Abstract
Prostaglandin E2 (PGE2) has been demonstrated to attenuate cardiac ischemia-reperfusion (I/R) injury. However, the underlying mechanism of PGE2 in cardiac I/R injury remains unknown. Upregulated expression levels of vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS) were reported in acute myocardial infarction (AMI), and were demonstrated to diminish I/R injury. In the current study the involvement of VEGF and eNOS in the myocardial protective effect of PGE2 were investigated in a catheter-based porcine model of AMI. Twenty-two Chinese miniature pigs were randomized into sham-surgery (n=6), control (n=8) and PGE2 (n=8) groups. PGE2 (1 µg/kg) was injected from 10 min prior to left anterior descending occlusion up to 1 h after reperfusion in the PGE2 group. Subsequently, the hemodynamic parameters were evaluated. Thioflavin-S and Evans Blue double staining were performed to evaluate the extent of the myocardial reperfusion area (RA) and no-reflow area (NRA). Immunohistochemical and western blot analysis were used to evaluate protein expression levels of VEGF and eNOS. Left ventricular (LV) systolic pressure significantly improved and LV end-diastolic pressure significantly decreased in the PGE2 group when compared with the control group 2 h after occlusion and 3 h after reperfusion (P<0.05, respectively). The RA and NRA were smaller in the PGE2 group than in the control group (P<0.05, respectively). Furthermore, PGE2 treatment increased the myocardial content of VEGF and eNOS when compared with the control group (P<0.05, respectively). Thus, the results of the present study demonstrate the cardio-protective mechanisms of PGE2, which may protect the heart from I/R injury via enhancement of VEGF and eNOS expression levels.
Keywords: prostaglandin E2, myocardial ischemia reperfusion injury, endothelial nitric oxide synthase, vascular endothelial growth factor
Introduction
Acute myocardial infarction (AMI) remains a leading cause of mortality worldwide (1), and reperfusion of the ischemic myocardium is a valuable approach for limiting infarct size (IS). However, reperfusion alone leads to reversible and irreversible injuries in the ischemic myocardium, which is called ischemia-reperfusion (I/R) injury (2,3). Prostaglandin E2 (PGE2) has been demonstrated to be beneficial during cardiac I/R (4,5). Previous studies indicate that endogenous PGE2 protects the heart from I/R injury in vivo and in vitro by promoting collateral vessel growth (4). However, the underlying mechanism of PGE2 in cardiac I/R injury remains unknown.
In AMI, expression levels of vascular endothelial growth factor (VEGF) have been reported to be upregulated, which diminished I/R injury (6). Endothelial nitric oxide synthase (eNOS), a rate-limiting enzyme for the synthesis of prostaglandins (PGs), has been reported to be induced in the heart during I/R (7). This result is consistent with the fact that production of PGE2 in the heart increases significantly during ischemia (8), suggesting that it is significant in cardiac I/R injury. The aim of the present study was to investigate whether PGE2 affected expression levels of VEGF and eNOS in a catheter-based porcine model of AMI.
Materials and methods
Animal experiment protocol
The Animal Research Committee of China-Japan Friendship Hospital (Beijing, China) provided ethical approval for the experiments. The investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication, 8th edition) (9).
Twenty-two male Chinese miniature pigs (weight, 25±3.2 kg; age, 6 months) procured from China Agricultural University (Beijing, China) were selected for the experiment. The pigs were housed separately in the Animal Lab Center of China-Japan Friendship Hospital at a temperature of 20°C and humidity of 50% under a 12-h light/dark cycle. They had free access to food of normal cholesterol content. The porcine model of AMI was created on the basis of a previous study by Suzuki et al (10) with modifications. The distal segment of the left anterior descending (LAD) coronary artery was completely occluded by a dilated balloon (2.0×10 mm) for 2 h. Successful construction of the AMI model was confirmed by findings of coronal artery angiography (CAG) and electrocardiogram (ECG). The LAD coronary artery was then reperfused for 3 h, followed by repeat CAG to ensure the presence of thrombolysis in MI (TIMI) grade 3 blood flow in the LAD coronary artery.
Twenty-two Chinese miniature pigs were randomized into 3 groups as follows: Sham-surgery (n=6), control (n=8) and PGE2 (n=8) groups. The distal segment of the LAD coronary artery of the control and PGE2 groups were occluded by dilated balloon for 2 h followed by a 3-h reperfusion. PGE2 (1 µg/kg; Beijing Tide Pharmaceutical Co., Ltd., Beijing, China) was injected from 10 min before LAD occlusion to 1 h after reperfusion in the PGE2 group. Saline was used instead of PGE2 in the control group. In the sham-surgery group animals, a balloon was placed in the LAD coronary artery, but was not dilated. There was no AMI reperfusion and no-reflow in the sham-surgery group.
Hemodynamic assessment
Left ventricular systolic pressure (LVSP), LV end-diastolic pressure (LVEDP) and heart rate (HR) were obtained via a 6F pigtail catheter method prior to AMI, 2 h after occlusion, and 1, 2 and 3 h after reperfusion for serial monitoring of cardiac function. The baseline hemodynamic parameters were measured prior to AMI.
