Abstract
Epidemiological and experimental evidence suggests that dietary flavonoids, including apigenin, have anticancer roles. Apigenin has been reported to elevate p53, a critical molecule in the induction of apoptosis. The present study aimed to investigate whether apigenin, a dietary flavonoid, improves the cytotoxic effect of cisplatin in a cancer cell culture system, and to elucidate the mechanism of this effect. Multiple tumor cell types were treated with apigenin, cisplatin or both drugs. Cell viability was evaluated, and the cytotoxic effect was determined biochemically and microscopically. Treatment with apigenin increased cisplatin-induced DNA damage and the apoptosis of tumor cells in a p53-dependent manner. Apigenin, when used with cisplatin, inhibited cell proliferation and promoted mitogen-activated protein kinase activation and subsequent p53 phosphorylation, leading to p53 accumulation and upregulation of proapoptotic proteins. Cisplatin is one of the most commonly used chemotherapeutic drugs for malignant tumors, but resistance to this drug occurs. The current results therefore demonstrate that dietary flavonoids may diminish the resistance of cancers to cisplatin.
Keywords: apigenin, cisplatin, apoptosis, p53, cancer
Introduction
Polyphenols, such as epigallocatechin gallate, resveratrol and flavonoids, exist in nature (1). Flavonoids are a group of polyphenols that share similar chemical features and are abundant in vegetables, fruits and tea, amongst other products (2). Humans absorb a large amount of flavonoids orally, and epidemiological studies have revealed that the risk of certain types of cancer, particularly cancers of the breast, digestive tract, skin, prostate and certain hematological malignancies, is inversely correlated with intake of flavonoids (3,4). Flavonoids possess antioxidative, anti-inflammatory and antitumor properties (5). A number of flavonoid compounds have previously been demonstrated to enhance the effects of conventional chemotherapeutic drugs in cancer cells, prompting increased attention (6–8).
Apigenin (4′,5,7-trihydroxyflavone) is a promising chemopreventive agent that is abundantly present in fruits, vegetables, and teas (3). This compound has anticarcinogenic properties via diverse mechanisms and is suggested to have a tumor preventative effect (3,9). The mechanism of action of apigenin appears to involve p53, as apigenin (15–60 µM) has previously been reported to induce the necrosis and apoptosis of neuroblastoma cells expressing wild-type, but not mutant, p53. Apigenin was suggested to elevate the levels of p53, and p53 effector gene expression in these neuroblastoma cells (10). Apigenin also causes cell cycle arrest and sensitizes leukemia cells to vincristine (11). Apigenin has additionally been reported to induce apoptosis of prostate and colon cancer cells through induction of death receptor 5, and to act synergistically with exogenous tumor necrosis factor-related apoptosis-inducing ligand to induce cell apoptosis (12). Furthermore, apigenin enhances the anticancer activity or minimizes the resistance of cancer cells to a number chemotherapeutics, including gemcitabine, paclitaxel, 5-fluorouracil and doxorubicin by inducing apoptosis (13–17). It was reported that apigenin sensitizes neuroblastoma cells to the anticancer drug etoposide by retention of p53 in cell nuclei (18).
The present study aimed to determine the effects of apigenin on cisplatin cytotoxic activity using several tumor cell lines, and to elucidate the potential mechanisms of this. Cisplatin is a member of a class of platinum-containing anticancer drugs, which has demonstrated therapeutic properties against a broad range of cancers (19,20). Cisplatin exerts a DNA-binding effect by cross-linking DNA in several different ways, interfering with mitotic cell division. The damaged DNA prompts induction of DNA repair mechanisms, which activates apoptotic mechanisms when repair proves impossible (21). Many tumors demonstrate good responsiveness to this drug, but drug resistance eventually develops, particularly in patients with lung, colorectal, prostate and ovarian cancer (22). Several approaches have been proposed to maintain cisplatin efficacy, including increased accumulation or stabilization of p53, upregulating reactive oxygen species production and inhibiting NF-κB and antiapoptotic proteins (23–26). The current study investigated the effects of apigenin on p53 accumulation, and its effect on cisplatin cytotoxic activity in several tumor cell lines. The present results demonstrated that apigenin enhances the inhibition of proliferation observed following cisplatin treatment in a p53-dependent manner, potentially enhancing the antitumor effects of cisplatin.
