Abstract
In Argentina, as in other countries of Latin America, cholera has occurred in an epidemic pattern. Vibrio cholerae O1 is native to the aquatic environment, and it occurs in both culturable and viable but nonculturable (VNC) forms, the latter during interepidemic periods. This is the first report of the presence of VNC V. cholerae O1 in the estuarine and marine waters of the Río de la Plata and the Argentine shelf of the Atlantic Ocean, respectively. Employing immunofluorescence and PCR methods, we were able to detect reservoirs of V. cholerae O1 carrying the virulence-associated genes ctxA and tcpA. The VNC forms of V. cholerae O1 were identified in samples of water, phytoplankton, and zooplankton; the latter organisms were mainly the copepods Acartia tonsa, Diaptomus sp., Paracalanus crassirostris, and Paracalanus parvus. We found that under favorable conditions, the VNC form of V. cholerae can revert to the pathogenic, transmissible state. We concluded that V. cholerae O1 is a resident of Argentinean waters, as has been shown to be the case in other geographic regions of the world.
Cholera reemerged in Latin America in 1991 after being absent from the continent for nearly a century. In Argentina, there have been seven epidemics since 1992, all of which were caused by El Tor biotype Vibrio cholerae O1. These cholera outbreaks occurred mainly during the summer months, and, consistent with reports from other geographic regions of the world, strains of V. cholerae O1 were isolated from water samples collected from rivers during epidemic periods. However, the microorganism could not be recovered from the environment during interepidemic periods (12).
V. cholerae is a natural inhabitant of aquatic environments, and it has been found to survive for extended periods in estuarine and brackish waters (12, 13, 15). Furthermore, in response to extreme environmental conditions, the bacteria may enter a dormant state (7, 30), which has been designated the viable but nonculturable (VNC) state, since the organism cannot be recovered by using traditional culture media. It has been hypothesized that it is in the VNC state that V. cholerae survives in the environment during interepidemic periods. Also, interaction with plankton appears to play an important role in the ecology of the microorganism and to facilitate persistence, mainly in response to low temperatures and reduced nutrient concentrations (12, 13, 14, 19, 21).
In Argentina, V. cholerae non-O1 was isolated from samples collected from the Río de la Plata in previous studies (5). Several facts, such as the seasonality of the outbreaks most likely associated with temperature shifts and plankton blooms, suggested that there are environmental reservoirs of V. cholerae. Additionally, in a recent study in which the genetic diversity of V. cholerae O1 strains isolated during the seven outbreaks that occurred in this country was analyzed, most of the cholera toxin (CT)-producing strains were found to belong to a single clonal group already recognized as the Latin American epidemic strain (22). Also, a new variant of isolates (the Tucumán variant [22]) from a northern district of Argentina which did not produce CT and was genetically related was identified. Both the Latin American epidemic clone and the Tucumán variant were repeatedly isolated in different outbreaks after long interepidemic intervals, and they produced exactly the same pulsed-field gel electrophoresis pattern as isolates from previous outbreaks. This finding strongly suggested that no new lineages were introduced into this region but instead the strains belonging to the two clones were present in the environment and sporadically reemerged as culturable, pathogenic agents, probably in response to favorable environmental conditions.
The aims of this study were to determine the environmental reservoir(s) of V. cholerae O1 in Argentina and to evaluate the association of this pathogen with plankton species. For these purposes, two different geographic areas were selected; these areas were a marine station in the Atlantic Ocean, at which there was little anthropogenic influence, and four stations in the Río de la Plata estuary, which serves as a vast funnel that collects most of the continental waters of Argentina.
MATERIALS AND METHODS
Sample collection sites.
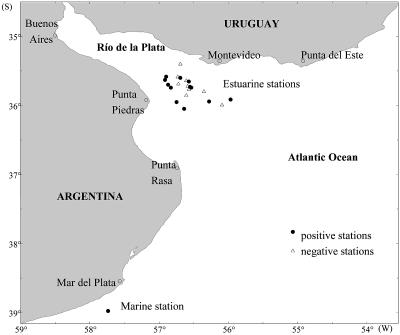
Samples were collected from five sites. One of the sites was located at a fixed station on the Argentine continental shelf at 38°28′S, 57°41′W and where the water depth was 48 m. The remaining four sites were located along a transect at the border between the Río de la Plata and its estuary (ecotone) (that is, from Punta Piedras in Argentina to Montevideo in Uruguay).
Between February 1999 and May 2000, a total of 13 cruises were conducted by R/V Captain Oca Balda, R/V E. L. Holmberg, and R/V Captain C´anepa of the Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP); these cruises included 7 cruises to the marine sampling station in the Argentine shelf and 6 cruises to the estuary of the Río de la Plata (Fig. 1).
FIG. 1.