Measurement of necrosis and no-reflow area (NRA)
Double-staining with 0.01 g/ml Evans blue dye and 0.04 g/ml Thioflavin-S was performed to delineate the reperfusion area (RA) and no-reflow area (NRA). Three hours after reperfusion, 1 ml/kg of 4% Thioflavin-S in saline was injected as a bolus. The reperfused area was stained, but the NRA was not stained. After another complete occlusion of the LAD coronary artery, 0.01 g/ml Evans Blue dye was infused into the left ventricle and the normal myocardium was stained to negatively mark the territory of the occluded artery (i.e., the risk area).
The deeply anesthetized swine were sacrificed by injection of 15% KCl (1 ml/kg). Then the heart was excised and rinsed in ice-cold saline solution to remove the blood and excess dye. The atria and right ventricular free wall were removed, and the remaining LV tissue was sectioned perpendicular to its long axis into six to seven sections and photographed. The risk area (the area unstained by Evans Blue) was traced and visualized under natural light. The NRA (the area not perfused by Thioflavin-S) was photographed using ultraviolet light (wavelength, 365 nm) and a yellow filter. The area between the risk area and NRA was the area of reflow. The normal area was defined as the area not including the RA, NRA and risk area. The RA, NRA and LV wall area (LVWA) were measured using image processing software IPP 6.0. Outcomes were calculated as follows: RA (%) = (RA/area of left ventricle) × 100; NRA (%) = (NRA/risk area) × 100%. In addition, RA/LVWA and NRA/LVWA were calculated.
The normal area, RA and NRA were then fixed using 10% formalin and embedded in paraffin for histopathological examination by hematoxylin and eosin (H&E) staining. Neutrophil infiltration in the area of reflow was semi-quantified by light microscopy (Nikon Eclipse E400; Nikon Corporation, Tokyo, Japan) at a magnification of ×400, in a blinded manner by a cardiac pathologist.
Immunohistochemical staining for VEGF and eNOS
In immunohistochemical analysis, cross-sectional myocardial slices at the level of LV papillary muscles were selected. Normal area, RA and NRA were fixed by 10% formalin and embedded in paraffin. Tissue sections (5 µm) were deparaffinized and re-hydrated. Samples were then subjected to 0.1% Triton X-100 for permeability. The endogenous peroxidase activity was subdued by treating with 3% hydrogen peroxide for 10 min. The paraffin-embedded sections were incubated with anti-VEGF antibody (Abcam, Cambridge, USA; cat. no. ab69479) and anti-eNOS antibody (Abcam; cat. no. ab66127) at a dilution of 1:200, or with negative control (normal serum) at 4°C overnight. The sections were stained with horseradish peroxidase conjugated secondary antibody (Histofine Simple Stain Mouse MAX PO (R), Nichirei Biosciences Inc. Tokyo, Japan; cat. no. 414341F) and 3,3′-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin. The percentage of positive staining for VEGF and eNOS were calculated and six fields selected in a clockwise direction were observed at a magnification of ×200 under a stereo microscope. The expression levels of VEGF and eNOS were observed as brown particles in the myocardial and perivascular tissue samples, and the sum of integrated optical density (IOD) and total positive areas of each group were measured using an image processing software IPP 6.0. IOD to area ratios were calculated as the sum of IOD divided by the total positive area. These measurements were performed three times and were analyzed by two independent observers who were blinded to the treatment allocation.
Western blot analysis for protein expression of VEGF and eNOS
Total protein was extracted from the left ventricle using cell lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) with protease inhibitor cocktail (BD Biosciences, San Jose, CA, USA). Protein samples (20 g) were denatured in SDS sample buffer [125 mmol/l Tris-HCl (pH 6.8), 50% glycerol, 2% SDS, 5% mercaptoethanol and 0.01% bromophenol blue] were subjected to SDS-PAGE and blotted onto Immobilon-FL transfer membranes (EMD Millipore, Billerica, MA, USA). The blotted membranes were blocked with 5% skimmed milk in Tris-buffered saline containing 0.1% Tween-20 for 2 h and subsequently incubated with the primary antibodies against VEGF, eNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Quanshijin Biotechnology Inc., Beijing, China; cat. no. HC301-02) overnight at 4°C. After three washes in Tris-buffered saline containing 0.1% Tween-20, the membranes were incubated with mouse anti-human IgM monoclonal secondary antibody (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA; cat. no. MA5-14712) diluted in phosphate-buffered saline for 50 min at room temperature. Immunoreactivity was quantified by using the Odyssey dual color infrared fluorescence imaging system (LI-COR, Lincoln, NE, USA) and normalized to GAPDH, which served as an internal control.
The signals from immunoreactive bands were visualized using an Amersham ECL system (GE Healthcare Life Sciences, Chalfont, UK) and quantified using densitometric analysis. The ratio for the protein examined was normalized against GAPDH.
Statistical analysis
All data were expressed as means ± standard deviation. Comparisons between two groups were performed using an unpaired Student's t-test. Differences among groups were evaluated by one-way ANOVA and P<0.05 was considered to indicate a statistically significant difference.