Materials and methods
Cell culture and inhibition of cell growth
The HeLa, A549, HCT 116, H1299, and MCF-7 cells were obtained from the Cell Bank of the Chinese Academy of Sciences and cultured in high-glucose Dulbecco's Modified Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA). H1299 cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific, Inc.). Apigenin, U0126 (a mitogen-activated protein kinase kinase inhibitor), MTT (all Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and cisplatin (Jiangsu Hansoh Pharm. Co., Ltd., Lianyungang, China) were obtained commercially. To determine the effect of the compounds on cell viability, MTT assays were performed. Briefly, ~5×103 cells per well were seeded in 96-well plates (Corning Incorporated, Corning, NY, USA) for 24 h at 37°C. The cells were treated in triplicate with apigenin (in DMSO; final concentration, ≤0.1%) or cisplatin (in PBS). These treatments were 30 µM apigenin for 2 h, followed by different doses of cisplatin (2.5, 5.0 and 10 µM) for another 48 h. At the end of treatment, MTT was added to determine cell viability as previously reported (27).
Western blotting
Western blotting was performed to monitor protein expression and modifications using a standard protocol (28). Briefly, total protein was extracted from the cultured cells using lysis buffer (Sigma-Aldrich; Merck Millipore). The proteins were separated by 10% SDS-PAGE and then transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 2% bovine serum albumin (Roche Diagnostics, Basel, Switzerland) at room temperature for 1 h and incubated overnight at 4°C with primary antibodies. The primary antibodies were against caspase-3 (catalog no. 9662S; dilution, 1:1,000), caspase-9 (catalog no. 9502S; dilution, 1:1,000), poly ADP ribose polymerase (PARP; catalog no. 9542S; dilution, 1:2,000), c-Jun N-terminal kinase (JNK1/2; catalog no. 9252S; dilution, 1:1,000), p-JNK (catalog no. 9251S; dilution, 1:1,000), p38 (catalog no. 9212S; dilution, 1:1,000), p-p38 (catalog no. 9211S; dilution, 1:1,000), cleaved caspase-9 (catalog no. 9501S; dilution, 1:1,000), cleaved caspase-3 (catalog no. 9661S; dilution, 1:2,000) (all Cell Signaling Technology, Inc., Danvers, MA, USA), extracellular signal-regulated kinase (Erk)2 (catalog no. sc-154; dilution, 1:4,000), p-Erk (catalog no. sc-7383; dilution, 1:1,000), caspase-8 (catalog no. sc-56070, dilution, 1:1,000), Bax (catalog no. sc-493; dilution, 1:200), p53 (catalog no. sc-47698; dilution, 1:1,000), p-p53/Ser15 (catalog no. sc-101762; dilution, 1:1,000), mouse double minute 2 homolog (MDM2; catalog no. sc-5304; dilution, 1:1,000) (all Santa Cruz Biotechnology, Inc., Dallas, TX, USA), cleaved caspase-8 (catalog no. PA5-35558; dilution, 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.) and GAPDH (catalog no. AG019; 1:500; Beyotime Institute of Biotechnology, Haimen, China). After washing, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibody (anti-mouse; catalog no. A3682-1ML; dilution, 1:80,000; and anti-rabbit; catalog no. A0545-1ML; dilution, 1:80,000; Abcam, Cambridge, UK) overnight at 4°C. The images were captured using a FluorChem HD2 imaging system (Protein Simple, San Jose, CA, USA) following incubation with an enhanced chemiluminescence reagent kit (Pierce; Thermo Fisher Scientific, Inc.). Protein expression levels were compared with GAPDH expression. The blots were quantified using Adobe Photoshop CS6 (Adobe Inc., San Jose, CA, USA).
DAPI staining
Cell nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) to reveal morphological changes. Briefly, cells (1×105/ml) were grown for 24 h at 37°C on sterilized, fibronectin-coated coverslips to allow cells to attach and spread. The cells were treated with the apigenin and cisplatin (50, 100, 150 or 200 mg/ml) for 48 h, fixed using 4% paraformaldehyde and stained with 50 µl/well of DAPI (1:2,000 dilution in TBST) for 5 min. The cells were then imaged under a BX-53 fluorescence microscope (Olympus Corporation, Tokyo, Japan) equipped with the QImaging system (Surrey, BC, Canada).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to determine p53 gene expression in apigenin and apigenin-cisplatin treated cells. Total RNA was extracted from the cultured cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. RT was performed using 5X AMV buffer, AMV enzyme, 10 mM dNTP and R-primer (Takara Bio Inc., Otsu, Japan) at 16°C for 15 min, 42°C for 1 h and 85°C for 5 min. p53 levels were determined with RT-qPCR. PCR was perfomed using 10X PCR buffer, 10 mM dNTP, Taq enzyme and primers from Takara Inc., and SYBR Green from Applied Biosystems (Thermo Fisher Scientific, Inc.). The following primers were used to amplify the p53 gene: Forward primer, 5′-AACGGTACTCCGCCACC and reverse primer, 5′-CGTGTCACCGTCGTGGA. The following primers were used for the GAPDH gene: Forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′. The cycling conditions were as follows: 95°C for 5 min, 95°C for 15 sec and 60°C for 1 min, for 40 cycles. The relative amount of gene normalized to control was calculated using the 2−ΔΔCq method (29). The mean Cq was calculated from triplicate PCRs.