Map showing the sampling stations for VNC V. cholerae O1.
Sample collection and processing.
Plankton samples were collected with 25- and 200-μm mesh nets. For each concentrated plankton sample (final volume, 20 ml), 10 ml was divided into three portions, and these portions were placed into three vials immediately after sampling. One of these vials, which contained 0.025% yeast extract and 0.002% nalidixic acid, was incubated for 6 to 12 h at 35°C and fixed with formaldehyde at a final concentration of 3% for analysis by the direct fluorescent antibody (DFA)-direct viable count (DVC) technique (9). The second vial was preserved with formaldehyde at a final concentration of 4% and used for identification of zooplankton and phytoplankton species. The sample in the last vial was kept at −20°C for PCR analysis. The remaining 10 ml of the plankton sample was enriched by addition of alkaline peptone broth for isolation of V. cholerae by conventional culture methods.
Water samples were collected in sterile 5-liter bottles and were filtered through 0.22-μm-pore-size membranes. Subsequently, the membranes were washed with 4 ml of phosphate buffer, and this buffer was fractioned for analysis by DFA-DVC, PCR, and direct culturing. Physical and chemical parameters (temperature and salinity) were measured in situ at all sites at the time of sampling.
DFA-DVC.
For detection of V. cholerae O1, formaldehyde-preserved samples were processed by using cholera DFA kits (3, 9) generously provided by New Horizons Diagnostics Corporation. Briefly, samples were incubated in the dark at room temperature for 6 to 8 h in the presence of yeast extract and nalidixic acid. Under these conditions, viable but nonculturable bacteria elongate from a coccoid shape to rod-like cells, yet they do not multiply due to the inhibitory effect of nalidixic acid (a DNA gyrase inhibitor). After incubation, samples were fixed with 3% formaldehyde and stained with monoclonal fluorescein isothiocyanate-conjugated anti-O1 antibodies. Stained preparations were observed by using an epifluorescence microscope (model IX 70; Olympus) at a maximum excitation wavelength of 490 nm.
Direct detection of the cholera toxin gene (ctxA).
A heminested PCR method was used to detect a region spanning the ctxA and ctxB genes in water and plankton samples; this method was based on a method used previously in our laboratory for detection in fecal specimens (29). For this assay, water and plankton samples were boiled for 10 min and centrifuged. The supernatant was used as the template DNA. For the first round of PCR, the primers used were 5′ GTGGGAATGCTCCAAGATCAAATCG 3′ (forward) and 5′ ATTGCGGCAATCGCATGAGGCGT 3′ (reverse). For the second round of PCR, the internal primer 5′ GATATGCAATCCTCAGGGTATCC 3′ was used along with the forward primer. The amplification reaction consisted of 5 min at 94°C and 30 cycles of 1 min at 94°C, 1 min at 60°C, and 2 min at 72°C.
DNA extraction.
DNA was extracted from estuarine and marine water samples and from plankton samples by a recently described protocol by using cetyltrimethylammonium bromide (25); the method used was based on the method of Sommerville (27). Briefly, a 1-ml aliquot of a plankton or water sample was homogenized, centrifuged, and suspended in SET buffer (20% sucrose, 50 mM EDTA, 50 mM Tris HCl; pH 7.6). After treatment with lysozyme, proteinase K, and ammonium dodecyl sulfate, DNA was extracted with cetyltrimethylammonium bromide and phenol-chloroform-isoamyl alcohol, concentrated with isopropanol, and suspended in tridistilled water.
PCR.
DNA extracted from water and plankton samples was used as the template to detect V. cholerae. First, an amplification assay was performed by using universal 16S rRNA primers to confirm the presence of bacterial DNA (1). The samples that showed positive results in the 16S rRNA PCR were subjected to another amplification reaction with primers specific for V. cholerae (4). Subsequently, a multiplex PCR assay was performed to detect genes coding for the somatic antigens O1 and O139 (25), and the positive samples were tested for the presence of the virulence-associated genes ctxA and tcpA (24). The primers used in all assays in this study are listed in Table 1.
TABLE 1.
Primers and PCR conditions used in this study
| Primer | Sequence (5′-3′) | Amplicon size (bp) | Annealing temp. (°C) | Extension time (min) |
|---|---|---|---|---|
| P16S-F | CAGCMGCCGCGGTAATWC | 888 | 55 | 3 |
| P16S-R | ACGGGCGGTGTGTRC | |||
| VC-F2 | TTAAGCSTTTTCRCTGAGAATG | 295 | 60 | 1 |
| VC-R1 | AGTCACTTAACCATACACCCG | |||
| VCO1F2 | CAACAGAATAGACTCAAGAA | 647 | ||
| VCO1R2 | TATCTTCTGATACTTTTCTAC | |||
| VCO139F2 | TTACCAGTCTACATTGCC | 741 | 55 | 3 |
| VCO139R2 | CGTTTCGGTAGTTTTTCTGG | |||
| 94F | CGGGCAGATTCTAGACCTCCTG | 564 | ||
| 614R | CGATGATCTTGGAGCATTCCCAC | |||
| 72F | CACGATAAGAAAACCGGTCAAGAG | 405 | 60 | 1 |
| 477R | CGAAAGCACCTTCTTTCACGTTG |
Virulence factors.