Results
Procedure success rate
Twenty-six male Chinese mini swines were used in the current study; however, four succumbed due to laryngeal edema as a result of intubation failure, sensitivity to anesthesia, thrombosis in the left main coronary artery due to balloon inflation and recurrent ventricular tachycardia following reperfusion. Complete occlusion of the LAD coronary artery by dilated balloon was confirmed by CAG in the control (n=8) and PGE2 (n=8) groups. CAG was performed again to ensure reperfusion following AMI, which was presented as TIMI grade 3 blood flow. The procedure success rate was 84.6%.
Hemodynamic effect of PEG2
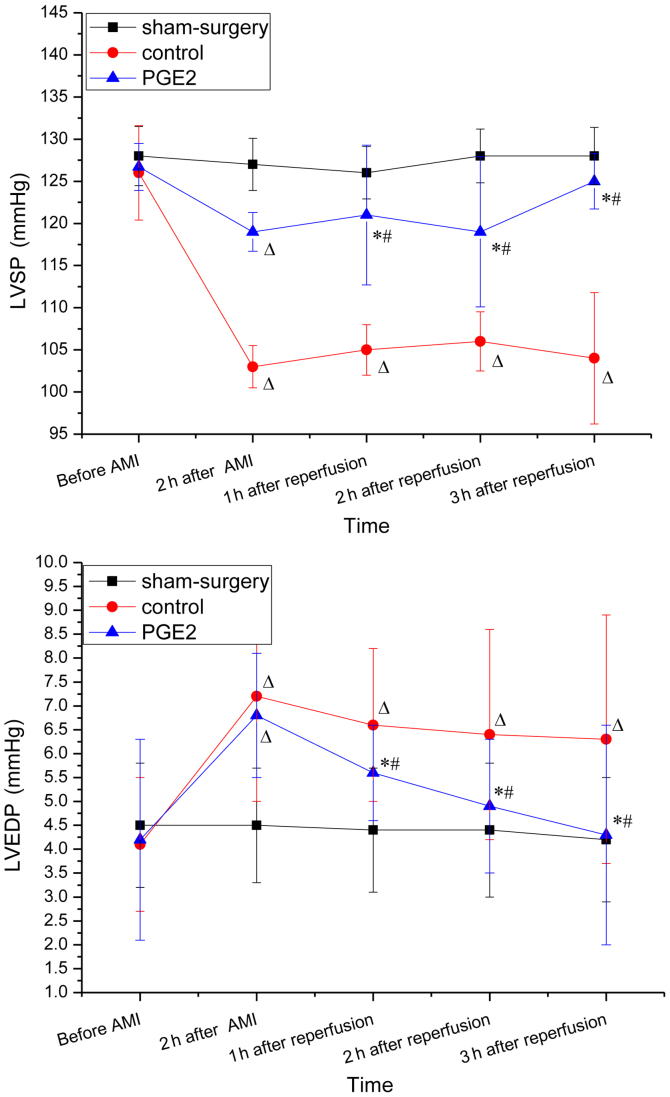
The baseline hemodynamic parameters (obtained prior to surgery) were similar in each of the three groups (Fig. 1). Two hours after occlusion and 3 h after reperfusion, LVEDP in the control group increased significantly when compared with the baseline (prior to AMI; P<0.05 for 2 and 3 h), whereas LVSP decreased significantly (P<0.05 for for 2 and 3 h). No changes in LVSP and LVEDP were observed 2 h after occlusion between the control and PGE2 groups, while 1, 2 and 3 h after reperfusion, increased LVSP and decreased LVEDP were observed in the PGE2 group when compared with the control group (P<0.05). For the PGE2 group, LVSP increased significantly after reperfusion compared with 2 h after occlusion, while the change of LVEDP exhibited the opposite trend (Fig. 1).
Figure 1.
Hemodynamics at different time-points in each group. (A) LVSP and (B) LVEDP were obtained via catheter method at five different time-points: i) Before AMI; ii) 2 h after occlusion; iii) 1 h; iv) 2 h; and v) 3 h after reperfusion. *P<0.05 vs. the control group, ΔP<0.05 vs. before AMI in the same group, #P<0.05 vs. 2 h after AMI in the same group. LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; PGE2, prostaglandin E2.
