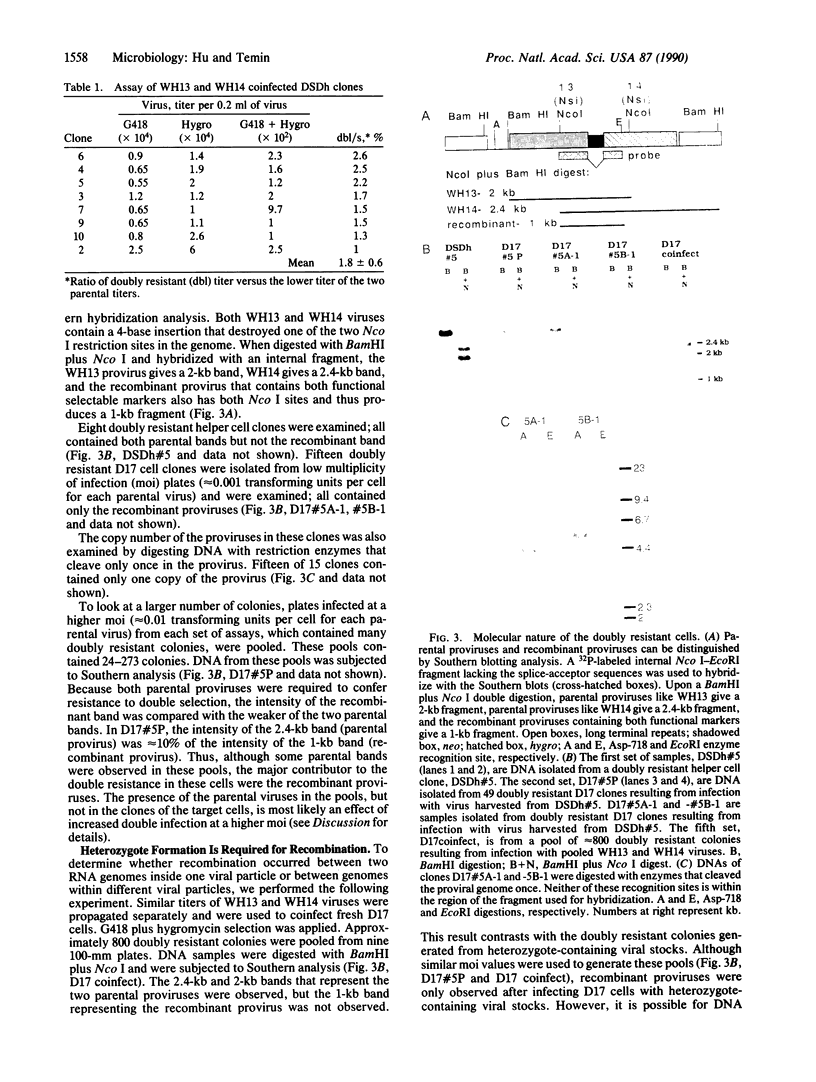
Abstract
Retroviruses contain two complete viral genomic RNAs in each virion. A system to study in a single round of replication the products of virions with two different genomic RNAs was established. A spleen necrosis virus-based splicing vector containing both the neomycin-resistance gene (neo) and the hygromycin B phosphotransferase gene (hygro) was used. Two frameshift mutants were derived from this vector such that the neo and the hygro genes were inactivated in separate vectors. Thus, each vector confers resistance to only one selection. The vectors with frameshift mutations were separately propagated and were pooled to infect DSDh helper cells. Doubly resistant cell clones were isolated, and viruses produced from these clones were used to infect D17 cells. This protocol allowed virions containing two different genomic RNAs (heterozygotes) to complete one round of retroviral replication. The molecular nature of progeny that conferred resistance to single or double selection and their ratio were determined. Our data demonstrate that each infectious heterozygous virion produces only one provirus. The rate of retroviral recombination is approximately 2% per kilobase per replication cycle. Recombinant proviruses are progeny of heterozygous virions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alevy M. C., Vogt P. K. Ts pol mutants of avian sarcoma viruses: mapping and demonstration of single cycle recombinants. Virology. 1978 Jun 1;87(1):21–33. doi: 10.1016/0042-6822(78)90154-x. [DOI] [PubMed] [Google Scholar]
- Beemon K. L., Faras A. J., Hasse A. T., Duesberg P. H., Maisel J. E. Genomic complexities of murine leukemia and sarcoma, reticuloendotheliosis, and visna viruses. J Virol. 1976 Feb;17(2):525–537. doi: 10.1128/jvi.17.2.525-537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G. Genetic recombination between avian leukosis and sarcoma viruses. Experimental variables and the frequencies of recombination. Virology. 1977 Apr;77(2):534–544. doi: 10.1016/0042-6822(77)90479-2. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. Determination of the rate of base-pair substitution and insertion mutations in retrovirus replication. J Virol. 1988 Aug;62(8):2817–2822. doi: 10.1128/jvi.62.8.2817-2822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. High mutation rate of a spleen necrosis virus-based retrovirus vector. Mol Cell Biol. 1986 Dec;6(12):4387–4395. doi: 10.1128/mcb.6.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. P., Wisniewski R., Yang S. L., Rhode B. W., Temin H. M. New retrovirus helper cells with almost no nucleotide sequence homology to retrovirus vectors. J Virol. 