In culturable non-O1 V. cholerae strains, virulence-associated genes were detected by colony blot hybridization with specific probes. The probes, kindly provided by J. Nataro and J. Kaper, Center for Vaccine Development, University of Maryland School of Medicine, Baltimore, included the ClaI fragment of pCVD628 for zot and ace gene sequences, the EcoRI fragment of pCVD27 for the ctxA gene, and the ClaI-XbaI fragment of pBB241 for the zot gene. The probes were labeled with digoxigenin by random priming (Boehringer). The tcpA gene, coding for the toxin coregulated pilus (TCP) pilin subunit, was detected by the multiplex PCR assay, which allows identification of both El Tor (471-bp) and Classical (617-bp) tcpA sequences (8). The primers used were 5′ GAAGAAGTTTGTAAAAGAAGAACAC 3′ (tcpA1 El Tor) and 5′ GAAAGGACCTTCTTTCACGTTG 3′ (tcpA2 El Tor); and 5′ CACGATAAGAAAACCGGTCAAGAG 3′ (tcpA3 Classical) and 5′ ACCAAATGCAACGCCGAATGGAG 3′ (tcpA4 Classical). The amplification conditions were 5 min at 94°C, 30 cycles of 1.5 min at 94°C, 1.5 min at 60°C, and 1.5 min at 72°C, and a final extension of 10 min a 72°C.
Statistics.
The relationships between the presence of culturable non-O1 V. cholerae or VNC V. cholerae O1 and two physicochemical parameters, temperature and salinity, were evaluated by using Pearson χ2 and χ2 for linear trends (a P value of ≤0.05 for the two-tailed test was considered significant) and logistic regression for multivariate analysis. The dependent variable presence of culturable non-O1 V. cholerae was defined with two categories: positive (value, 1), when non-O1 V. cholerae was isolated in at least one sample of water or plankton from a single site; and negative (value, 0), when no non-O1 V. cholerae was found in any sample from a single site. The same categories were defined for V. cholerae O1 as a dependent variable.
The independent variables salinity and temperature were each divided into three categories, as follows: salinity, less than 10 g/ml, 10 to 25 g/ml, and more than 25g/ml; and temperature, less than 15°C, 15 to 20°C, and more than 20°C. A likelihood ratio test was used to assess linear trends in the multivariate analysis. The nonparametric test Spearman coefficient was used to evaluate the relationship between the presence of non-O1 V. cholerae or VNC V. cholerae O1 and the different kinds of samples collected at a single site. Data analyses were performed by using the Epi Info v6.02 and Stata v7.0 programs.
RESULTS
Isolation of V. cholerae.
A total of 21 samples were obtained from water collected at the marine sampling station on the Argentine continental shelf, and 63 samples were collected from the Río de la Plata estuary; these samples included water and plankton samples. Based on the net mesh size, the plankton were divided into two categories: microplankton (∼25 to 200 μm), which included mainly phytoplankton and protozooplankton; and mesoplankton (>200 μm), which included mainly multicellular zooplankton. While no isolates of V. cholerae O1 were recovered by conventional culture methods, 97 strains of V. cholerae non-O1 were isolated. None of these strains carried the virulence-associated genes of V. cholerae O1 (i.e., ctxA, zot, ace, and tcpA), as determined by colony blot hybridization and PCR. The rate of isolation of non-O1 V. cholerae increased as the temperature increased (χ2 for trend [2 df] = 10.26; P = 0.001) and decreased as the salinity increased (χ2 for trend [1 df] = 8.72; P = 0.003). The relationship was statistically significant even when the two variables were considered together; the β coefficients were 1.40 (P = 0.024) for temperature and −1.37 (P = 0.040) for salinity (multivariate analysis; linear model; LR test χ2 = 1, 42; P > 0.05). Thus, culturable non-O1 V. cholerae strains were recovered from the estuarine stations only during the warmer months (November through March).
There was a positive correlation between isolation of non-O1 V. cholerae strains from water and isolation of non-O1 V. cholerae strains from mesoplankton (diameter, >200 μm) at a single site, indicating that plankton samples positive for V. cholerae were frequently accompanied by positive water samples (Spearman rho = 0.69; P < 0.05).
Viable but nonculturable V. cholerae.