Effect of PEG2 on pathological changes
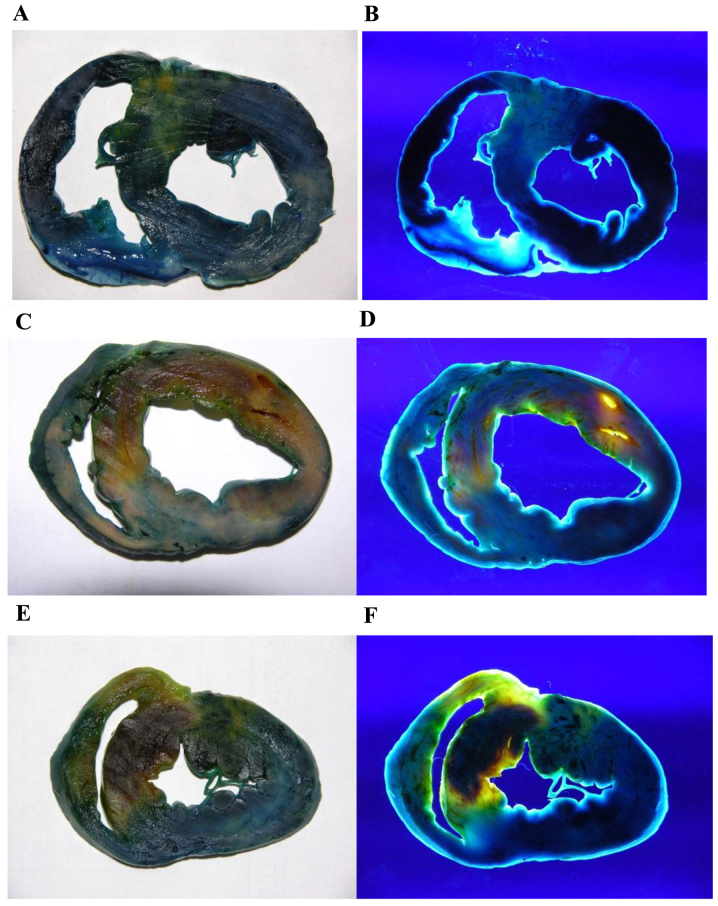
Pathological changes were analyzed by double-staining and H&E staining. For double-staining, the normal myocardium, RA and NRA appeared dark blue, yellow and dark red, respectively under natural light, whereas their color changed to black, bright yellow, and deep red, respectively under UV light. Infarct size was expressed as a percentage of the myocardium at risk. Following reperfusion, no significant difference in RA/LVWA was identified between the PGE2 and the control groups (54.37±8.72 vs. 50.73±3.93%; P>0.05). NRA/LVWA of the PGE2 group was found to be significantly lower than that of the control group (14.83±5.51 vs. 25.39±3.49%; P<0.01). The modified reperfusion area of LV [(RA-NRA)/LVWA] of the PGE2 group increased significantly when compared with the control group (39.54±7.55 vs. 25.34±2.68%; P<0.01) (Table I and Fig. 2).
Table I.
RA, NRA of the three groups following double staining.
| Group | n | RA/LVWA (%) | NRA/RA (%) | NRA/LVWA (%) | (RA-NRA)/LVWA (%) |
|---|---|---|---|---|---|
| Control | 8 | 50.73±3.93 | 49.84±5.04 | 25.39±3.49 | 25.34±2.68 |
| PGE2 | 8 | 54.37±8.72 | 27.13±8.71a | 14.83±5.51a | 39.54±7.55a |
(RA-NRA)/LVWA (%) represents the modified RA of the left ventricle.
P<0.01 vs. control group. RA, reperfusion area; NRA, no-reflow area; LVWA, left ventricular wall area; PGE2, prostaglandin E2.
Figure 2.
Double staining of myocarium in the different groups (A and B) Sham-surgery (C and D) control and (E and F) PGE2 groups under natural (left column) and UV (right column) light. Normal myocardium, RA and NRA appeared dark blue, yellow, and dark red under natural light, respectively. Under UV light, the colors changed to black, bright yellow, and deep red, respectively. RAs of the PGE2 and control groups were not significantly different, whereas NRA of the PGE2 group was markedly smaller than of the control group. PGE2, prostaglandin E2; UV, ultraviolet; RA, reperfusion area; NRA, non-reflow area.
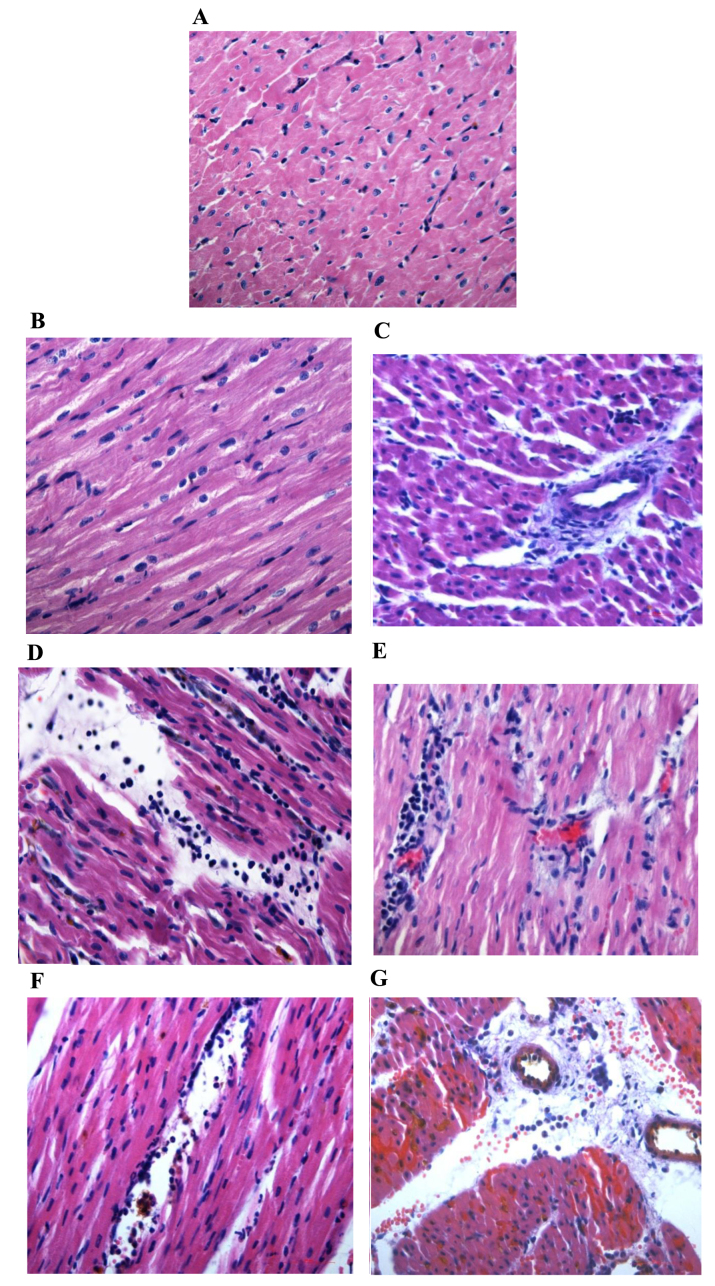
