Abstract
Objective
The aim of the present study was to compare the effects of clinical Pilates exercises with those of the standard lymphedema exercises on lymphedema developing after breast cancer treatment.
Materials and Methods
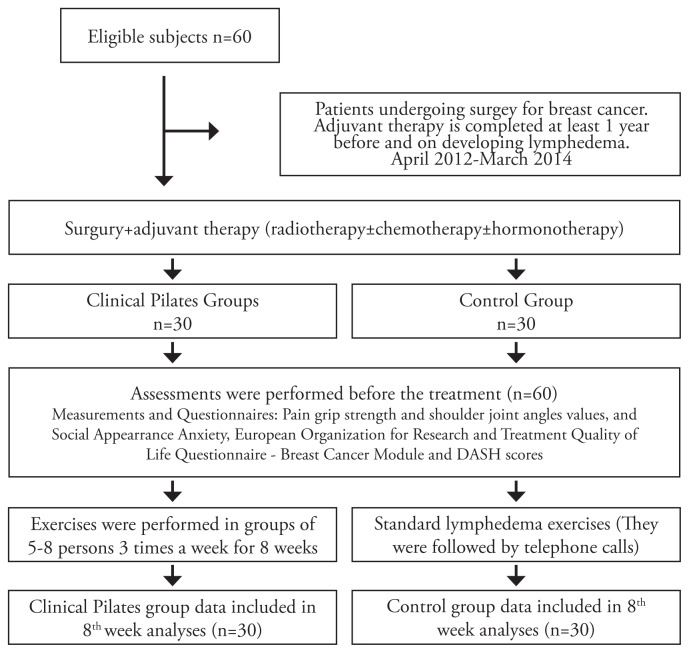
The study comprised 60 female patients with a mean age of 53.2±7.7 years who developed lymphedema after having breast cancer treatment. The patients were randomized into two groups: the clinical Pilates exercise group (n=30), and the control group (n=30). Before, and at the 8th week of treatment, the following parameters were measured: the severity of lymphedema, limb circumferences, body image using the Social Appearance Anxiety Scale, quality of life with the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ-BR23), and upper extremity function using the Disabilities of the Arm, Shoulder and Hand (DASH) outcome measure. Both groups performed one-hour exercises three days a week for 8 weeks.
Results
After treatment, the symptoms recovered significantly in both groups. Reductions in the severity of lymphedema, improvements in the social appearance anxiety scale scores, quality of life scores, and upper extremity functions scores in the clinical Pilates exercise group were greater than those in the control group. Clinical Pilates exercises were determined to be more effective on the symptoms of patients with lymphedema than were standard lymphedema exercises.
Conclusions
Clinical Pilates exercises could be considered a safe model and would contribute to treatment programs.
Keywords: Lymphedema, clinical Pilates, breast cancer, physiotherapy, Combined Decongestive Therapy
Introduction
Breast cancer is the most common type of cancer among women, and the number of women who develop breast cancer is increasing with each passing day. Today, approximately one in every eight women in the world has a risk of developing breast cancer. Although advances in breast cancer treatment such as radiotherapy, chemotherapy and surgical treatment reduce breast cancer-related mortality rates, they also lead to the development of serious complications such as lymphedema (1, 2). In the literature, the incidence of lymphedema after breast cancer treatment is reported to range from 2% to 83% (3–5).
Vignes et al. (6) reported that there was a relationship between the amount of radiotherapy administered after surgery and the frequency of lymphedema development. Chandra et al. (7) defined lymphedema as an irreversible chronic swelling and stated that if it was detected early, the amount of axillary radiotherapy administered could be less and the progress of lymphedema would be prevented. Several researchers reported that patients with breast cancer who presented to cancer research centers developed lymphedema caused by postoperative radiotherapy administered to axillary lymph nodes (8–11).
Lymphedema is classified into three groups according to the clinical stage. Both of the upper extremities are measured at regular intervals using a tape measure, starting from the nail root of the middle finger, up to the axillary region, and then the measurements are compared. If the difference between the two upper extremities is:
less than 3 cm, lymphedema is classified as mild,
between 3 and 5 cm, lymphedema is classified as moderate
more than 5 cm, lymphedema is classified as severe (11, 12).
Another measurement technique used in lymphedema classification is volumetric measurement. In this measurement, if the difference between the two extremities is:
less than 250 mL, lymphedema is classified as mild,
between 250 and 500 mL, lymphedema is classified as moderate
more than 500 mL, lymphedema is classified as severe (13).
In the literature, many publications have indicated that patients who underwent surgery for breast cancer and then had radiotherapy, chemotherapy, hormone therapy and/or medical therapy developed lymphedema due to tissue fibrosis, and their shoulder girdle joint movements deteriorated or became restricted. These publications report that in particular, shoulder flexion, abduction, and external rotation angles decreased, that patients’ activities of daily living were restricted, and that their quality of life was adversely affected (14–17).