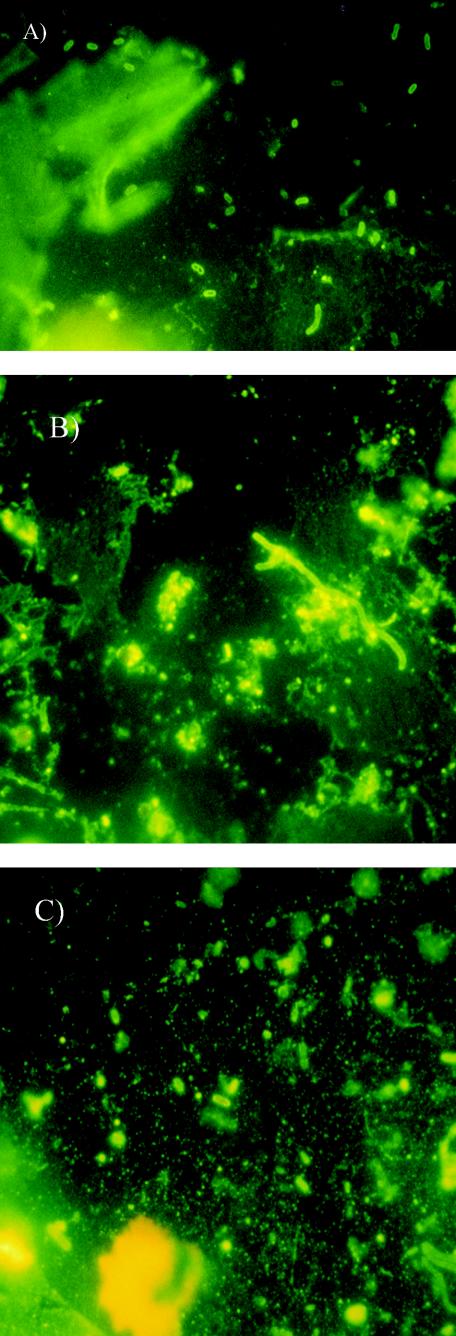
Despite the fact that no strains of V. cholerae O1 were obtained by conventional culture, DFA-DVC revealed the presence of VNC V. cholerae O1, which appeared as rod-shaped bacteria after incubation with yeast extract and nalidixic acid (Fig. 2). The results obtained by DFA-DVC were confirmed by heminested PCR and multiplex PCR. The amplification reactions showed the presence of V. cholerae O1-specific genes, as well as genes coding for CT and TCP (Fig. 3, 4, and 5).
FIG.2.
Fluorescent photomicrographs of V. cholerae O1 attached to microplankton (A), in a water sample (B), and attached to zooplankton (C).
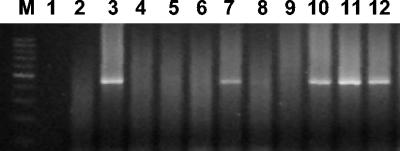
FIG. 3.
Heminested PCR for ctxAB detection. Lane M, 100-bp ladder; lane 1, negative control (PCR master mixture); lanes 2, 4, 5, 6, 8, and 9, negative samples; lanes 3, 7, 10, and 11, positive samples; lane 12 positive control (V. cholerae O1 strain O425).
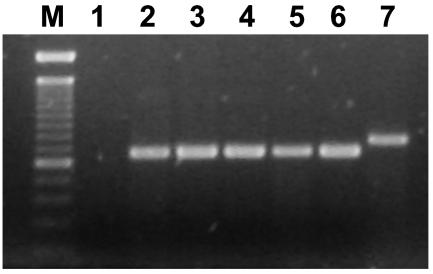
FIG. 4.
Multiplex PCR for the detection of O1/O139 genes. Lane M, 100-bp ladder; lane 1, negative control (PCR master mixture); lanes 2 to 5, positive samples; lane 6, O1 positive control strain ATCC 14035; lane 7, O139 positive control strain MO4045.
FIG. 5.
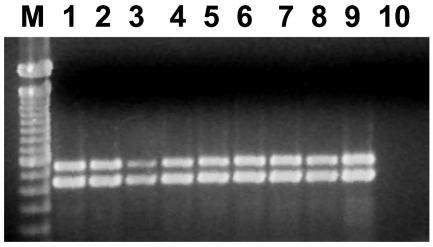
Multiplex PCR for the detection of ctxA/tcpA. Lane M, 100-bp ladder; lanes 1 to 8, positive samples; lane 9, positive control V. cholerae O1 strain O425; lane 10, negative control (PCR master mixture).
