental room were inoculated with a liquid B. atrophaeus spore suspension containing 103 CFU and allowed to air dry. Wet and dry surface samples (area, 1 m2) were collected with the BiSKit. The lower limit of detection was calculated based on the sampling efficiency measured and the detection of 1 CFU in a 1-ml filter sample. The data obtained from sampler comparison and sampling efficiency trials were analyzed statistically by comparing the mean values by the analysis of variance and Student's t test methods.
RESULTS
Sampler comparison.
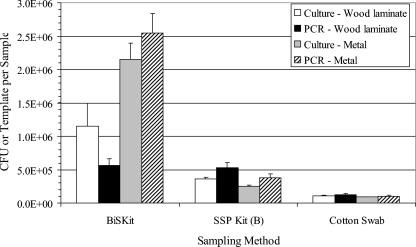
Three sampling methods, the BiSKit, SSP kit, and cotton swab methods, were compared by using experimental room trials. Wood laminate or metal surfaces were contaminated by aerosolization of B. atrophaeus spores into the room. The results demonstrated that the BiSKit collected significantly more B. atrophaeus spores per sample, regardless of the test material or assay, than the SSP kit or the cotton swab method (P ≤ 0.003) (Fig. 2). There was one exception, in which there was no difference between the BiSKit and the SSP kit in terms of the amount of B. atrophaeus measured on wood surfaces as determined by QPCR analysis (P = 0.79). For BiSKit samples, more B. atrophaeus spores were detected on metal surfaces than on wood laminate by both culturing and QPCR. When the data were expressed as the number of B. atrophaeus spores measured per unit area, both the SSP kit and cotton swab methods collected greater numbers of B. atrophaeus spores per square centimeter (data not shown). The BiSKit values per unit of area ranged from 4- to 10-fold less than the values for the SSP kit and cotton swab sampling methods, with the exception of the QPCR values for wood laminate samples, which were more than 1 order of magnitude less than the values obtained with the other sampling methods. Surface samples were also analyzed with B. atrophaeus HHAs, and the results were positive for all sampling methods, although the strongest signal was noted with the BiSKit samples (Table 1).
FIG. 2.
Comparison of sampling methods for experimental room trials. Wood laminate or metal surfaces were contaminated by aerosolization of B. atrophaeus spores into the room. Duplicate samples were collected by each sampling method and were analyzed by culturing and QPCR. The bar heights indicate the means for eight samples (wood laminate) or four samples (metal), and the error bars indicate standard errors. The sample areas were as follows: BiSKit, 1 m2; SSP kit, 317 cm2; cotton swab, 100 cm2.
TABLE 1.
Sample volume measurements and HHA results for BiSKit evaluation trials in the experimental room (n = 8 for wood laminate and n = 4 for metal)
| Sampling method | Surface material
|
|||
|---|---|---|---|---|
| Wood laminate
|
Metal
|
|||
| Sample vol (ml) (mean ± SE) | HHA resultsa | Sample vol (ml) (mean ± SE) | HHA resultsa | |
| BiSKit | 2.1 ± 0.3 | +++ | 4.6 ± 0.5 | +++ |
| SSP kit | 1.4 ± 0.1 | ++ | 1.4 ± 0.1 | ++ |
| Cotton swab | 3.3 ± 0.05 | + | 3.5 ± 0.03 | + |
The scale ranged from − to ++++.
The BiSKit samples were processed a second time to determine the concentration of B. atrophaeus spores remaining in the foam collection material. The results showed that a high percentage of the B. atrophaeus spores removed by surface sampling remained trapped in the foam after the initial processing (data not shown). PCR amplifications performed with BiSKit samples with an internal positive control present in the reaction mixture showed that there was no inhibition of the PCR due to the foam material.
Wet sampling versus dry sampling: efficiency and lower detection limit.
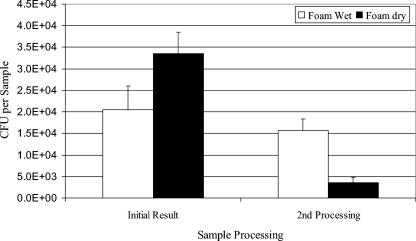
Wet and dry sampling formats were evaluated for the BiSKit in laboratory and experimental room trials. Laboratory trials were conducted with B. atrophaeus spore suspensions that were applied to 1-m2 metal surfaces (inoculum, 1.82 × 105 ± 8.69 × 103 [mean ± standard error]), allowed to dry, and sampled with the BiSKit. The culture results showed that dry sampling was more effective (efficiency, 18.4%; n = 8) than wet sampling (efficiency, 11.3%; n = 4) (Fig. 3); however, the difference was not significant (P = 0.13). A second processing of the foam collection material showed that more B. atrophaeus remained in the foam after wet sampling than after dry sampling. The sum of the data from the first sample processing and the second sample processing indicated that wet and dry sampling collected equivalent numbers of B. atrophaeus spores from surfaces, but the initial processing of the dry samples yielded more B. atrophaeus spores from the foam. This observation may be related to differences in sample volume after processing. The average sample volumes were 3.3 ml after wet sample processing and 16.1 ml after dry sample processing. This indicates that much of the buffer present on the wet foam was lost to the surface during sampling and that the low buffer volume remaining in the foam resulted in decreased B. atrophaeus spore removal from the foam during processing. Recovery of B. atrophaeus spores from the foam after wet sampling may be improved by adding more buffer to the BiSKit before processing.