Due to the lymphedema developing after the breast cancer surgery and radiotherapy, in addition to the reduction in arm function, deterioration is observed in body image and in posture accordingly. The flow rate of the lymphatic fluid throughout the body depends on the intermittent external pressure by skeletal muscles. Therefore, researchers emphasize that the treatment of a patient with lymphedema should include the reactivation of the patient’s arm functions and also the exercises that activate the whole body (18–21).
Studies conducted to investigate breast cancer and breast cancer-related lymphedema reported that physical activity contributed to patients’ quality of life and emotional status extremely positively and that it would be very necessary and useful to increase the diversity of physical activities. Implementing different exercise models, group works, social activities and Pilates training are reported to be useful for patients with breast cancer (22).
The exercise program for patients with lymphedema should also include cardiovascular exercises contributing to the development of aerobic fitness, breathing exercises stimulating the lymph system in the thoracic region, and the appropriate amount of resistance exercises to improve muscle strength and endurance. Therefore, the exercise program to be selected should be multipurpose, but not boring for the patient. In order to use the same terminology with health professionals, it will be useful to use termed exercise models. In recent years, although attention has been paid to some exercise models such as Pilates, yoga, tai chi, and aquatic exercises, studies on this issue are few (23, 24). In the literature, it is reported that core stability-based clinical Pilates exercises in particular activate all the body muscles. Thus, it is reported that when combined with breathing exercises, the intermittent external pressure that results from muscle contraction would stimulate ductus thoracicus due to the contraction of the diaphragm and facilitate the lymphatic flow, and thus reduce lymphatic load, and promote the immune system (22–25).
However, with the exception of one study that reported that lymphedema could be prevented if Pilates exercises were implemented, there are no studies in the literature in which Pilates exercises were used for the treatment of lymphedema (26). Therefore, the present study was planned to examine the impact of clinical Pilates exercises on the severity of lymphedema that developed after breast cancer treatment, grip strength, shoulder function, quality of life, and social image concerns, and to compare the effectiveness of clinical Pilates exercises with that of standard lymphedema physiotherapy education. The main hypothesis of this present study was that core stability-based clinical Pilates exercises that can activate all the body muscles would reduce the severity of lymphedema developing after breast cancer.
Materials and Methods
Design
All the participants were evaluated at Dokuz Eylül University School of Physical Therapy and Rehabilitation. Informed consent was obtained from patients who participated in this study.
Inclusion criteria:
Presence mild, moderate or severe lymphedema in upper extremities after breast cancer treatment
aged over 18 years
Exclusion criteria:
Presence of metastatic breast cancer
Presence of diagnosis of severe heart failure and / or arrhythmia
Presence of infection in the affected limb
Presence of severe psychological disorders
Presence of severe pain of unknown cause in the axillary region
Presence of musculoskeletal problems in the upper extremity before the treatment of breast cancer
Presence of other health problems that would prevent participation in the evaluation and treatment program
Informed consents were obtained from all the participants included in the study.
The study was approved by the ethics committee (decision April 12, 2012; number 2012 / 14–14).
Randomization
Based on their admission to the clinic without looking at the severity of lymphedema, the patients were asked to draw one of the two cards colored blue or red, and thus they were assigned to the two study groups through this simple random sampling method. Those who drew red cards were assigned to the clinical Pilates exercise group (n=2) and those who drew blue cards were assigned to the control group (n=30). When the study started, there were 32 patients in the clinical Pilates exercise group. However, two patients were excluded from the study because one developed liver metastasis and the other discontinued treatment. Therefore, the study was completed with 60 patients, 30 in the clinical Pilates group and 30 in the control group.
Randomization Scheme
Measurements and Questionnaires
All evaluations were done by another physiotherapist to avoid mistakes. Cases and controls were chosen as the concurrent controls in an attempt to prevent bias. The patients’ heights and weights were measured and recorded in centimeters (cm) and kilograms (kg) respectively. Other relevant data such as sex, site of cancer, post mastectomy duration were obtained via face-to-face interviews.
Grip strength was measured using a Jamar hand dynamometer. Measurements were consecutively repeated three times while the patient was in the standing position, with the arm close to the body and the elbow bent at 90 degrees. The highest one of these three measurements was recorded (27).
Upper limb circumferences were measured with a tape measure starting from the proximal nail fold of the middle finger up to the armpit at 5 cm intervals when the patient was in the supine position (28).
Upper extremity range of motion measurements were performed using a goniometer. Flexion, abduction, and internal and external rotation angles of the shoulder joint were measured when the patient was in the supine position (29).
Social Appearance Anxiety was assessed using the Social Appearance Anxiety (SAA) Scale. The scale was developed as a self-report scale to measure a patient’s cognitive, behavioral and emotional anxieties. The SAA Scale is a 16-item, 5-point Likert-type scale. High scores indicated poor performance. The Turkish validity and reliability study of the scale was performed by Doğan (30).