There was good correlation between the results of PCR and the results of DFA-DVC (Table 2); a total of 19 of 84 samples yielded positive results with both PCR and DFA-DVC. This group included nine water, five mesoplankton, and five microplankton samples. Additionally, one microplankton sample was positive as determined by PCR and negative as determined by DFA-DVC; this sample was considered positive for VNC V. cholerae O1, since all the PCRs were positive, which proved that V. cholerae O1 CT and TCP genes were present. A total of 59 of the remaining samples were negative as determined by both methods, while six samples having different origins showed positive results when fluorescent staining was used but were negative as determined by PCR. These samples were considered negative, since the observations were confounded by high background fluorescence. Also, two PCR-positive zooplankton samples that gave confusing, weak results when DFA-DVC was used were clearly positive as determined by this technique after trituration; this was most likely a case of the bacteria being attached to unexposed areas of the plankton.
TABLE 2.
Correlation between DFA-DVC and PCR for detection of VNC V. cholerae O1
| Site | Samples
|
No. DFA-DVC positive and PCR positive | No. DFA-DVC positive and PCR negative | No. DFA-DVC negative and PCR negative | No. DFA-DVC negative and PCR positive | Total no. of VNCa | |
|---|---|---|---|---|---|---|---|
| Type | No. | ||||||
| Estuary | Water | 21 | 6 | 1 | 14 | 6 | |
| Mesoplankton (>200 μm) (n = 21) | 21 | 4 | 0 | 17 | 4 | ||
| Microplankton (25-200 μm) | 21 | 4 | 1 | 15 | 1 | 5 | |
| Marine station | Water | 7 | 3 | 1 | 3 | 3 | |
| Mesoplankton (>200 μm) | 7 | 1 | 2 | 4 | 1 | ||
| Microplankton (25-200 μm) | 7 | 1 | 1 | 5 | 1 | ||
| Total | 84 | 84 | 19 | 6 | 58 | 1 | 20 |
Total number of samples that were considered positive for VNC V. cholerae O1.
VNC V. cholerae O1 was found during all seasons examined at both the marine and estuarine stations (Table 3). In the Río de la Plata estuary, VNC V. cholerae was identified in samples collected during the six cruises conducted in the summer, winter, and spring (there were no cruises in the autumn), and at the continental shelf marine station the samples were positive for five of the seven cruises in the summer and autumn (there were no cruises in the spring). There was not a significant correlation between the presence of V. cholerae O1 and temperature (Pearson χ2 [2 df] = 0.399; P = 0.819) or salinity (Pearson χ2 [2 df] = 0.9604; P = 0.619) at the sampling site (the β coefficients were 0.49 [P = 0.334] for salinity and 0.31 [P = 0.525] for temperature; multivariate). Also, there was no correlation between the types of samples collected at a single site; i.e., a positive microplankton sample was not frequently accompanied by a positive water and/or mesoplankton sample (Spearman rho [water-mesoplankton] = 0.16; Spearman rho [water-microplankton] = −0.03; Spearman rho [mesoplankton-microplankton] = −0.06; P > 0.05).
TABLE 3.
Seasonal occurrence of VNC V. cholerae O1 in surface water and plankton in the estuarine and marine environments monitored by inmunofluorescence and PCR techniques
| Season | No. of positive samples/total no. (%)
|
|||
|---|---|---|---|---|
| Water | Microplankton (25-200 μm.) | Mesoplankton (>200 μm.) | Total | |
| Summer | 2/11 (18.2) | 4/11 (36.4) | 1/11 (9.1) | 7/33 (21.2) |
| Fall | 2/3 (66.7) | 1/3 (33.3) | 1/3 (33.3) | 4/9 (44.4) |
| Winter | 1/5 (20.0) | 0/5 (0) | 2/5 (40.0) | 3/15 (20.0) |
| Spring | 4/8 (50.0) | 1/8 (12.5) | 1/8 (12.5) | 6/24 (25.0) |
Association with plankton.
Several zooplankton species collected with a 200-μm-mesh net were associated with V. cholerae O1, including Acartia tonsa, Diaptomus sp., Paracalanus crassirostris, and Paracalanus parvus at the estuarine stations and Corycaeus amazonicus, Centropages furcatus, and Ctenocalanus vanus at the marine station. All plankton species were separated, identified, and individually confirmed to be associated with V. cholerae O1 by DFA-DVC.
For the microplankton, one marine and four estuarine samples were positive for VNC V. cholerae O1 as determined by PCR and DFA-DVC; there was also one estuarine sample that was positive as determined by PCR and negative as determined by DVA-DVC (Table 2). In these samples, in spite of isolation and staining of the more frequent species, none of the microplankton could be unquestionably observed as carrying V. cholerae by microscopy. The major species present in positive samples from the Río de la Plata estuary were the diatoms Thalassionema nitzschioides, Pleurosigma cf. normanii, and species of the genera Thalassiosira and Coscinodiscus, together with the dinoflagellates Ceratium furca, Noctiluca scintillans, Prorocentrum scutellum, Diplopelta asymmetrica, and species of Protoperidinium. At the continental shelf marine station, the most abundant species identified in the sample that was positive for VNC V. cholerae O1 were the diatoms Meunieria membranacea, Cerataulina pelagica, Leptocylindrus minimus, Hemiaulus sinensis, Thalassionema nitzschioides, and Guinardia flaccida and species of Protoperidinium. Members of other taxonomic groups, such as silicoflagellates, tintinnids, and ebriaceans, were too infrequent in samples associated with V. cholerae to be relevant.