Statistical analysis
All data were performed at least in triplicate. The results were presented as the mean ± standard deviation of at least three independent experiments. Statistical analysis was performed using Student's t-test by utilizing IBM SPSS Statistics, version 20.0 (IBM, Armonk, NY, USA). P<0.05 was used to indicate a statistically significant difference.
Results and Discussion
Apigenin enhances the cytotoxic effect of cisplatin
To study the effect of apigenin on cisplatin-induced cell death, A549, MCF-7, HCT 116 and HeLa cells that possess wild-type p53 and H1299 cells, which are p53-null due to homozygous deletion of the TP53 gene (30), were treated with apigenin for 2 h, followed by different doses of cisplatin for 48 h, as indicated. The cells were visually inspected under an inverted fluorescence microscope at 24 and 48 h after cisplatin treatment. Cisplatin treatment caused cell death at 48 h. The effect was quantitatively measured using an MTT assay. As reported in Fig. 1, apigenin treatment alone caused a low viablity of cells to 10–30% in all cell lines, while cisplatin treatment alone caused a viability of 3% at low doses to 42% at higher concentrations in HeLa cells. Co-treatment of apigenin at 30 µM with cisplatin at 2.5, 5.0, and 10.0 µM increased the inhibitory effect of cisplatin in all cell lines tested, except the H1299 cells.
Figure 1.
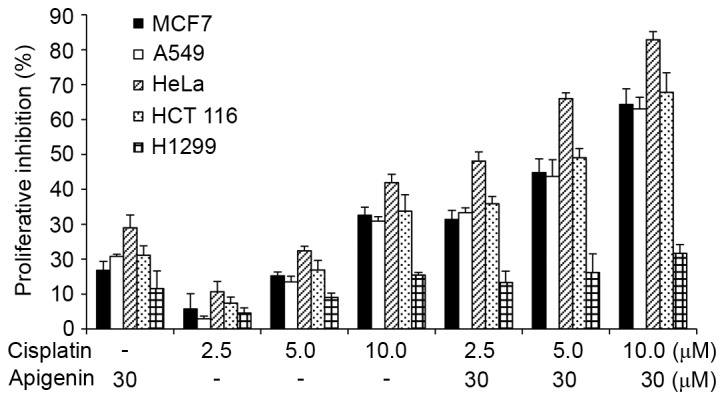
Effect of apigenin and cisplatin on proliferative inhibition of different cancer cell lines, determined by MTT assay. MCF-7, A549, HeLa, HCT 116, or H1299 cells were treated with 30 µM apigenin for 2 h, followed by different concentrations (0, 2.5, 5 or 10 µM) of cisplatin for a further 48 h. The bars represent mean + standard deviation of results obtained from three independent experiments.
Apigenin enhances cisplatin-induced apoptosis
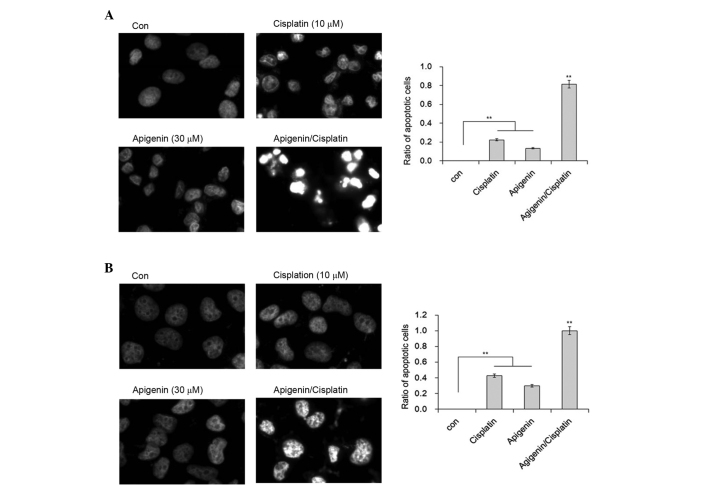
Cisplatin appeared to exert a cytotoxic effect via the induction of apoptosis. Marked cell shrinkage resembling cell apoptosis was observed in apigenin plus cisplatin-treated samples. To confirm whether this shrinkage was due to apoptosis, DAPI staining was performed to illustrate the nuclear morphology of A549 and H1299 cells. Although a proportion of A549 cells demonstrated chromatin condensation in cells treated with cisplatin, inclusion of apigenin resulted in marked changes to chromatin condensation, nuclear shrinkage and the formation of apoptotic bodies in A549 cells (Fig. 2A). In comparison, no nuclear fragmentation was observed in the H1299 cells under the same conditions, although the formation of heterochromatin foci was apparent in these cells (Fig. 2B). These results indicate that apigenin is likely responsible for the apoptotic effect of cisplatin in A549 cells.
