Sir,
Hereditary hemorrhagic telangiectasia (HHT) is one of the rare diseases associated with a variety of manifestations such as skin lesions, mucosal bleed, and visceral arteriovenous malformations, and their nonfatal and fatal complications such as hemothorax and cerebral abscess. It is inherited as an autosomal dominant pattern and many times it may manifest later in life like pregnancy when there is altered hemodynamics. Here, we present to you a case of Osler-Weber-Rendu syndrome (hereditary HHT) in pregnant patient highlighting role of its prompt diagnosis and appropriate, timely treatment to save life of the mother and the fetus.
A 25-year-old female, housewife, resident of Nashik, non-addict, 23rd weeks of gestation (Gravida 6, Para 4) presented with sudden onset left-sided pleuritic chest pain and breathlessness at rest following severe bout of cough since 11 days. The patient had received antituberculous treatment 3 years ago in view of right-sided exudative pleural effusion.
On examination, the patient was afebrile, heart rate of 110 beats/min, respiratory rate of 26 breaths/min with use of accessory muscles of respiration, and blood pressure 110/80 mm Hg. Trachea was deviated toward the right side, and breath sounds were absent on left side. Per abdomen revealed 22 weeks size uterus with fetal heart sound present. Cardiovascular and CNS examination were normal. The patient had undergone pleural aspiration, in other center before referral to our center, under ultrasound guidance in view of chest radiograph suggestive of massive pleural effusion and 150 mL blood aspirated from hemothorax. Chest radiograph was suggestive of massive left pleural effusion with a shift of mediastinum to the right [Figure 1]. After reviewing above scenario computed tomography of chest (plain and angiography) with double lead abdominal shield was done was suggestive of Left massive hemothorax with lingular arteriovenous malformation [Figure 2]; aneurysmal dilatation of arteriovenous malformation to 1.5 cm diameter from left pulmonary artery draining into a left pulmonary vein. Pulmonary artery dil. 3.0 cm mediastinal shift to the right, bulging of the chest and splaying of ribs. For which patient underwent successful transcatheter embolotherapy (TCE) with various 0.018–14-4 Nester and 0.018–4-6 Hilal coils after super selective cannulation of feeder artery by 2.7 Fr microcatheter (Progreat, Terumo). Postembolization angiogram showed complete exclusion of pseudoaneurym from the pulmonary circulation [Figure 3]. Postprocedure chest radiograph showed endovascular coils and decrease in the size of hemothorax.
Figure 1.
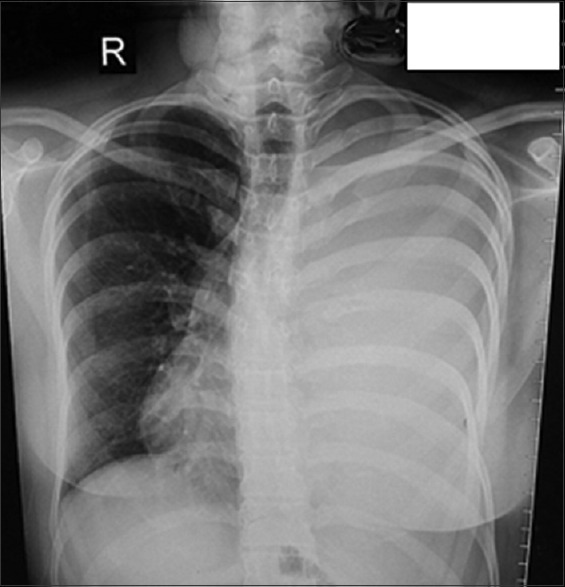
Massive left sided pleural effusion with shift of mediastinum to right
Figure 2.
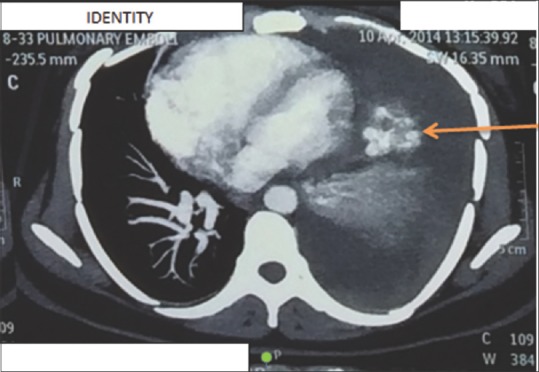
Computed tomography pulmonary angiography suggestive of lingular arteriovenous malformation with massive pleural effusion
Figure 3.