VNC V. cholerae O1 was identified in microplankton samples in the spring and summer at the estuary stations and in the autumn at the marine station, whereas VNC V. cholerae O1 was associated with zooplankton in the summer, winter, and spring in the estuary and in the autumn at the marine station.
DISCUSSION
V. cholerae is an aquatic microorganism, and several reports have shown that there are environmental reservoirs of this pathogen in different geographical areas of the world (2, 17, 18, 20). In Argentina, as in the rest of Latin America, cholera has occurred since 1991 in an epidemic pattern. It has been suggested that V. cholerae O1 remains in the environment during interepidemic periods in a viable but nonculturable state. Prior to this study, many cruises had been conducted in the Río de la Plata and at the marine platform in Argentina in a search for culturable forms of V. cholerae in which traditional culture methods were used, and only non-O1 strains were recovered. No studies had been carried out to look for VNC V. cholerae O1. Thus, this is the first study which proves that VNC V. cholerae O1 is present in estuarine and marine waters in the Río de la Plata estuary and the continental shelf of Argentina in the southwest Atlantic Ocean.
Results of previous studies suggested that V. cholerae is present in the environment in association with plankton, mainly attached to copepods and some aquatic hydrophytes (6, 15, 16, 28). In 1990, Huq et al. (12) reported that V. cholerae O1 cells could be observed by DFA microscopy to be attached to a variety of phytoplankton and zooplankton species, including Volvox, Desmida, Rotifera, Cladocera, and Daphnia spp. In this study, VNC V. cholerae O1 was associated with both microplankton and zooplankton. In the zooplankton fraction collected from the Río de la Plata estuary, species of marine origin (Acartia tonsa, Paracalanus crassirostris, Paracalanus parvus) and species of continental water origin (Diaptomus sp.) were associated with VNC V. cholerae O1. Acartia tonsa is a marine euryhaline species that occurs predominantly in the estuary of the Río de la Plata. Diaptomus has been repeatedly found in the continental waters of Argentina (23; F. C. Ramirez, D. R. Soarrain, H. Mianzan, and N. Fernandez Araoz, submitted for publication). Furthermore, at the continental shelf marine station, the species associated with VNC V. cholerae O1 (Corycaeus amazonicus, Centropages furcatus, and Ctenocalanus vanus) are common inhabitants of the northern coastal waters of Argentina. It would be useful to examine the plankton in northern Argentina, where most of the cholera cases occurred during the seven epidemic outbreaks, since the water of the Parano-Platense basin is carried down to the Río de la Plata.
In both the estuarine and marine environments, a larger number of water samples than of plankton fractions were positive for VNC V. cholerae O1. The samples that were positive for V. cholerae O1 but negative for plankton-attached V. cholerae O1 most likely included species that were smaller than the pore size of the plankton net used, as well as organic detritus, exuviae, and phytoplankton and zooplankton remains. Plankton nets with smaller mesh should be used for future studies. It must be considered that during passage through this wide plexus of bodies of water, chitinous remains of copepod exoskeletons disintegrate and are reduced to fine debris. It is well known that copepods, like other crustaceans, undergo a growth cycle that includes a change in the tegument, which due to the action of biological and/or mechanical agents, results in an abundance of microscopic substrates that are suitable for attachment of bacteria. Biofilms also are formed. Thus, the finding that positive water samples are more numerous than positive plankton samples is logical.
Culturable V. cholerae non-O1 was identified predominantly during warmer months and in the lower-salinity waters of the estuary. This finding is in agreement with reports for other areas (10, 11, 20). A similar relationship was not evident for the presence of nonculturable V. cholerae O1, since this organism was found during all seasons at both the marine and estuarine stations. Although the numbers of cruises conducted in the different seasons and in the two geographical areas were not equal, it can be assumed that the VNC state makes it possible for this bacterium to persist at a wide range of salinities and temperatures. Nevertheless, since the methodologies used to detect viable but nonculturable forms were specific for V. cholerae O1, the possibility that non-O1 strains could also be found in nonculturable forms cannot be ruled out. Furthermore, VNC non-O1 V. cholerae has been found in the River Ganga in Varanasi in India (26). If this was the case in the Río de la Plata and in the Argentine marine platform, it could be speculated that non-O1 V. cholerae (or at least some strains in this group) could be more readily transformed to the culturable state than the O1 strains in the presence of lower salinity and at higher temperatures.