FIG. 3.
Comparison of wet and dry sampling with the BiSKit in the experimental room. Metal surfaces (1 m2) were contaminated with 105 B. atrophaeus spores in a liquid suspension and allowed to dry. Samples were collected and were analyzed by culturing. Following initial processing, a sample was processed a second time by removing the foam collection material and hand mixing in buffer for 1 min. The bar heights indicate the means for eight samples (dry) or four samples (wet), and the error bars indicate standard errors.
The sensitivity of detection of B. atrophaeus spores was measured for metal surfaces inoculated with B. atrophaeus spore suspensions in the experimental room. Culture analysis showed that the lower limits of detection, based on 1 CFU in a 1-ml filter sample, were 42 ± 5.8 CFU/m2 (mean ± standard error) for wet sampling (n = 8) and 100.5 ± 10.2 CFU/m2 for dry sampling (n = 8). It should be noted that although the sampling efficiency was lower for the wet sampling method than for the dry sampling method, the wet samples were more concentrated due to the low sample volume and therefore had a greater sensitivity of detection when they were used directly for biological detection assessment.
Surface sampling after B. atrophaeus aerosolization.
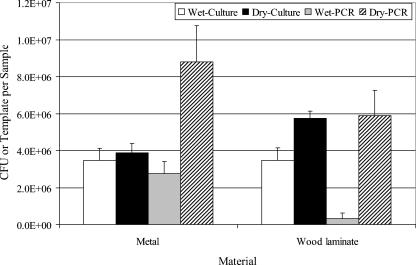
Experimental room trials were performed with dry aerosols of B. atrophaeus spores that were released and allowed to settle onto 1-m2 metal and wood laminate surfaces. The surfaces were sampled with wet or dry foam BiSKits and analyzed by culturing and PCR. The culture data from these experiments confirmed the seeding experiment results in that dry sampling of surfaces yielded greater concentrations of B. atrophaeus spores than wet sampling yielded (Fig. 4). The difference was significant for wood laminate surfaces (P = 0.03) but not significant for metal surfaces (P = 0.64). PCR data also demonstrated that dry sampling yielded greater concentrations of B. atrophaeus spores than wet sampling yielded (P = 0.03 for metal; P = 0.007 for wood laminate). The PCR measurements for B. atrophaeus spore concentrations were greater than the culture measurements obtained with dry sampling but were less than the culture measurements obtained with wet sampling. A second processing of the BiSKit foam collection material showed that equivalent numbers of B. atrophaeus spores were removed from surfaces by wet and dry sampling (data not shown); however, initial processing of the dry samples yielded more B. atrophaeus spores from the foam, which confirmed laboratory results.
FIG. 4.
Comparison of wet and dry sampling with the BiSKit in the experimental room. Metal and wood laminate surfaces (1 m2) were contaminated by aerosolization and deposition of dry B. atrophaeus spores in the room. Samples were collected and were analyzed by culturing and PCR. The bar heights indicate the means for four samples, and the error bars indicate standard errors.
DISCUSSION
Several sampling methods were used in attempts to detect biocontamination on surfaces following the Washington, D.C., area bioterrorist attacks, including swabs, wipes, and HEPA vacuum socks (3, 5, 6). While the data obtained with these sampling methods were compared (5), the sampling efficiencies and lower detection limits for these sampling methods have not been established, with the exception of a study in which the sampling efficiencies of four swab materials were compared (4). Another concern is the large number of samples that can be generated by sampling methods, such as small cotton swabs, that are capable of sampling only small surface areas that are approximately the size of a postcard. For example, 3,000 swab samples were collected in a 3-week period in one study (3). A potential advantage of the BiSKit is the ability to rapidly sample large areas (1 m2), which can result in increased total recovery and greater sensitivity while generating fewer samples. Preliminary experiments showed that BiSKit samples contained approximately 10-fold-greater numbers of B. atrophaeus spores than the cotton swab and SSP kit samples contained. While the numbers of B. atrophaeus spores collected per unit of area were less for the BiSKit than for the other two methods, the large surface area sampled with the BiSKit increased the total amount of B. atrophaeus collected per sample.
Experimental room trials were performed with B. atrophaeus spore aerosolization and subsequent sampling of B. atrophaeus spore-contaminated surfaces. The BiSKit was evaluated and compared to the cotton swab and SSP kit methods. The culture results showed that the BiSKit sampler collected more B. atrophaeus spores per sample than the SSP kit or cotton swab methods collected due to the large surface area sampled with the BiSKit. It was also observed that the type of surface sampled influenced the data obtained. In room trials, more B. atrophaeus spores were recovered from metal surfaces than from wood laminate surfaces with the BiSKit.