The European Organization for Research and Treatment Quality of Life Questionnaire – Breast Cancer Module (EORTC QLQ-BR23) was developed to assess challenges of daily life faced by patients with breast cancer and was used to assess the Quality of Life of the participants. It is a 4-point Likert scale ranging from 1 (not at all) to 4 (very much). The total high scores obtained from the QLQ-BR 23 questionnaire with which patients’ quality of daily lives is analyzed indicate difficulties in performing daily living activities, functional activities and reduction in the quality of life. The Turkish validity and reliability study of the scale was conducted by Demirci et al. (31).
To assess the functional level of upper limbs, the 30-item DASH questionnaire was administered. The measurements were compared after all the items were given scores ranging from 0 (no disability) to 100 (the most severe disability). A lower score indicated an improvement in functional status. The score for the disability/symptom scale was defined as the DASH score (32).
Assessments were performed before the treatment and at the 8th week of the treatment. Both groups wore pressure garments during exercises.
Clinical Pilates exercise group; before starting the clinical Pilates exercise program, the patients were trained on Pilates exercises and postures. Exercises were performed in groups of 5–8 persons 3 times a week for 8 weeks. During training, the patients were taught how to create lumbopelvic stability (core stabilization), which is the basis for Pilates exercises, and spinal stabilization and appropriate posture techniques. Each patient was taught how to create lumbar and spinal stabilization in the prone, supine, and side-lying positions using a stabilizer.
Clinical Pilates exercises were performed as group training sessions and included the following exercises:
Roll Down, upper-extremity proprioceptive neuromuscular facilitation (PNF) methods, Dumb Waiter, Cleopatra, Toy Soldier, Chester stretch, and swinging exercises in the standing position
Spine stretch, the Saw, Mermaid, and oblique roll up exercises in the sitting position
Abdominal preparation, Hundreds, one-leg stretch, double-leg stretch, scissors, shoulder bridge, and hip twist exercises in the supine position
Clam, arm openings, sidekick, lift lower, and leg lift exercises in the side-lying position
Swan Dive, one-leg kick, and swimming exercises in the prone position
After four weeks, this exercise program was continued by adding a yellow elastic resistance band exercises. Through exercises performed by concentrating on spinal stabilization, the ductus thoracicus was stimulated and lymphatic flow was induced through continuous contraction of muscles in this region where the lymph nodes are intense. By adding hand-arm-shoulder movements in all positions and pumping activities (opening and closing of fingers), it was aimed to accelerate the lymphatic flow.
The patients in the clinical Pilates exercise group, which was supervised by physiotherapists, were also asked to practice a home program every day that included manual lymphatic drainage training, wall extension, and Wand exercises used to improve shoulder flexibility and skin care training.
Control Group; lumbopelvic stability (core stabilization) was taught to the control group patients. They were taught how to protect core stabilization while performing activities of daily living and they were recommended to maintain a home exercise program. They were also taught how to conduct manual lymphatic drainage included in the complex decongestive therapy method, skincare, and shoulder exercises, and were instructed to perform each exercise every day. To increase their shoulder function and to reduce joint limitations, the participants were taught wall extension and Wand exercises, head and neck exercises, and exercises to improve shoulder girdle stability. In addition they were recommended to perform pumping activities and breathing exercises. The participants were given a brochure that described these exercises and were recommended to repeat these exercises at least 10 times. They were also advised to pay attention to skin care and to walk 1 hour every day. The participants were followed up through telephone calls.
Statistical analysis
The data of this study were analyzed using the Statistical Package for the Social Sciences for Windows software version 16.0 (SPSS Inc.; Chicago, IL, USA). The anthropometric data are presented as means and standard deviation. The numerically determined data are expressed in numbers and percentages. To compare the difference between the two groups, the independent samples t-test was used. For the analysis of the intra-group pre- and post-treatment results, the dependent samples t-test was used. In addition, for the analysis of survey results calculated at certain periods and rates, the Wilcoxon test was used, which is the non-parametric counterpart of the t-test. A p value of <0.05 was considered statistically significant.
Results
The study was completed with 60 patients, 30 in the clinical Pilates group and 30 in the control group.
The patients’ pre-treatment demographic characteristics were similar in both groups and there was no statistically significant difference between them (Table 1).
Table 1.
Comparison of demographic and anthropometric characteristics of the participants. Lymphedema Development Time in Patients (after completion of radio- and chemotherapy)
| Clinical Pilates exercise group (n=30) X±SD | Control group (n=30) X±SD | p | |
|---|---|---|---|
| Age (years) | 53.17±7.66 | 54.03±12.57 | 0.748 |
| Height (cm) | 1.61±0.06 | 1.61±0.07 | 0.891 |
| Body weight (kg) | 73.57±11.61 | 77.83±11.41 | 0.156 |
| BMI (kg/m2) | 28.53±4.51 | 30.35±4.99 | 0.144 |
| Lymphedema Development time in years | 5.0±3.57 | 4.95±4.87 | p>0.05 |
When the patients were compared to determine how much time later they developed lymphedema after the completion of the treatment (surgery, radiotherapy, chemotherapy) implemented following the diagnosis of breast cancer, no statistically significant difference was determined between them (p>0.05). The groups were similar in terms of the duration of lymphedema development (Table 1).