The heminested PCR technique (29) employed for detection of V. cholerae when the concentrations of bacteria were very low (2 to 3 CFU/ml) proved to be a useful tool for analysis of environmental samples. Moreover, the combination of the PCR and DFA-DVC methods used in this study allowed us to confirm not only the viability of the V. cholerae O1 identified but also the pathogenic potential of the nonculturable V. cholerae population. The DFA-DVC method confirmed the presence of viable forms of V. cholerae O1, while PCR allowed detection of virulence-associated genes that could be found not only in V. cholerae O1 but also in V. cholerae non-O1 strains.
In summary, prior to this study, there was no evidence that V. cholerae O1 is part of the normal flora of southwest Atlantic Ocean waters. Therefore, the present study is the first report of the existence of an environmental reservoir of this pathogen in this geographical area, both in coastal water and in the Río de la Plata and its estuary. The VNC V. cholerae O1 detected by the methods used in this study possessed genetic information typical for pathogenic V. cholerae O1 (e.g., the ctxAB and tcpA genes). Given that under certain climate conditions VNC V. cholerae O1 could revert to a transmissible state, programs for prevention of cholera in South America should not be abandoned. Instead, there is a need for systematic environmental monitoring and strategic surveillance for V. cholerae O1 in this region.
Acknowledgments
We thank I. Rivera for her support in the confirmation of PCR results. We gratefully acknowledge M. I. Caffer for performing identification and serotyping of the V. cholerae strains, J. Labbé for assistance with the heminested PCR, and M. Panagopulos, A. Garbini, and N. Martinez for excellent technical assistance.
This research was supported by grants 05-00083-01943 and PICTR2000-00010 from the Agencia Nacional de Promoción Científica y Tecnológica, Secretaría de Ciencia y Tecnología, Ministerio de Cultura y Educación, Argentina.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakraborty, S., A. K. Mukhopadhyay, R. K. Bhadra, A. N. Ghosh, R. Mitra, T. Shimada, S. Yamasaki, S. M. Faruque, Y. Takeda, R. R. Colwell, and G. B. Nair. 2000. Virulence genes in environmental strains of Vibrio cholerae. Appl. Environ. Microbiol. 66:4022-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdhury, M. A. R., B. Xu, R. Montilla, J. A. K. Hasan, A. Huq, and R. R. Colwell. 1995. A simplified immunofluorescence technique for detection of viable cells of Vibrio cholerae O1 and O139. J. Microbiol. Methods 24:165-170. [Google Scholar]
- 4.Chun, J., A. Hug, and R. Colwell. 1999. Analysis of 16S-23S rRNA intergenic spacer regions of Vibrio cholerae and Vibrio mimicus. Appl. Environ. Microbiol. 65:2202-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costagliola, M., A. López, A. Malaspina, R. Guerrero, D. Medina, M. Odizzio, A. Abelenda, and C. Friss de Kereki. 2000. Study of the presence of Vibrio cholerae on the Argentine-Uruguayan common fisheries zone. Period 1992-1996. Frente Maritimo 18A:53-58. [Google Scholar]
- 6.Dastidar, S. G., and A. Narayanaswami. 1968. The occurrence of chitanase in vibrios. Indian J. Med. Res. 56:654-658. [PubMed] [Google Scholar]
- 7.Gauthier, M. J. 2000. Environmental parameters associated with the viable but nonculturable state, p. 87-112. In R. R. Colwell and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 8.Ghosh, C., R. K. Nandy, S. K. Dasgupta, G. B. Nair, R. H. Hall, and A. C. Ghose. 1997. A search for cholera toxin (CT), toxin coregulated pilus (TCP), the regulatory element ToxR and other virulence factors in non-O1/non-O139 V. cholerae. Microb. Pathog. 22:199-208. [DOI] [PubMed] [Google Scholar]
- 9.Hasan, J., D. Bernstein, A. Huq, L. Loomis, M. Tamplin, and R. Colwell. 1994. Cholera DFA: an improved direct fluorescent monoclonal antibody staining kit for rapid detection and enumeration of Vibrio cholerae O1. FEMS Microbiol. Lett. 120:143-148. [DOI] [PubMed] [Google Scholar]
- 10.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Seasonality of Chesapeake Bay bacterioplankton species. Appl. Environ. Microbiol. 68:5488-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshino, K., S. Yamasaki, A. K. Mukhopadbyay, S. Chakraborty, A. Basu, S. K. Bhattacharya, G. B. Nair, T. Shimada, and Y. Takeda. 1998. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol. Med. Microbiol. 20:201-207. [DOI] [PubMed] [Google Scholar]
- 12.Huq, A., R. R. Colwell, R. Rahman, A. Ali, M. A. R. Chowdhury, S. Parveen, D. A. Sack, and E. Russek-Cohen. 1990. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl. Environ. Microbiol. 56:2370-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huq, A., E. B. Small, P. A. West, and R. R. Colwell. 1984. The role of planktonic copepods in the survival and multiplication of Vibrio cholerae in the aquatic environment, p. 521-534. In R. R. Colwell (ed.), Vibrios in the environment. John Wiley & Sons, Inc., New York, N.Y.