Figure 2.
Effect of apigenin and cisplatin on nuclear morphology of cancer cells. (A) A549 or (B) H1299 cells were treated with 30 µM apigenin for 2 h, followed by 10 µM cisplatin for another 48 h. The cells were stained with DAPI and examined under a fluorescence microscope (magnification, ×40). **P<0.01. Con, control.
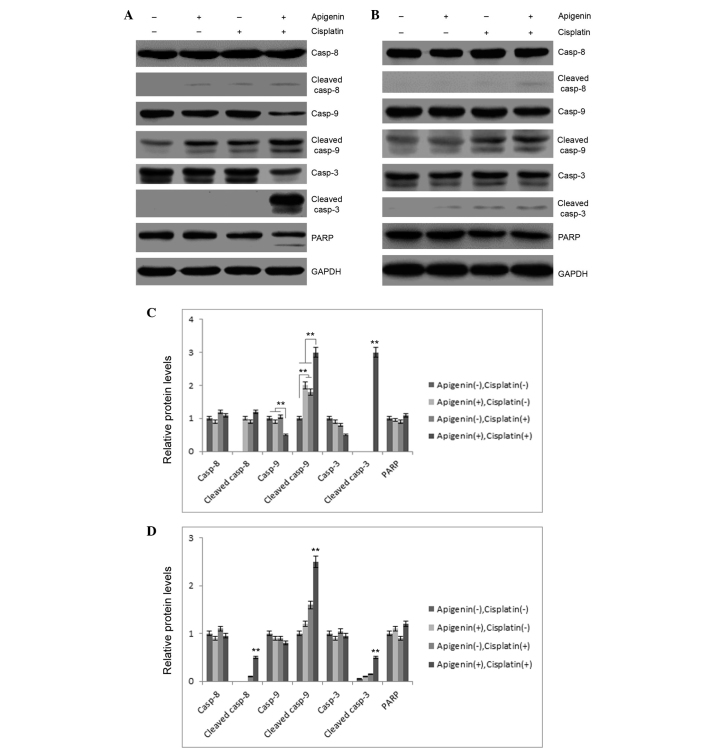
To investigate these results, the role of apigenin and cisplatin treatment in caspase activation was examined. As reported in Fig. 3A, apigenin (30 µM) or cisplatin (10 µM) treatment in A549 cells resulted in partial cleavage of caspase-9, while no marked cleavage of caspase-3 or −8 was detected in these samples. Combined application of apigenin and cisplatin caused significant cleavage of caspase-3 and −9. Concomitantly, PARP cleavage was also detected in apigenin and cisplatin-treated cells (Fig. 3A), indicating that combined application of apigenin and cisplatin promoted activation of caspase-3 and −9. However, combinatorial treatment did not cause significant cleavage of these caspases in H1299 cells, which are p53-null (Fig. 3B). These results indicated that apigenin amplified the apoptosis-inducing activity of cisplatin in A549 cells.
Figure 3.
Effect of apigenin and cisplatin on caspase activation. (A) A549 or (B) H1299 cells were treated with 30 µM apigenin for 2 h, followed by 10 µM cisplatin for another 48 h. Casp-3, −8 and −9 expression and their cleavage or PARP degradation in the two cell lines was evaluated by western blotting. (C) Semi-quantification of (A). (D) Semi-quantification of (B). Casp, caspase; PARP, poly ADP ribose polymerase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.**P<0.01.