Of the patients in the different treatments, 66.7% of the clinical Pilates exercise group and 43.3% of the control group received complex decongestive therapy.
When the two groups were compared in terms of their pre- and post-treatment scores for pain in the lymphedematous arm; severity of lymphedema; grip strength; shoulder range of motion; and disabilities of the arm, shoulder and hand (DASH); quality of life with breast cancer (QLQ-BR23); and social appearance anxiety (SAA), although there were significant improvements in all aspects in the clinical Pilates exercise group (p<0.05), the control group had no improvements in grip strength, shoulder flexion, and external rotation angles (p>0.05) (Table 2).
Table 2.
Comparison of the Clinical Pilates exercise group with the control group in terms of their pre- and post-treatment pain (VAS), grip strength and shoulder joint angles values, and Social Appearance Anxiety (SAA), European Organization for Research and Treatment Quality of Life Questionnaire (EORTC) - Breast Cancer Module (QLQ-BR23) and DASH scores
| Clinical Pilates exercise group (n=30) | Control group (n=30) | Clinical pilates and control groups | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| GROUPS | Pre-treatment X ± SD | Post-treatment X ± SD | P* | Pre-treatment X ± SD | Post-treatment X ± SD | P* | Post-treatment P*** |
| VAS (pain) | 3.47±3.18 | 0.67±0.84* | <0.01* | 2.30±3.30 | 0.87±1.43* | 0.02* | 0.51 |
| Grip Strength (kg) | 17.53±6.71 | 19.80±6.16* | 0.01* | 20.73±6.63 | 21.90±5.38* | 0.08 | 0.05* |
| Shoulder Flexion (0–180°C) | 165.33±21.45 | 179.17±2.65* | 0.01* | 172.67±14.13 | 177.50±6.40* | 0.08 | 0.19 |
| Shoulder Abduction (0–180°C) | 155.50±35.70 | 177.17±7.39* | 0.01* | 163.67±25.90 | 173.50±16.56* | 0.01* | 0.27 |
| Shoulder Ext. Rotation (0–45°C) | 77.17±22.65 | 88.67±3.46* | 0.05* | 81.83±15.00 | 85.67±10.73* | 0.22 | 0.15 |
| SAA** | 24.83±7.98 | 19.67±3.66** | <0.01** | 27.57±9.08 | 26.17±8.09** | 0.04** | <0.01** |
| QLQ-BR 23** | 32.44±10.27 | 38.51±8.42** | 0.04** | 34.10±9.63 | 38.37±7.48** | 0.02** | 0.94 |
| DASH (0–100)** | 44.24±15.33 | 37.99±15.02** | <0.01** | 34.82±11.96 | 32.15±12.11** | <0.01** | 0.39 |
Paired Samples Test, p<0.05;
Wilcoxon Signed-Rank Test, p<0,05;
post-treatment Comparison of the two groups, p<0.05*;
VAS: Visual Analogue Scale
The main objective of the study was to evaluate the efficacy of clinical Pilates exercises in reducing the severity of lymphedema. Swelling caused by lymphedema is not even across the limb. Therefore, measurement of lymphedema must be performed from distal to proximal at frequent intervals. In the present study, measurements were performed bilaterally in the upper extremities at 5 cm intervals starting from the nail root of the middle finger up to the axilla. The effectiveness of the treatment was assessed by measuring the severity of edema at the beginning and end of the treatment. When the two groups were compared in terms of the reduction of the severity of edema in line with the data related to the upper extremity measurements, clinical Pilates exercises were found more effective than standard exercises. Statistical comparisons revealed that measurements of each region of the upper extremity in the clinical Pilates group were more significant than those in the control group (p<0.05) (Table 3).
Table 3.