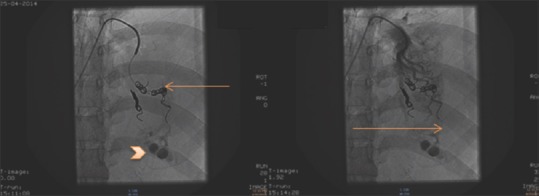
Digital subtraction angiography and endovascular coiling (embolotherapy): Third coil placed with residual trapped contrast (arrowhead) in arteriovenous malformation. No blush of contrast in arteriovenous malformation after embolotherapy
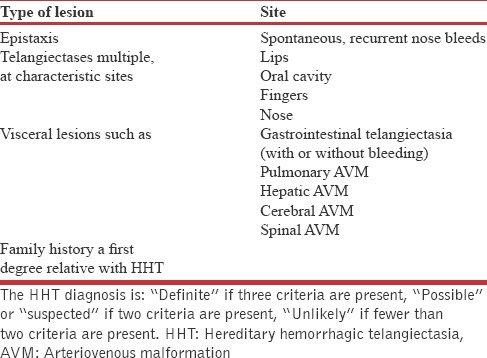
In our patient, after knowing history of recurrent epistaxis and computed tomography suggestive of arteriovenous malformation, when enquired also revealed history of family history of brain hemorrhage in five second-degree relatives and on examination found to have one small pin head size hemangioma on right buccal mucosa and hence the diagnosis of hereditary HHT was made as per the Curaçao criteria as presented in Shovlin et al. [Table 1].[1]
Table 1.
The Curaçao criteria for diagnosis of Hereditary hemorrhagic telangiectasia
Pulmonary arteriovenous malformations (PAVMs) with feeding arteries of ≥3 mm in diameter are associated with stroke; brain abscess, hemothorax, and life-threatening hemoptysis.[2] Patients with HHT and a PAVM with a feeding artery of ≥3 mm have improved life expectancy and quality-adjusted survival with immediate embolotherapy. Embolotherapy should be the standard of care in such circumstances.[3,4]
Fetal-related complications as a result of exposure to radiation include spontaneous abortion, teratogenesis, growth retardation, and developmental delay; the dose of radiation believed to produce these complications is very large compared to the doses used in our modified version of TCE. Usually, the generous estimates of radiation dose to the fetus ranges from 50 to 220 mrad, less than half of the maximum recommended occupational exposure (500 mrad) for a pregnant worker.
In literature, 8% PAVM related complications occur in the first trimester as compared to 85% during the second trimester. Therefore, the best approach to managing women with PAVMs who present early is to closely observe them through the first trimester, and then perform TCE in the second trimester.[5]
In a study done by Shovlin et al.,[6] all 262 pregnancies in the 111 women with HHT and PAVMs reviewed between 1999 and 2005 were studied. Eighty-two women (74%) did not have a diagnosis of HHT/PAVM at the time of pregnancy. Thirteen women experienced life-threatening events during pregnancy: 1.0% (95% confidence interval 0.1–1.9) of pregnancies resulted in a major PAVM bleed; 1.2% (0.3–2.2%) in stroke (not all were HHT related); and 1.0% (0.13–1.9%) in maternal death. All deaths occurred in women previously considered well. In women experiencing a life-threatening event, prior awareness of HHT or PAVM diagnosis was associated with improved survival (P = 0.041, Fisher's exact test).
Our patient underwent TCE successfully in the second trimester and delivered healthy newborn at full-term.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome) Am J Med Genet. 2000;91:66–7. doi: 10.1002/(sici)1096-8628(20000306)91:1<66::aid-ajmg12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Khurshid I, Downie GH. Pulmonary arteriovenous malformation. Postgrad Med J. 2002;78:191–7. doi: 10.1136/pmj.78.918.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Faughnan ME, Bayoumi AM. Embolization for pulmonary arteriovenous malformation in hereditary hemorrhagic telangiectasia: A decision analysis. Chest. 2009;136:849–58. doi: 10.1378/chest.09-0334. [DOI] [PubMed] [Google Scholar]
- 4.Shovlin CL, Letarte M. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations: Issues in clinical management and review of pathogenic mechanisms. Thorax. 1999;54:714–29. doi: 10.1136/thx.54.8.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershon AS, Faughnan ME, Chon KS, Pugash RA, Clark JA, Bohan MJ, et al. Transcatheter embolotherapy of maternal pulmonary arteriovenous malformations during pregnancy. Chest. 2001;119:470–7. doi: 10.1378/chest.119.2.470. [DOI] [PubMed] [Google Scholar]
- 6.Shovlin CL, Sodhi V, McCarthy A, Lasjaunias P, Jackson JE, Sheppard MN. Estimates of maternal risks of pregnancy for women with hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): Suggested approach for obstetric services. BJOG. 2008;115:1108–15. doi: 10.1111/j.1471-0528.2008.01786.x. [DOI] [PubMed] [Google Scholar]