Apigenin promotes p53 phosphorylation and accumulation
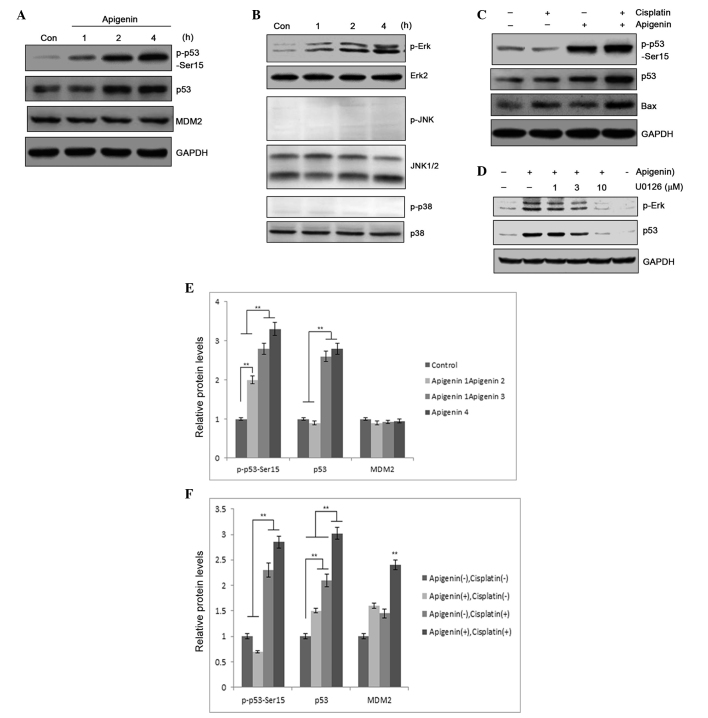
p53 may be functionally compromised by its interaction with several proteins. Among those proteins, MDM2 serves as a pivotal negative regulator and counteracts p53 activation (31,32). p53 is maintained at constant levels in quiescent cells as the protein undergoes constant degradation mediated by MDM2 and the proteasome (33). Factors that promote N-terminal phosphorylation of p53 protein cause disruption of p53-MDM2 interaction, resulting in p53 accumulation and transcriptional regulation (34). In the present study, immunoblotting was performed in A549 cells to determine whether apigenin treatment caused p53 accumulation. In addition to increased accumulation of p53 protein in apigenin-treated samples, apigenin also dose-dependently induced p53 phosphorylation, as detected by a phospho-Ser15 specific antibody (Fig. 4A). Compared with treatment using cisplatin alone, combined treatment with apigenin and cisplatin significantly elevated the levels of p53 protein in the sample (Fig. 4B). Elevated p53 expression could may result from increased gene expression or from posttranslational modification, an event that can lead to p53 stabilization and accumulation (35). However, the levels of p53 mRNA in the A549 cells that were treated with apigenin and cisplatin for 6, 12 and 24 h were not significantly altered, as determined by RT-PCR (data not shown); this suggests that elevated expression of p53 protein was likely to be associated with decreased degradation. Notably, increased p53 accumulation was associated with increased detection of Bax, a proapoptotic protein, in the samples (Fig. 4B), suggesting that apigenin amplified the cisplatin cytotoxic effect through induction of p53 accumulation and p53-regulated proapoptotic gene expression.
Figure 4.
(A) p53 expression and p53 Ser-15 phosphorylation was determined by western blotting, following treatment of A549 cells with 30 µM apigenin for 1, 2 or 4 h. (B) p53 and Bax expression, and p53 phosphorylation, as detected by western blotting, following treatment of A549 cells with 30 µM apigenin for 2 h, then 10 µM cisplatin for another 24 h. (C) MAPK pathway molecule activation, determined by protein phosphorylation levels, in A549 cells treated with 30 µM apigenin for 15, 30, 60 or 120 min, reported by western blotting. (D) Erk/MAPK activation relative to p53 accumulation in A549 cells pretreated with U0126, an Erk/MAPK inhibitor, then treated with 10 µM apigenin to induce Erk/MAPK activation. (E) Semi-quantification of (A). (F) Semi-quantification of (B). Con, control; MDM2, mouse double minute 2 homolog; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Erk, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase.**P<0.01.
Erk/mitogen-activated protein kinase (MAPK) activation is responsible for induced p53 phosphorylation
MAPK activation has a critical role in p53 phosphorylation, which may promote p53 stabilization (36). An immunoblotting analysis was performed in A549 cells to determine whether apigenin treatment activated MAPK. Apigenin treatment was revealed to selectively activate the Erk/MAPK pathway, while no activation of JNK or p38 MAPK was detected in apigenin-treated A549 cells (Fig. 4C). Erk/MAPK activation was determined to be responsible for p53 phosphorylation, as pretreatment with U0126, a specific inhibitor that blocks Erk/MAPK pathway activation (37), dose-dependently blocked apigenin-induced p53 phosphorylation (Fig. 4D). This suggests that Erk/MAPK pathway activation is a contributing factor in apigenin-induced p53 accumulation. These results indicate that apigenin sensitizes tumor cells to the cisplatin-associated cytotoxic effect via modification of the p53 protein.
Apigenin is a dietary flavonoid that is hypothesized to have a beneficial role in cancer chemotherapy (38). This compound has been reported to induce cell cycle arrest, in addition to apoptosis when used at varying concentrations (39). In the current study, apigenin had the ability to amplify cisplatin-induced apoptosis of cancer cells. Apigenin treatment induced MAPK activation and subsequent p53 phosphorylation, leading to its accumulation and increased effector gene activation. In addition, increased caspase activation was also detected in samples treated with both cisplatin and apigenin, suggesting that apigenin sensitizes A549 cells to induced apoptosis through p53 accumulation and altered effector gene regulation.