Comparison of clinical Pilates exercise and control group patients’ pre- and post-treatment upper limb circumferences measured starting from proximal nail fold of the middle finger up to the armpit of the arm with lymphedema at 5-cm intervals
| Clinical Pilates group | Control group | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| GROUPS | Pre-treatment X ± SD | Post-treatment X ± SD | P* | Pre-treatment X ± SD | Post-treatment X ± SD | P* |
| Proximal nail fold | 4.93±0.39 | 4.77±0.34 | 0.01* | 5.33±0.46 | 5.32±0.44 | 0.32 |
| 5 cm↑ | 6.60±0.91 | 6.50±0.82 | 0.18 | 7.13±0.97 | 6.95±0.71 | 0.02* |
| 10 cm↑ | 19.92±2.11 | 18.73±3.49 | 0.02* | 21.32±2.36 | 21.13±2.20 | 0.01* |
| 15 cm↑ | 18.38±2.23 | 17.67±2.96 | 0.03* | 20.33±3.82 | 19.65±3.94 | 0.06 |
| 20 cm↑ | 20.25±4.38 | 19.80±3.48 | 0.24 | 23.08±3.99 | 22.63±3.76 | 0.01* |
| 25 cm↑ | 24.13±4.06 | 23.48±3.78 | 0.02* | 26.68±4.56 | 26.20±4.39 | <0.00* |
| 30 cm↑ | 27.68±4.41 | 26.98±4.14 | 0.02* | 30.22±4.49 | 29.73±4.29 | <0.01* |
| 35 cm↑ | 28.98±4.22 | 28.27±4.12 | 0.01* | 31.40±4.18 | 31.00±3.99 | 0.03* |
| 40 cm↑ | 29.38±4.49 | 28.70±4.41 | 0.09 | 32.38±4.47 | 32.15±4.38 | 0.06 |
| 45 cm↑ | 31.60±5.00 | 30.65±4.59 | 0.01* | 34.78±5.47 | 34.45±5.41 | 0.01* |
| 50 cm↑ | 33.67±4.67 | 32.43±4.20 | 0.01* | 36.80±5.16 | 36.38±4.82 | 0.07 |
| 55 cm↑ | 34.97±4.28 | 33.72±3.87 | 0.01* | 37.48±5.06 | 37.03±4.90 | <0.01* |
| 60 cm↑ | 36.22±4.16 | 34.98±3.73 | 0.01* | 36.68±3.76 | 36.57±3.78 | 0.38 |
Proximal nail fold, 10cm-, 15cm-, 25cm-, 30cm-, 35cm-, 45cm-, 50cm-, 55cm-, 60cm- levels are statistically significant (*p<0.05).
5cm-, 10cm-, 20 cm-, 25 cm-, 30 cm-, 35cm-, 45cm-, 55cm- levels are statistically significant (*p<0.05).
However, when the two groups were compared in terms of reduction in the severity of lymphedema after treatment, there were significant decreases in all regions except the axillary region (p<0.05), which supports the fact that the severity of lymphedema decreased more in patients in the clinical Pilates group (Table 4).
Table 4.
Comparison of the two groups in terms of the severity of lymphedema after treatment
| GROUPS | Clinical Pilates group X ± SD | Control group X ± SD | p |
|---|---|---|---|
| Proximal nail fold | 4.77±0.34 | 5.32±0.44 | <0.01* |
| 5 cm↑ | 6.50±0.82 | 6.95±0.71 | 0.02* |
| 10 cm↑ | 18.73±3.49 | 21.13±2.20 | 0.02* |
| 15 cm↑ | 17.67±2.96 | 19.65±3.94 | 0.03* |
| 20 cm↑ | 19.80±3.48 | 22.63±3.76 | 0.04* |
| 25 cm↑ | 23.48±3.78 | 26.20±4.39 | 0.01* |
| 30 cm↑ | 26.98±4.14 | 29.73±4.29 | 0.01* |
| 35 cm↑ | 28.27±4.12 | 31.00±3.99 | 0.01* |
| 40 cm↑ | 28.70±4.41 | 32.15±4.38 | 0.04* |
| 45 cm↑ | 30.65±4.59 | 34.45±5.41 | 0.05* |
| 50 cm↑ | 32.43±4.20 | 36.38±4.82 | 0.01* |
| 55 cm↑ | 33.72±3.87 | 37.03±4.90 | 0.06* |
| 60 cm↑ | 34.98±3.73 | 36.57±3.78 | 0.20 |
Proximal nail fold, 5 cm-, 10 cm-, 15 cm-, 25 cm-, 30 cm-, 35 cm-, 40 cm-, 45 cm-, 50 cm-, 55 cm-, levels are statistically significant (*p<0.05).
Discussion and Conclusions
In Turkey, breast cancer ranks the first of the 10 most common cancers among women (33).
Therefore, in the present study, the efficacy of clinical Pilates exercises in the treatment of lymphedema that develops after breast cancer treatment was investigated. At the end of the present study, it was determined that lymphedema decreased, and upper limb function and quality of life increased in the clinical Pilates exercises group supervised by physiotherapists, and that clinical Pilates exercises were more effective than standard exercises according to the comparison of the results of both groups.
Lymphedema after breast cancer treatment is caused by inflammation, infection, and disruption of the lymphatic system due to radiation-related fibrosis of soft tissues, which disrupts patients’ upper extremity functions (34, 35). All patients included in this present study underwent surgery. Ninety-six percent of the patients underwent radio-chemo-medical therapy and / or hormonal therapy. The duration of lymphedema development among them was close to each patient’s.
Schmitz et al. (35) argued that exercise training increased the capacity of the muscular and cardiovascular systems by loading controlled physiologic stress onto the body, and could improve collaterals as in the arterial system, which would thus facilitate lymphatic flow in patients with lymphedema (36).