For H&E staining, no necrosis or neutrophil infiltration was observed in the myocardium of the normal area in the three groups, whereas the myocardium in the RA and NRA of the control group exhibited myocardial necrosis, local tissue swelling, fibrosis, large quantities of neutrophil infiltration and a greater leucocyte count when compared with the normal area of the control group (P<0.01 and P<0.05, respectively). In RA and NRA of the PGE2 group, myocardial cells with normal structure and shape were apparent, and the myocardial cells were mildly swollen or partially ruptured with fewer leucocytes compared with the RA and NRA of the control group (P<0.01 and P<0.05, respectively; Table II and Fig. 3).
Table II.
Leucocyte count per single field of view from the three groups following hematoxylin and eosin staining).
| Group | Normal area (per field of view) | RA (per field of view) | NRA (per field of view) |
|---|---|---|---|
| Sham-surgery | 3±1 | – | – |
| Control | 4±2 | 70±5a | 57±8b |
| PGE2 | 3±1 | 30±3c | 45±5d |
RA, reperfusion area; NRA, no-reflow area.
P<0.01 vs. normal area
P<0.05 vs. normal area
P<0.01 vs. control group
P<0.05 vs. control group.
Figure 3.
Hematoxylin and eosin staining of myocarium in the different groups (magnification, ×400). Normal area of the three groups exhibited normal size cardiomyocytes, no hemorrhaging or neutrophil granulocyte infiltration. (A) Sham-surgery group, and normal areas of the (B) control and (C) PGE2 groups. The RA and NRA in the control group exhibited cardiomyocyte degeneration, hemorrhaging, edema, and significant interstitial neutrophil granulocyte infiltration compared with the normal area of the same group. (D) Reperfusion area and (E) NRA of the control group. No significant cardiomyocyte degeneration was identified in the RA and NRA in the PGE2 group, however, slight edema between the myocardial fibers, and mild neutrophil granulocyte infiltration was observed compared with the RA and NRA of the control group. (F) Reperfusion area and (G) NRA of the PGE2 group. The intravascular yellow staining is Thioflavin-S stain. PGE2, prostaglandin E2; RA, reperfusion area; NRA, no-reflow area.