As a tumor suppressor, p53 has an important role in genome stability and functions as a critical tumor suppressor that is involved in preventing cancer (40). Multiple studies have verified the important role p53-dependent apoptosis has in inhibiting carcinogenesis (41). Cell lines of lung cancer (A549), breast cancer (MCF-7), colorectal cancer (HCT 116) and cervical cancer (HeLa) that possess wild-type p53, and the lung cancer line H1299, which does not generate functional p53, were therefore examined, and the effects of combined treatment of cisplatin with apigenin was determined in these cells. Apigenin notably amplified the inhibitory effect on proliferation of cisplatin in cells with wild-type p53, but not in the p53-null H1299 cells. This was supported by the observed increased activation of caspases-9 and −3, and the nuclear morphological changes in A549 cells.
Posttranslational modification has a critical role in p53 function (42,43). Consistent with this, increased phosphorylation and accumulation of p53 was detected in apigenin-treated samples. Apigenin significantly enhanced p53 phosphorylation. Increased p53 phosphorylation was revealed to be modulated by MAPK, as apigenin treatment promoted MAPK activation; this was blocked by application of U0126, a specific inhibitor of the Erk/MAPK pathway. It is of note that the timing of MAPK (60 min) and p53 (2 h) activation potentially suggest a sequential effect of these events, indicating that MAPK activation may have a crucial role in p53 accumulation and the proapoptotic effect.
Apigenin has demonstrable activity in reducing oxidative stress, inducing cell cycle inhibition and apoptosis, and as an anti-inflammatory agent (44). Foods high in apigenin include celery, parsley, tomatoes and red wine (45,46). Apigenin is generally considered to be safe when consumed in plant foods, with no toxic or mutagenic effects previously reported (47). In a population-based case-control study that included 1,141 patients with ovarian cancer and 1,183 women of similar age to assess the content of their diets, it was reported that flavonoid intake lowered the risk of ovarian cancer (48). The current results demonstrate that dietary flavonoids like apigenin sensitize tumor cells to chemotherapeutic agents. Therefore, combined treatment of tumor cells with conventional chemotherapeutic drugs and dietary supplements provides a promising direction for cancer chemotherapy.
Apigenin enhances the cytotoxic effect of cisplatin during apoptosis in tumor cells, promotes p53 phosphorylation and accumulation, and the Erk/MAPK pathway was revealed to be focal in apigenin-induced p53 accumulation.
Acknowledgements
The present work was supported by National Natural Science Foundation of China (grant nos. 81201946 and 81372394), Ministry of Education Research Fund for Doctoral Programmes (grant no. 2012202120013), the Science Foundation of the Medical University Of Tianjin (grant no. 2011KY15), the National Research Platform of Clinical Evaluation Technology for New Anticancer Drugs (grant no. 2013ZX09303001). The authors thank Xiaoyan Li and Ziying Yang for their technical assistance and Dr Erguang Li for English editing.
References
- 1.Afzal M, Safer AM, Menon M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology. 2015;23:151–161. doi: 10.1007/s10787-015-0236-1. [DOI] [PubMed] [Google Scholar]
- 2.Altemimi A, Watson DG, Kinsel M, Lightfoot DA. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem Cent J. 2015;9:39. doi: 10.1186/s13065-015-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shukla S, Gupta S. Apigenin: A promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71S–88S. doi: 10.1016/S0002-9343(01)00995-0. (Suppl 9B) [DOI] [PubMed] [Google Scholar]
- 5.Yang S, Zhang H, Yang X, Zhu Y, Zhang M. Evaluation of antioxidative and antitumor activities of extracted flavonoids from Pink Lady apples in human colon and breast cancer cell lines. Food Funct. 2015;6:3789–3798. doi: 10.1039/C5FO00570A. [DOI] [PubMed] [Google Scholar]
- 6.Trudel D, Labbé DP, Bairati I, Fradet V, Bazinet L, Têtu B. Green tea for ovarian cancer prevention and treatment: A systematic review of the in vitro, in vivo and epidemiological studies. Gynecol Oncol. 2012;126:491–498. doi: 10.1016/j.ygyno.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 7.Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011;1215:150–160. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nessa MU, Beale P, Chan C, Yu JQ, Huq F. Synergism from combinations of cisplatin and oxaliplatin with quercetin and thymoquinone in human ovarian tumour models. Anticancer Res. 2011;31:3789–3797. [PubMed] [Google Scholar]