Several studies conducted to investigate lymphedema after breast cancer treatment reported that physical activity generally contributed to patients’ quality of life and emotional status extremely positively. However, they also reported that increasing the diversity of physical activities was of great importance because this may create alternative exercise options in the treatment of lymphedema. It has been reported that different exercise models, group exercise, social activity-oriented exercises, and Pilates training might be useful in patients with lymphedema (19, 20, 22, 24, 26).
A review of the literature in line with this information revealed that patients with breast cancer used Pilates exercises to improve their quality of life, shoulder function, and body image. However, no detailed exercise program was implemented in the treatment of lymphedema, a chronic disease that develops after breast cancer treatment. Studies demonstrated that when combined with breathing exercises, Pilates exercises were capable of activating the whole body together and promote the quality of life and functionality by improving body image; therefore, they could be used in the treatment of chronic diseases such as lymphedema that deteriorate life quality (26, 37–39).
In the present study, which was conducted to investigate the effects of clinical Pilates exercises as a treatment option in patients who develop lymphedema after breast cancer treatment with group exercises, the patients’ socialization improved, their awareness of their own body increased after they learned how to control their body in general, and their quality of life improved. Moreover, the severity of lymphedema decreased due to improvements in functionality and the constant union of mind and body through body stability. In the present study, the results obtained by the patients in the clinical Pilates exercise group were compared with those obtained by the control group, which performed standard lymphedema exercises, and it was determined that clinical Pilates exercises were more effective than standard lymphedema exercises in all the parameters investigated.
These effects result from the fact that spinal stabilization, which is the basis for clinical Pilates exercises, can be maintained in all activities of daily life. It is considered that spinal stabilization contributes to continuous contraction of the muscles of the trunk and the diaphragm, and thus stimulates ductus thoracicus and abdominal lymph nodes in patients with lymphedema, which facilitates lymphatic flow, and acts as a pump that accelerates the flow of lymph when combined with limb exercises (21, 36, 40). In addition, the patients’ awareness and union of mind and body were increased, and through isolated muscle exercises, they were taught that they themselves could control their muscles. In line with this cognitive restructuring, this stabilization of the trunk maintained its effects on all the body movements, and patients who learned how to correct inappropriate movements during exercises developed a positive perception of recovery. The survey results obtained after the treatment also supported this view. The results indicate that both functional independence and quality of life improved.
The results of this present study indicate that clinical Pilates exercises had positive effects on the amount of lymphedema, functional status, grip strength, and quality of life of patients with lymphedema. Patients in both the clinical Pilates and control group were recommended to wear pressure garments on the arm with lymphedema during the treatment sessions. In conclusion; given the positive effects of clinical Pilates exercises on patient with breast cancer who developed lymphedema after their treatment in terms of functionality, mood and quality of life, it was decided that it would be appropriate to include clinical Pilates exercises in physiotherapy programs as a safe exercise model. It was also considered that Clinical Pilates exercises would be a good exercise regimen for patients with lymphedema and they might adopt them as a lifestyle exercise model. However, if this view is to be supported, new studies should be performed with a greater number of patients
Acknowledgement
We thank all participants who agreed to participate in the study.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from Dokuz Eylül University İzmir Clinical Research Ethics Committee (decision dated April 12, 2012 and Research Protocol No: 2012 / 14–14).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Conflict of Interest: No conflict of interest was declared by the authors.