1989 Jul;63(7):3209–3212. doi: 10.1128/jvi.63.7.3209-3212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Jaenisch R., MacIsaac P. Low-multiplicity infection of Moloney murine leukemia virus in mouse cells: effect on number of viral DNA copies and virus production in producer cells. J Virol. 1978 Dec;28(3):802–809. doi: 10.1128/jvi.28.3.802-809.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. doi: 10.1016/s0042-6822(72)80005-9. [DOI] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. Structure, subunit composition, and molecular weight of RD-114 RNA. J Virol. 1975 Aug;16(2):397–411. doi: 10.1128/jvi.16.2.397-411.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Hu S., Bender W., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. RD-114, baboon, and woolly monkey viral RNA's compared in size and structure. Cell. 1976 Apr;7(4):609–620. doi: 10.1016/0092-8674(76)90211-7. [DOI] [PubMed] [Google Scholar]
- Linial M., Brown S. High-frequency recombination within the gag gene of Rous sarcoma virus. J Virol. 1979 Jul;31(1):257–260. doi: 10.1128/jvi.31.1.257-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L. Evidence for intrachromosomal gene conversion in cultured mouse cells. Cell. 1983 Nov;35(1):157–165. doi: 10.1016/0092-8674(83)90218-0. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Fiore D. Ordered interstrand and intrastrand DNA transfer during reverse transcription. Science. 1988 Aug 26;241(4869):1064–1069. doi: 10.1126/science.2457948. [DOI] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka A. M., Boone L., Junghans R., Luk D. Genetic recombination in avian retroviruses. J Cell Biochem. 1982;19(3):293–304. doi: 10.1002/jcb.240190311. [DOI] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. 8. Glycolysis and cell multiplication. Int J Cancer. 1968 Mar 15;3(2):273–282. doi: 10.1002/ijc.2910030213. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The DNA provirus hypothesis. Science. 1976 Jun 11;192(4244):1075–1080. doi: 10.1126/science.58444. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Genetically stable reassortment of markers during mixed infection with avian tumor viruses. Virology. 1971 Dec;46(3):947–952. doi: 10.1016/0042-6822(71)90093-6. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke J. A., Beamand J. A. Genetic recombination in Rous sarcoma virus: the genesis of recombinants and lack of evidence for linkage between pol, env and src genes in three factor crosses. J Gen Virol. 1979 May;43(2):349–364. doi: 10.1099/0022-1317-43-2-349. [DOI] [PubMed] [Google Scholar]