- 9.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: Progress, potential and promise (review) Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 10.Torkin R, Lavoie JF, Kaplan DR, Yeger H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol Cancer Ther. 2005;4:1–11. doi: 10.1186/1476-4598-4-1. [DOI] [PubMed] [Google Scholar]
- 11.Ruela-de-Sousa RR, Fuhler GM, Blom N, Ferreira CV, Aoyama H, Peppelenbosch MP. Cytotoxicity of apigenin on leukemia cell lines: Implications for prevention and therapy. Cell Death Dis. 2010;1:e19. doi: 10.1038/cddis.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Sakai T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Ther. 2006;5:945–951. doi: 10.1158/1535-7163.MCT-05-0431. [DOI] [PubMed] [Google Scholar]
- 13.Strouch MJ, Milam BM, Melstrom LG, McGill JJ, Salabat MR, Ujiki MB, Ding XZ, Bentrem DJ. The flavonoid apigenin potentiates the growth inhibitory effects of gemcitabine and abrogates gemcitabine resistance in human pancreatic cancer cells. Pancreas. 2009;38:409–415. doi: 10.1097/MPA.0b013e318193a074. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Xin Y, Diao Y, Lu C, Fu J, Luo L, Yin Z. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS One. 2011;6:e29169. doi: 10.1371/journal.pone.0029169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelini A, Di Ilio C, Castellani ML, Conti P, Cuccurullo F. Modulation of multidrug resistance p-glycoprotein activity by flavonoids and honokiol in human doxorubicin- resistant sarcoma cells (MES-SA/DX-5): Implications for natural sedatives as chemosensitizing agents in cancer therapy. J Biol Regul Homeost Agents. 2010;24:197–205. [PubMed] [Google Scholar]
- 16.Choi EJ, Kim GH. 5-Fluorouracil combined with apigenin enhances anticancer activity through induction of apoptosis in human breast cancer MDA-MB-453 cells. Oncol Rep. 2009;22:1533–1537. doi: 10.3892/or_00000598. [DOI] [PubMed] [Google Scholar]
- 17.Wong IL, Chan KF, Tsang KH, Lam CY, Zhao Y, Chan TH, Chow LM. Modulation of multidrug resistance protein 1 (MRP1/ABCC1)-mediated multidrug resistance by bivalent apigenin homodimers and their derivatives. J Med Chem. 2009;52:5311–5322. doi: 10.1021/jm900194w. [DOI] [PubMed] [Google Scholar]
- 18.Cai X, Liu X. Inhibition of Thr-55 phosphorylation restores p53 nuclear localization and sensitizes cancer cells to DNA damage. Proc Natl Acad Sci USA. 2008;105:16958–16963. doi: 10.1073/pnas.0804608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chabner BA, Roberts TG., Jr Timeline Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 20.Billecke C, Finniss S, Tahash L, Miller C, Mikkelsen T, Farrell NP, Bögler O. Polynuclear platinum anticancer drugs are more potent than cisplatin and induce cell cycle arrest in glioma. Neuro Oncol. 2006;8:215–226. doi: 10.1215/15228517-2006-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendes F, Groessl M, Nazarov AA, Tsybin YO, Sava G, Santos I, Dyson PJ, Casini A. Metal-based inhibition of poly (ADP-ribose) polymerase-the guardian angel of DNA. J Med Chem. 2011;54:2196–2206. doi: 10.1021/jm2000135. [DOI] [PubMed] [Google Scholar]
- 22.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 23.Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFκB transcription factors. J Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Musich PR, Zou Y. Differential DNA damage responses in p53 proficient and deficient cells: Cisplatin-induced nuclear import of XPA is independent of ATR checkpoint in p53-deficient lung cancer cells. Int J Biochem Mol Biol. 2011;2:138–145. [PMC free article] [PubMed] [Google Scholar]
- 25.Losert D, Pratscher B, Soutschek J, Geick A, Vornlocher HP, Müller M, Wacheck V. Bcl-2 downregulation sensitizes nonsmall cell lung cancer cells to cisplatin, but not to docetaxel. Anticancer Drugs. 2007;18:755–761. doi: 10.1097/CAD.0b013e3280adc8c8. [DOI] [PubMed] [Google Scholar]
- 26.He F, Wang Q, Zheng XL, Yan JQ, Yang L, Sun H, Hu LN, Lin Y, Wang X. Wogonin potentiates cisplatin-induced cancer cell apoptosis through accumulation of intracellular reactive oxygen species. Oncol Rep. 2012;28:601–605. doi: 10.3892/or.2012.1841. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Wang Z, Yang Z, Wang J, Xu Y, Tan RX, Li E. Houttuynia cordata blocks HSV infection through inhibition of NF-κB activation. Antiviral Res. 2011;92:341–345. doi: 10.1016/j.antiviral.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Lee J, Ahn HJ, Chong CK, Dias RF, Nam HW. Western Blot Detection of Human Anti-Chikungunya Virus Antibody with Recombinant Envelope 2 Protein. Korean J Parasitol. 2016;54:239–241. doi: 10.3347/kjp.2016.54.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Lin DL, Chang C. p53 is a mediator for radiation-repressed human TR2 orphan receptor expression in MCF-7 cells, a new pathway from tumor suppressor to member of the steroid receptor superfamily. J Biol Chem. 1996;271:14649–14652. doi: 10.1074/jbc.271.25.14649. [DOI] [PubMed] [Google Scholar]