Author Contributions: Concept - H.O.S, M.M., D.K.; Design - H.O.S., M.M.; Supervision M.M.; Funding - D.K.; Materials - T.Y.; Data Collection and/or Processing - H.O.S.; Analysis and/or Interpretation - H.O.S., G.E.; Literature Review - H.O.S.; Writing - H.O.S.; Critical Review - H.O.S., M.M.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Didem K, Ufuk YS, Serdar S, Zümre A. The comparison of two different physiotherapy methods in treatment of lymphedema after breast surgery. Breast Cancer Res Treat. 2005;93:49–54. doi: 10.1007/s10549-005-3781-2. https://doi.org/10.1007/s10549-005-3781-2. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, SR, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. https://doi.org/10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Cheifetz O, Haley L. Management of secondary lymphedema related to breast cancer. Can Fam Physician. 2010;56:1277–1284. [PMC free article] [PubMed] [Google Scholar]
- 4.Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Physical Therapy. 2001;81:1192–1205. [PubMed] [Google Scholar]
- 5.Moseley AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Annals of Oncology. 2007;18:639–646. doi: 10.1093/annonc/mdl182. https://doi.org/10.1093/annonc/mdl182. [DOI] [PubMed] [Google Scholar]
- 6.Vignes S, Arrault M, Ebelin M. [Poor influence of surgery on upper limb lymphedema volume in patients after breast cancer treatment]. Journal des maladies vasculaires. 2006;31(4 Pt 1):202–5. doi: 10.1016/s0398-0499(06)76544-1. https://doi.org/10.1016/S0398-0499(06)76544-1. [DOI] [PubMed] [Google Scholar]
- 7.Chandra RA, Miller CL, Skolny MN, Warren LE, Horick N, Jammallo LS, et al. Radiation therapy risk factors for development of lymphedema in patients treated with regional lymph node irradiation for breast cancer. International Journal of Radiation Oncology* Biology* Physics. 2015;91(4):760–4. doi: 10.1016/j.ijrobp.2014.12.029. https://doi.org/10.1016/j.ijrobp.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herd Smith A, Russo A, Muraca MG, Del Turco MR, Cardona G. Prognostic factors for lymphedema after primary treatment of breast carcinoma. Cancer. 2001;92(7):1783–7. doi: 10.1002/1097-0142(20011001)92:7<1783::aid-cncr1694>3.0.co;2-g. https://doi.org/10.1002/1097-0142(20011001)92:7<1783::AID-CNCR1694>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 9.Mortimer P, Bates D, Brassington H, Stanton A, Strachan D, Levick J. The prevalence of arm oedema following treatment for breast cancer. Qjm. 1996;89(5):377–80. https://doi.org/10.1093/qjmed/89.5.377. [Google Scholar]
- 10.Meek AG. Breast radiotherapy and lymphedema. Cancer. 1998;83(S12B):2788–97. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2788::aid-cncr27>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Mondry T. Part II Physical therapy. 2000. Current Problems in Cancer. [PubMed] [Google Scholar]
- 12.Gary D. Lymphedema diagnosis and management. Journal of the American Academy of Nurse Practitioners. 2007;19(2):72–8. doi: 10.1111/j.1745-7599.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 13.Mortimer The pathophysiology of lymphedema. Cancer. 1998 doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2798::aid-cncr28>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Bosompra K, Ashikaga T, O’Brien PJ, Nelson L, Skelly J. Swelling, numbness, pain, and their relationship to arm function among breast cancer survivors: a disablement process model perspective. The breast journal. 2002;8(6):338–48. doi: 10.1046/j.1524-4741.2002.08603.x. https://doi.org/10.1046/j.1524-4741.2002.08603.x. [DOI] [PubMed] [Google Scholar]
- 15.Ebaugh D, Spinelli B, Schmitz KH. Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors. Medical hypotheses. 2011;77(4):481–7. doi: 10.1016/j.mehy.2011.06.015. https://doi.org/10.1016/j.mehy.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Hayes S, Battistutta D, Newman B. Objective and subjective upper body function six months following diagnosis of breast cancer. Breast cancer research and treatment. 2005;94(1):1–10. doi: 10.1007/s10549-005-5991-z. https://doi.org/10.1007/s10549-005-5991-z. [DOI] [PubMed] [Google Scholar]
- 17.Lash TL, Silliman RA. Long-Term Follow-Up of Upper-Body Function Among Breast Cancer Survivors. The breast journal. 2002;8(1):28–33. doi: 10.1046/j.1524-4741.2002.08006.x. https://doi.org/10.1046/j.1524-4741.2002.08006.x. [DOI] [PubMed] [Google Scholar]
- 18.O’Toole JA, Ferguson CM, Swaroop MN, Horick N, Skolny MN, Brunelle CL, et al. The impact of breast cancer-related lymphedema on the ability to perform upper extremity activities of daily living. Breast cancer research and treatment. 2015;150(2):381–8. doi: 10.1007/s10549-015-3325-3. https://doi.org/10.1007/s10549-015-3325-3. [DOI] [PubMed] [Google Scholar]