Effect of PEG2 on expression levels of VEGF and eNOS
Content and distribution of VEGF and eNOS proteins: Immunohistochemical analysis
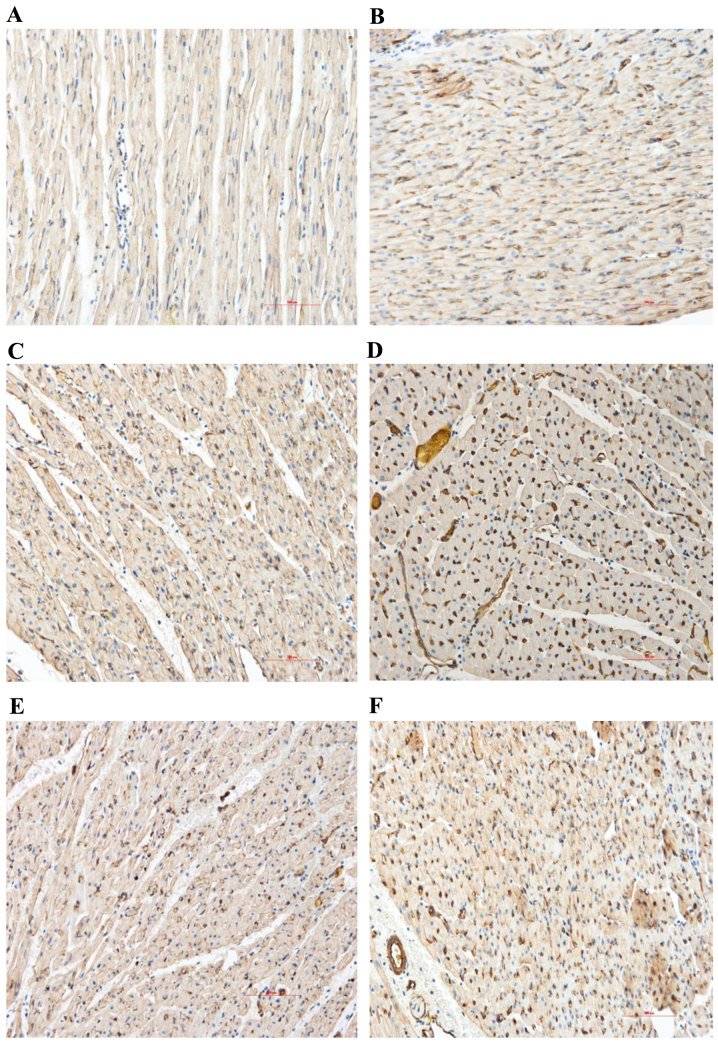
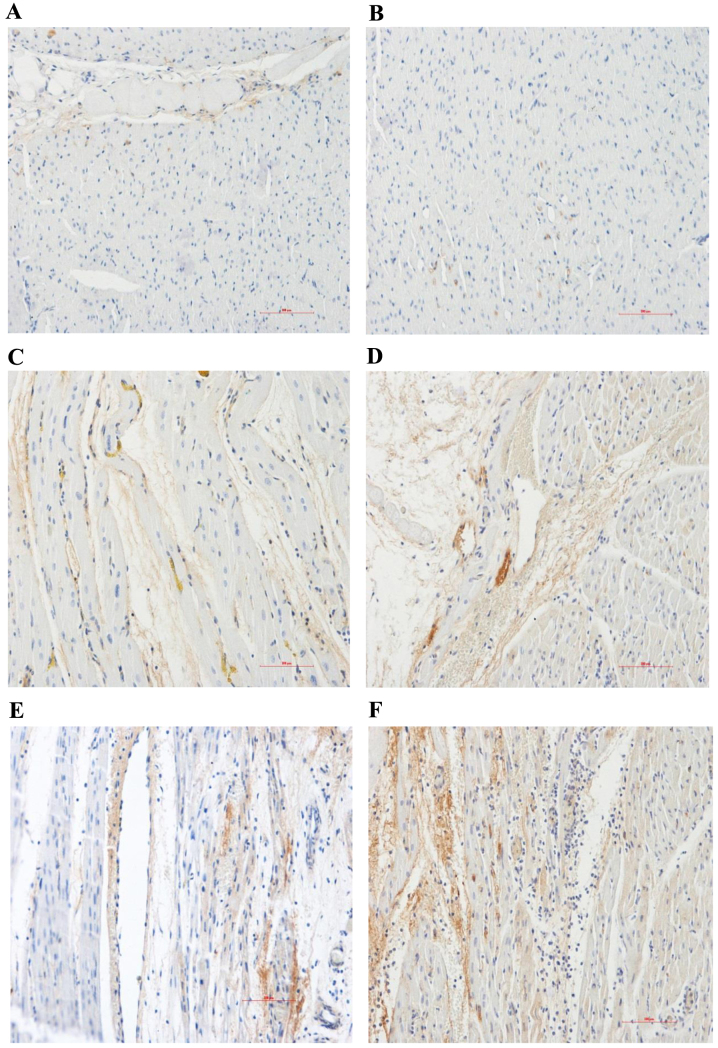
The expression levels of VEGF and eNOS in the myocardial sections were evaluated by immunohistochemical analysis. Compared with the normal area, the myocardial VEGF and eNOS protein expression levels of the control group significantly increased in the RA and NRA. These expression levels were significantly upregulated in the PGE2 group compared with the control group, particularly in the NRA (Table III and Figs. 4 and 5; P<0.05).
Table III.
Immunohistochemical analysis of the content and distribution of eNOS and VEGF proteins (integrated optical density/area ratio).
| Protein | Group | Normal area | Reperfusion area | No-reflow area |
|---|---|---|---|---|
| eNOS | Sham-surgery | 0.13±0.05 | – | – |
| Control | 0.12±0.01 | 0.34±0.08a | 0.41±0.04a | |
| PGE2 | 0.13±0.01 | 0.48±0.05a,b | 0.62±0.04a,b | |
| VEGF | Sham-surgery | 0.13±0.03 | – | – |
| Control | 0.12±0.05 | 0.24±0.03a | 0.26±0.06a | |
| PGE2 | 0.13±0.02 | 0.46±0.03a,b | 0.66±0.05a,b |
P<0.05 vs. normal area
P<0.05 vs. control group. eNOS, endothelial nitric oxide synthase; VEGF, vascular endothelial growth factor; PGE2, prostaglandin E2.
Figure 4.
Immunohistochemical analysis of the content and distribution of VEGF proteins (magnification, ×200). Myocardial VEGF protein expression levels of the control group markedly increased in the RA and NRA compared with the normal area. PGE2 markedly upregulated the expression level of VEGF in the RA and NRA further when compared with the control group. Normal area of the (A) control and (B) PGE2 groups; reperfusion area of the (C) control and (D) PGE2 groups; NRA of the (E) control and (F) PGE2 groups. VEGF, vascular endothelial growth factor; RA, reperfusion area; NRA, no-reflow area; PGE2, prostaglandin E2.