- 31.Stindt MH, Muller PA, Ludwig RL, Kehrloesser S, Dötsch V, Vousden KH. Functional interplay between MDM2, p63/p73 and mutant p53. Oncogene. 2015;34:4300–4310. doi: 10.1038/onc.2014.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heyne K, Förster J, Schüle R, Roemer K. Transcriptional repressor NIR interacts with the p53-inhibiting ubiquitin ligase MDM2. Nucleic Acids Res. 2014;42:3565–3579. doi: 10.1093/nar/gkt1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn HJ, Kim KS, Shin KW, Lim KH, Kim JO, Lee JY, Kim J, Park JH, Yang KM, Baek KH, et al. Ell3 stabilizes p53 following CDDP treatment via its effects on ubiquitin-dependent and -independent proteasomal degradation pathways in breast cancer cells. Oncotarget. 2015;6:44523–44537. doi: 10.18632/oncotarget.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Z, Wu L, Shi H, Wu C. p53 N-terminal binding and stabilisation by PIAS3 inhibits MDM2-induced p53 ubiquitination and regulates cell growth. Mol Med Rep. 2014;9:1903–1908. doi: 10.3892/mmr.2014.1993. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Wang L, Zhang S, Qu G, Zhang D, Li S, Liu S. Downregulation of ubiquitin E3 ligase TNF receptor-associated factor 7 leads to stabilization of p53 in breast cancer. Oncol Rep. 2013;29:283–287. doi: 10.3892/or.2012.2121. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura T, Fukuyo Y, Inoue M, Horikoshi NT, Shindoh M, Rogers BE, Usheva A, Horikoshi N. Mutant p53 disrupts the stress MAPK activation circuit induced by ASK1-dependent stabilization of Daxx. Cancer Res. 2009;69:7681–7688. doi: 10.1158/0008-5472.CAN-09-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui C, Wang P, Cui N, Song S, Liang H, Ji A. Sulfated polysaccharide isolated from the sea cucumber Stichopus japonicas promotes the SDF-1α/CXCR4 axis-induced NSC migration via the PI3K/Akt/FOXO3a, ERK/MAPK, and NF-κB signaling pathways. Neurosci Lett. 2016;616:57–64. doi: 10.1016/j.neulet.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 38.Kim B, Jung N, Lee S, Sohng JK, Jung HJ. Apigenin Inhibits Cancer Stem Cell-Like Phenotypes in Human Glioblastoma Cells via Suppression of c-Met Signaling. Phytother Res. 2016;30:1833–1840. doi: 10.1002/ptr.5689. [DOI] [PubMed] [Google Scholar]
- 39.Seo HS, Ku JM, Choi HS, Woo JK, Jang BH, Go H, Shin YC, Ko SG. Apigenin induces caspase-dependent apoptosis by inhibiting signal transducer and activator of transcription 3 signaling in HER2-overexpressing SKBR3 breast cancer cells. Mol Med Rep. 2015;12:2977–2984. doi: 10.3892/mmr.2015.3698. [DOI] [PubMed] [Google Scholar]
- 40.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: Understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 41.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7:979–987. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 42.Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: A post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol. 2011;12:439–452. doi: 10.1038/nrm3143. [DOI] [PubMed] [Google Scholar]
- 43.Meek DW. Tumour suppression by p53: A role for the DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 44.Guo H, Kong S, Chen W, Dai Z, Lin T, Su J, Li S, Xie Q, Su Z, Xu Y, Lai X. Apigenin mediated protection of OGD-evoked neuron-like injury in differentiated PC12 cells. Neurochem Res. 2014;39:2197–2210. doi: 10.1007/s11064-014-1421-0. [DOI] [PubMed] [Google Scholar]
- 45.Gazzani G, Daglia M, Papetti A. Food components with anticaries activity. Curr Opin Biotechnol. 2012;23:153–159. doi: 10.1016/j.copbio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Tang D, Chen K, Huang L, Li J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin Drug Metab Toxicol. 2016;2:1–8. doi: 10.1080/17425255.2017.1251903. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, Zhao W. Apigenin protects against bleomycin-induced lung fibrosis in rats. Exp Ther Med. 2016;11:230–234. doi: 10.3892/etm.2015.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gates MA, Vitonis AF, Tworoger SS, Rosner B, TitusErnstoff L, Hankinson SE, Cramer DW. Flavonoid intake and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2009;124:1918–1925. doi: 10.1002/ijc.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]