- 14.Huq, A., E. B. Small, P. A. West, M. I. Huq, R. Rahman, and R. R. Colwell. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Islam, M. S., B. S. Drasar, and D. J. Bradley. 1989. Attachment of toxigenic Vibrio cholerae O1 to various freshwater plants and survival with a filamentous green alga, Rhizoclonium fontanum. J. Trop. Med. Hyg. 92:396-401. [PubMed] [Google Scholar]
- 16.Islam, M. S., B. S. Drasar, and R. B. Sack. 1996. Ecology of Vibrio cholerae: role of aquatic fauna and flora, p. 187-227. In B. S. Drasar and B. D. Forrest (ed.), Cholera and the ecology of Vibrio cholerae. Chapman & Hall, London, United Kingdom.
- 17.Jesudason, M. V., V. Balaji, U. Mukundan, and C. J. Thomson. 2000. Ecological study of Vibrio cholerae in Vellore. Epidemiol. Infect. 124:201-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipp, E., I. N. Rivera, A. I. Gil, E. M. Espeland, N. Choopun, V. R. Louis, E. Russek-Cohen, A. Huq, and R. R. Colwell. 2003. Predictability of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 69:3676-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobitz, B., L. Beck, A. Huq, B. B. Woods, G. Fuchs, A. S. Faruque, and R. Colwell. 2000. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. USA 97:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis, V. R., E. Russek-Cohen, N. Choopun, I. N. Rivera, B. Gangle, S. C. Jiang, A. Rubin, J. A. Patz, A. Huq, and R. R. Colwell. 2003. Predictability of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 69:2773-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin, Y. P., and M. A. Bianchi. 1980. Structure, diversity and catabolic potentialities of aerobic heterotrophic bacterial populations associated with continuous culture of natural marine phytoplankton. Microb. Ecol. 5:265-279. [DOI] [PubMed] [Google Scholar]
- 22.Pichel, M., M. Rivas, F. Martin, C. Ibarra, and N. Binsztein. 2003. Genetic diversity and emergence of a new variant of Vibrio cholerae O1 isolated in Argentina. J. Clin. Microbiol. 41:124-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringuelet, R. A. 1958. Los crustáceos copépodos de las aguas continentales en la República Argentina. Sinopsis sistemática. Universidad de Buenos Aires. Facultad de Ciencias Exactas y Naturales. Contribuciones científicas. Senè zoología. 1:35-126. [Google Scholar]
- 24.Rivera, I. N. G., J. Chun, A. Huq, R. B. Sack, and R. R. Colwell. 2001. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl. Environ. Microbiol. 67:2421-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera, I. N., E. Lipp, A. Gil, N. Choopun, A. Huq, and R. R. Colwell. 2003. Method of DNA extraction and application of multiplex polymerase chain reaction to detect toxigenic Vibrio cholerae O1 and O139 from aquatic environments. Environ. Microbiol 5:599-606. [DOI] [PubMed] [Google Scholar]
- 26.Shukla, B. N., D. V. Singh, and S. C. Sanyal. 1995. Attachment of non-culturable toxigenic Vibrio cholerae O1 and non-O1 and Aeromonas spp. to the aquatic arthropod Gerris spinolae and plants in the rRiver Ganga, Varanasi. FEMS Immunol. Med. Microbiol. 12:113-120. [DOI] [PubMed] [Google Scholar]
- 27.Sommerville, C. C., I. T. Knight, W. L. Straube, and R. R. Colwell. 1989. Simple, rapid method for direct isolation of nucleic acids from aquatic environments. Appl. Environ. Microbiol. 55:548-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamplin, M. L., A. L. Gauzens, A. Huq, D. A. Sack, and R. R. Colwell. 1990. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 56:1977-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varela, P., G. Pollevick, M. Rivas, I. Chinen, N. Binsztein, A. Frasch, and R. Ugalde. 1994. Direct detection of Vibrio cholerae in stool samples. J. Clin. Microbiol. 32:1246-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu, H.-S., N. Roberts, F. L. Singleton, R. W. Attwell, D. J. Grimes, and R. R. Colwell. 1982. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in estuarine and marine environment. Microb. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]