Figure 5.
Immunohistochemical analysis of the content and distribution of eNOS proteins (magnification, ×200). Myocardial eNOS protein expression levels of the control group markedly increased in the RA and NRA compared with the normal area. PGE2 markedly upregulated the expression level of eNOS in the RA and NRA further when compared with the control group. Normal area of the (A) control and (B) PGE2 groups; reperfusion area of the (C) control and (D) PGE2 groups; NRA of the (E) control and (F) PGE2 groups. eNOS, endothelial nitric oxide synthase; RA, reperfusion area; NRA, no-reflow area; PGE2, prostaglandin E2.
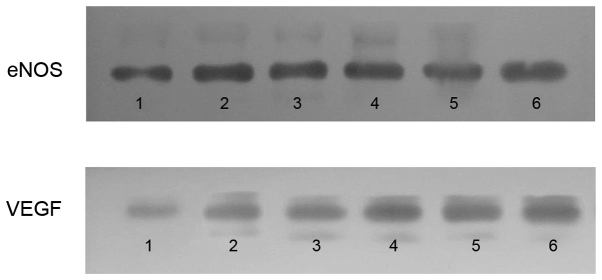
VEGF and eNOS protein expression levels: Western blot analysis. Western blot analysis was performed to detect the protein expression levels of VEGF and eNOS. The myocardial VEGF and eNOS expression levels of the control group significantly increased in the RA and NRA compared with the normal area. PGE2 significantly upregulated the expression of VEGF and eNOS in the RA and NRA when compared with the control group (Fig. 6).
Figure 6.
Western blot analysis of VEGF and eNOS protein expression levels. The myocardial eNOS and VEGF protein expression levels of the control group significantly increased in the RA and NRA compared with the normal area. PGE2 significantly upregulated the expression levels of VEGF and eNOS in the RA and NRA further when compared with the control group. Lanes 1–3, normal area, RA and NRA of the control group; lanes 4–6, normal area, RA and NRA of the PGE2 group. VEGF, vascular endothelial growth factor; eNOS, endothelial nitric oxide synthase; RA, reperfusion area; NRA, no-reflow area; PGE2, prostaglandin E2.
Discussion
AMI is currently the leading cause of morbidity and mortality worldwide (1). Increasing numbers of AMI patients receive early restoration of coronary flow from the emergency medical services, such as percutaneous coronary intervention and thrombolysis, which significantly improves the prognosis (11). However, I/R injury of the heart, which is caused by reperfusion itself, affects the cardiac function and prognosis of patients. Micro-vascular spasms, neutrophil infiltration and micro-thrombus, amongst others, have been identified as mechanisms of I/R injury in a recent study (12).
PGE2 is an endogenous lipid mediator, which is important in the control of vascular tone and platelet aggregation (13). A recent study has shown that PGE2 is protective against MI (14). In the present porcine model of AMI, significant reductions in myocardial injury were observed following PGE2 therapy, which improved LVSP (by alleviating myocardial ischemia and improving impaired ventricular systolic function), and reduced LVEDP and NRA following reperfusion in AMI. In the pathological analysis, myocardial cells were mildly swollen with fewer leucocytes in the RA and NRA of the PGE2 group when compared with the control group. These findings provide evidence of the potential benefits of PGE2 in reducing NRA 2 h after AMI and 3 h after reperfusion. It is hypothesized that this occurs by alleviating neutrophil infiltration and myocardial cell edema.
To date, studies examining the role of VEGF in ischemia over extended periods of time suggest promising efficacy (15). VEGF has been shown to cause NO release, resulting in vasodilation and increased blood flow (16). Recent studies have revealed that trans-coronary arterial delivery of VEGF to an isolated heart immediately prior to ischemia improves myocardial functional recovery (17). In the current study, exogenous PGE2 increased the expression level of VEGF 2 h after AMI and 3 h after reperfusion when compared with the control group, and simultaneously reduced the NRA of the myocardium. VEGF may be an important intermediate factor of PGE2 in attenuating the deleterious effects of I/R. Protection of PGE2 against myocardial I/R injury may be achieved by enhancement of VEGF formation. The underlying mechanism of this protection may be via vasodilation, and subsequently the improvement of collateral blood flow and alleviation of micro-vascular spasms in the coronary artery.
eNOS is one of the key enzymes in the synthesis and release of NO. Previous studies have shown that activation of eNOS is important for mediating the cardioprotective effect of NO against I/R injury (18). NO generation elicits vasodilation, which exerts protective effects during I/R by influencing platelet aggregation, leukocyte adhesion and neutrophil infiltration (19). In the present study, PGE2 enhanced protein production of eNOS and VEGF following myocardial reperfusion. The myocardial protection of PGE2 may be achieved by diminishing platelet aggregation, leukocyte adhesion and neutrophil infiltration, which are performed by VEGF and eNOS. This is hypothesized to be an important cardio-protective mechanism of PGE2. In conclusion, PGE2 induces myocardial protection against myocardial I/R injury via enhancement of VEGF and eNOS expression levels.
Acknowledgements
The present study was supported by the Beijing Natural Science Foundation (grant no. 7152128).