If it is impractical to analyze liquid samples in a timely manner, it may be preferable to sample with a dry format and resuspend the sample at a later time just prior to analysis. Dry sampling with the BiSKit indicated that dry sampling was more efficient (efficiency, 18.4%) than wet sampling (efficiency, 11.3%) in terms of the amount of B. atrophaeus spores collected per sample. Analysis of the foam material after processing showed that the wet and dry sampling methods collected equivalent numbers of B. atrophaeus spores from the surfaces, but more B. atrophaeus spores were released from the dry foam with additional processing. This was probably due to the fact the only 3 to 4 ml of buffer could be squeezed from the foam after wet sampling; the remainder of the 20 ml of hydration liquid was lost to the surface during sampling. In contrast, the dry foam was hydrated with 20 ml after sampling, so there was more liquid available to remove the B. atrophaeus spores from the foam during processing. Although wet sampling was less efficient than dry sampling, the samples were approximately threefold more concentrated due to the low sample volume after wet sampling (sample volume, approximately 3 ml) than after dry sampling (sample volume, approximately 16 ml). Therefore, the samples tested following wet sampling provided a greater sensitivity of detection (42 ± 5.8 CFU/m2) than the samples tested following dry sampling (100.5 ± 10.2 CFU/m2).
Additional work is being conducted to improve both the wet and dry sampling protocols to increase the overall sampling efficiency. For dry sampling, it was observed that there was a considerable drag of the BiSKit during sampling due to friction between the foam and the surfaces sampled. Reducing friction by applying a lubricant coating to the foam may increase the ease of use and the efficiency of dry sampling. Another alternative is prewetting the area to be sampled with a light mist from a spray bottle, sampling with a dry BiSkit, and then washing the BiSkit sponge with the bottled buffer.
The results of these experiments demonstrate the utility of the BiSKit for sampling biocontaminated surfaces. The primary advantage of the BiSKit is its ability to rapidly sample a larger area and deliver biological agents in a concentrated sample. Large-area surface sampling translates into greater sensitivity for detection and generates fewer samples compared with other methods. The liquid sample can be analyzed by a variety of methods, including culturing, PCR and HHAs. In addition, dry sampling is a potential alternative to wet sampling with the BiSKit, which increases the capability of the sampler and should be useful when analysis of recovered samples is expected to be delayed.
Acknowledgments
This work was supported by the Joint Program Executive Office for Chemical and Biological Defense.
We thank engineers from the Computer Aided Engineering Team at the U.S. Army Edgewood Chemical Biological Center, Aberdeen Proving Ground, Md., for their work in designing and supplying BiSKit units; Veridian Systems Division, Charlottesville, Va., for evaluating alternative collection matrices for use with the BiSKit; and MIT Lincoln Laboratory, Lexington, Mass., for testing potential PCR sample preparation methods.
REFERENCES
- 1.Buttner, M. P., and L. D. Stetzenbach. 1993. Monitoring of fungal spores in an experimental indoor environment to evaluate sampling methods and the effects of human activity on air sampling. Appl. Environ. Microbiol. 59:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buttner, M. P., P. Cruz-Perez, and L. D. Stetzenbach. 2001. Enhanced detection of surface-associated bacteria in indoor environments by quantitative PCR. Appl. Environ. Microbiol. 67:2564-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins, J. A., M. Cooper, L. Schroeder-Tucker, S. Black, D. Miller, J. S. Karns, E. Manthey, R. Breeze, and M. L. Perdue. 2003. A field investigation of Bacillus anthracis contamination of U.S. Department of Agriculture and other Washington, D.C., buildings during the anthrax attack of October 2001. Appl. Environ. Microbiol. 69:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose, L., B. Jensen, A. Peterson, S. N. Banerjee, and M. J. Arduino. 2004. Swab materials and Bacillus anthracis spore recovery from nonporous surfaces. Emerg. Infect. Dis. 10:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanderson, W. T., M. J. Hein, L. Taylor, B. D. Curwin, G. M. Kinnes, T. A. Seitz, T. Popovic, H. T. Holmes, M. E. Kellum, S. K. McAllister, D. N. Whaley, E. A. Tupin, T. Walker, J. A. Freed, D. S. Small, B. Klusaritz, and J. H. Bridges. 2002. Surface sampling methods for Bacillus anthracis spore contamination. Emerg. Infect. Dis. 8:1145-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weis, C. P., A. J. Intrepido, A. K. Miller, P. G. Cowin, M. A. Durno, J. S. Gebhardt, and R. Bull. 2002. Secondary aerosolization of viable Bacillus anthracis spores in a contaminated U.S. Senate office. JAMA 288:2853-2858. [DOI] [PubMed] [Google Scholar]