- 19.Brown JC, Schmitz KH. Weight lifting and physical function among survivors of breast cancer: a post hoc analysis of a randomized controlled trial. Journal of Clinical Oncology. 2015;33(19):2184–9. doi: 10.1200/JCO.2014.57.7395. https://doi.org/10.1200/JCO.2014.57.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arya R, Siamakpour-Reihani S, Palta M, Massa L, Broadwater G, Blitzblau RC, et al. Exercise behavior and patient-reported outcomes in women with early breast cancer receiving locoregional radiation therapy. Practical radiation oncology. 2015;5(4):e275–e81. doi: 10.1016/j.prro.2015.01.003. https://doi.org/10.1016/j.prro.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Kampman E, Vrieling A, van Duijnhoven FJ, Winkels RM. Impact of diet, body mass index, and physical activity on cancer survival. Current nutrition reports. 2012;1(1):30–6. doi: 10.1007/s13668-011-0004-9. https://doi.org/10.1007/s13668-011-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz K. Physical Activity and Cancer. Springer; 2010. Physical activity and breast cancer survivorship; pp. 189–215. [DOI] [PubMed] [Google Scholar]
- 23.Lane KN, Dolan LB, Worsley D, McKenzie DC. Upper extremity lymphatic function at rest and during exercise in breast cancer survivors with and without lymphedema compared with healthy controls. Journal of Applied Physiology. 2007;103(3):917–25. doi: 10.1152/japplphysiol.00077.2007. https://doi.org/10.1152/japplphysiol.00077.2007. [DOI] [PubMed] [Google Scholar]
- 24.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Journal of Clinical Oncology. 2007;25(28):4396–404. doi: 10.1200/JCO.2006.08.2024. https://doi.org/10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 25.Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Annals of plastic surgery. 2007;59(4):464–72. doi: 10.1097/01.sap.0000257149.42922.7e. https://doi.org/10.1097/01.sap.0000257149.42922.7e. [DOI] [PubMed] [Google Scholar]
- 26.Keays KS, Harris SR, Lucyshyn JM, MacIntyre DL. Effects of Pilates exercises on shoulder range of motion, pain, mood, and upper-extremity function in women living with breast cancer: a pilot study. Physical Therapy. 2008;88(4):494–510. doi: 10.2522/ptj.20070099. https://doi.org/10.2522/ptj.20070099. [DOI] [PubMed] [Google Scholar]
- 27.Shechtman O, Gestewitz L, Kimble C. Reliability and validity of the DynEx dynamometer. Journal of Hand Therapy. 2004;17(4):438. doi: 10.1197/j.jht.2005.04.002. https://doi.org/10.1016/S0894-1130(04)00204-2. [DOI] [PubMed] [Google Scholar]
- 28.Mayrovitz HN, Sims N, Macdonald J. Assessment of limb volume by manual and automated methods in patients with limb edema or lymphedema. Advances in skin & wound care. 2000;13(6):272. [PubMed] [Google Scholar]
- 29.Otman S, Demirel H, Sade A. Hacettepe Üniversitesi Fizik Tedavi ve Rehabilitasyon Yüksekokulu Yayınları. 1995. Tedavi hareketlerinde temel değerlendirme prensipleri; p. 16. [Google Scholar]
- 30.DOĞAN T. Sosyal görünüş kaygısı ölçeği’nin (SGKÖ) Türkçe uyarlaması: Geçerlik ve güvenirlik çalışması. Hacettepe Üniversitesi Eğitim Fakültesi Dergisi. 2010;39(39) [Google Scholar]
- 31.Demirci S, Eser E, Ozsaran Z, Tankisi D, Aras AB, Ozaydemir G, et al. Validation of the Turkish versions of EORTC QLQ-C30 and BR23 modules in breast cancer patients. Asian Pac J Cancer Prev. 2011;12(5):1283–7. [PubMed] [Google Scholar]
- 32.Düger T, Yakut E, Öksüz Ç, Yörükan S, Bilgütay B, Ayhan Ç. Kol, omuz ve el sorunları (disabilities of the arm, shoulder and hand-DASH) anketi Türkçe uyarlamasının güvenirliği ve geçerliği. Fizyoter Rehabil. 2006;17(3):99–107. [Google Scholar]
- 33.Thomas MacLean RLHT, Kwan W, Towers A, Miedema B, Tilley A, editors. Oncology nursing forum. 2008. Arm morbidity and disability after breast cancer: new directions for care. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. Journal of Clinical Oncology. 2008;26(35):5689–96. doi: 10.1200/JCO.2008.16.4731. https://doi.org/10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MDS, NK Upper body pain and functional disorders in patients with breast cancer. PM&R. 2014:170–83. doi: 10.1016/j.pmrj.2013.08.605. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz KH. Balancing lymphedema risk: exercise versus deconditioning for breast cancer survivors. Exercise and sport sciences reviews. 2010;38(1):17. doi: 10.1097/JES.0b013e3181c5cd5a. https://doi.org/10.1097/JES.0b013e3181c5cd5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzarino M, Kerr D, Wajswelner H, Morris ME. Pilates Method for Women’s Health: Systematic Review of Randomized Controlled Trials. Archives of physical medicine and rehabilitation. 2015;96(12):2231–42. doi: 10.1016/j.apmr.2015.04.005. https://doi.org/10.1016/j.apmr.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Stan DLRS, Sundt K, Cheville AL, Youdas JW, Krause DA, Boughey JC, Walsh MF, Cha SS, Pruthi S. Pilates for breast cancer survivors. Clinical journal of oncology nursing. 2012;16(2):131–41. doi: 10.1188/12.CJON.131-141. https://doi.org/10.1188/12.CJON.131-141. [DOI] [PubMed] [Google Scholar]
- 39.Eyigor S, Karapolat H, Yesil H, Uslu R, Durmaz B. MINERVA MEDICA COPYRIGHT®. European journal of physical and rehabilitation medicine. 2010;46:481–7. [PubMed] [Google Scholar]
- 40.Aaronson N. Exercise for the breast cancer survivor: what you need to know about designing a program for clients at risk of post-breast-cancer lymphedema. IDEA Fitness Journal. 2007;4(4):29–33. [Google Scholar]