References
- 1.Bulluck H, Yellon DM, Hausenloy DJ. Reducing myocardial infarct size: Challenges and future opportunities. Heart. 2016;102:341–348. doi: 10.1136/heartjnl-2015-307855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 3.Wei M, Xin P, Li S, Tao J, Li Y, Li J, Liu M, Li J, Zhu W, Redington AN. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res. 2011;108:1220–1225. doi: 10.1161/CIRCRESAHA.110.236190. [DOI] [PubMed] [Google Scholar]
- 4.Hishikari K, Suzuki J, Ogawa M, Isobe K, Takahashi T, Onishi M, Takayama K, Isobe M. Pharmacological activation of the prostaglandin E2 receptor EP4 improves cardiac function after myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2009;81:123–132. doi: 10.1093/cvr/cvn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang L, Cai Y, Tang EH, Irwin MG, Ma H, Xia Z. Prostaglandin E receptor subtype 4 signaling in the heart: role in ischemia/reperfusion injury and cardiac hypertrophy. J Diabetes Res. 2016;2016:1324347. doi: 10.1155/2016/1324347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Infanger M, Faramarzi S, Grosse J, Kurth E, Ulbrich C, Bauer J, Wehland M, Kreutz R, Kossmehl P, Paul M, et al. Expression of vascular endothelial growth factor and receptor tyrosine kinases in cardiac ischemia/reperfusion injury. Cardiovasc Pathol. 2007;16:291–299. doi: 10.1016/j.carpath.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Cao J, Xie H, Sun Y, Zhu J, Ying M, Qiao S, Shao Q, Wu H, Wang C. Sevoflurane post-conditioning reduces rat myocardial ischemia reperfusion injury through an increase in NOS and a decrease in phopshorylated NHE1 levels. Int J Mol Med. 2015;36:1529–1537. doi: 10.3892/ijmm.2015.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siu KL, Lotz C, Ping P, Cai H. Netrin-1 abrogates ischemia/reperfusion-induced cardiac mitochondrial dysfunction via nitric oxide-dependent attenuation of NOX4 activation and recoupling of NOS. J Mol Cell Cardiol. 2015;78:174–185. doi: 10.1016/j.yjmcc.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, corp-author. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press (US); Washington, DC: 2011. [Google Scholar]
- 10.Suzuki Y, Lyons JK, Yeung AC, Ikeno F. In vivo porcine model of reperfused myocardial infarction: in situ double staining to measure precise infarct area/area at risk. Catheter Cardiovasc Interv. 2008;71:100–107. doi: 10.1002/ccd.21329. [DOI] [PubMed] [Google Scholar]
- 11.Fordyce CB, Gersh BJ, Stone GW, Granger CB. Novel therapeutics in myocardial infarction: Targeting microvascular dysfunction and reperfusion injury. Trends Pharmacol Sci. 2015;36:605–616. doi: 10.1016/j.tips.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G, Chen L. An update of microsomal prostaglandin E synthase-1 and PGE2 receptors in cardiovascular health and diseases. Oxid Med Cell Longev. 2016;2016:5249086. doi: 10.1155/2016/5249086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kezeli T, Rukhadze T, Gongadze N, Sukoyan G, Dolidze N, Chipashvili M, Mirziashvili M. Effect of calcitonin gene-related peptide antagonist on the cardiovascular events, mortality, and prostaglandin E2 production by nitrate-induced tolerant rats with acute myocardial infarction. EPMA J. 2016;7:6. doi: 10.1186/s13167-016-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Zhang X, Pang N, Xiao L, Li Y, Chen N, Ren M, Deng X, Wu J. Glycation of vitronectin inhibits VEGF-induced angiogenesis by uncoupling VEGF receptor-2-αvβ3 integrin cross-talk. Cell Death Dis. 2015;6:e1796. doi: 10.1038/cddis.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park YS, Jeon YJ, Kim HS, Chae KY, Oh SH, Han IB, Kim HS, Kim WC, Kim OJ, Kim TG, et al. The role of VEGF and KDR polymorphisms in moyamoya disease and collateral revascularization. PLoS One. 2012;7:e47158. doi: 10.1371/journal.pone.0047158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J, Wang H, Wang Y, Ren F, Yi W, Zhao K, Li Z, Zhao Q, Liu Z, Wu H, et al. Induction of angiogenesis by controlled delivery of vascular endothelial growth factor using nanoparticles. Cardiovasc Ther. 2013;31:e12–e18. doi: 10.1111/j.1755-5922.2012.00317.x. [DOI] [PubMed] [Google Scholar]
- 18.Simon JN, Duglan D, Casadei B, Carnicer R. Nitric oxide synthase regulation of cardiac excitation-contraction coupling in health and disease. J Mol Cell Cardiol. 2014;73:80–91. doi: 10.1016/j.yjmcc.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Li XD, Yang YJ, Geng YJ, Zhao JL, Zhang HT, Cheng YT, Wu YL. Phosphorylation of endothelial NOS contributes to simvastatin protection against myocardial no-reflow and infarction in reperfused swine hearts: partially via the PKA signaling pathway. Acta Pharmacol Sin. 2012;33:879–887. doi: 10.1038/aps.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]