Abstract
Objective
To assess the relationship between background parenchymal enhancement (BPE) and fibroglandular tissue (FGT) proportion on breast magnetic resonance imaging (MRI) and hormone receptor expression and molecular subtypes in invasive breast cancer.
Materials and Methods
This retrospective study enrolled 75 breast cancer patients who underwent breast MRI before treatment. T1-weighted images were reviewed to determine the FGT proportion, and contrast-enhanced fat-suppressed T1-weighted images were reviewed to determine BPE. Estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor 2-neu (HER2) status, and molecular subtypes of the tumors were compared with the BPE and FGT proportions.
Results
Women with high BPE tended to have increased rate of ER and PR positive tumors (p=0.018 and p=0.013). FGT proportion was associated with ER positivity (p=0.009), but no significant differences between FGT proportion and PR positivity were found (p=0.256). There was no significant difference between HER2 status and any of the imaging features (p=0.453 and p=0.922). For premenopausal women, both FGT proportion and BPE were associated with molecular subtypes (p=0.025 and p=0.042). FGT proportion was also associated with BPE (p<0.001).
Conclusion
In women with invasive breast cancer, both high FGT containing breasts and high BPE breasts tended to have ER positive tumors.
Keywords: Breast neoplasms, magnetic resonance imaging, molecular subtypes, enhancement, hormone receptor
Introduction
Breast cancer is the most common malignancy of women with a life-time risk up to 12% in USA (1). It is the second most common cause of death from cancer in women (2). In recent years, advancement in the knowledge of the biology of breast cancer has contributed to better understanding the nature of the disease. For therapeutic reasons, breast cancer is divided into four different molecular subtypes using molecular biomarkers: estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor 2 (HER2). Four molecular subtypes are identified: Luminal A, Luminal B, HER2 positive, and Triple Negative (3). Different subtypes present with distinct epidemiological risk factors, distinct disease prognoses, and different responses to systemic and local therapy (4–8). This knowledge has opened a new door in disease management toward personalized therapy. For example, luminal A cancers require hormonotherapy whereas triple negative tumors respond better to chemotherapy (3). Trastuzumab, which is directed against the HER2-neu receptor, is considered in the treatment of HER2 positive tumors (3). Luminal A and luminal B subtypes are more likely to develop bone metastases whereas triple negative subtypes are more likely to develop lung and brain metastases (9).
Breast MRI is a common diagnostic tool in the management of breast diseases. It can be used for screening in a high-risk population, for determining the disease extent, or for problem solving to contribute in discordant results of mammography, ultrasonography or clinical findings. FGT proportion, which refers to the proportion of FGT to fat tissue of the breast, and BPE, which refers to the enhancement of normal breast parenchyma after contrast agent administration, are two imaging features of breast MRI. Breast density which reflects FGT composition is a well-known risk factor for breast malignancy. Women having a high amount of FGT content are also more likely to develop breast cancer (10). Similarly, a recent study reported that moderate or marked BPE is associated with a greater risk of developing breast cancer than minimal or mild BPE (11). Considering those known risk factors and the spectrum of breast cancers, one may suspect that FGT proportion and BPE of the same molecular subtypes may have some common properties. If this relationship is demonstrated, we may further increase our knowledge of which mechanism the FGT proportion and BPE influence the risk of developing breast cancer. Furthermore, it could be used for guiding the initial treatment planning and predicting the disease prognosis. This is still unclear and more studies are needed to determine whether the FGT proportion or BPE can predict receptor positivity, which also determines the molecular subtypes of the breast cancer. Therefore, in this retrospective study, we aimed to demonstrate whether there is any correlation between the FGT proportion and BPE of cancerous breast tissue and hormone receptor expression and molecular subtypes of invasive breast cancer.
Materials and Methods
This study was approved by the institutional review board. Owing to the retrospective nature of the study, the requirement for informed consent was waived.
Patient selection and characteristics
Retrospective analysis of the medical records between January 2010 and April 2016 of our university hospital computer database was queried. A total of 115 patients who had pre-treatment breast MRI were identified. Of these, 40 patients were excluded from the study because of unavailable or poor quality of MR images (n=5), previous history of breast cancer (n=13), unknown receptor status (n=12), and unknown menopausal status (n=10). The remaining 75 cases were enrolled in the study. Eligible patients had not used any hormone replacement or anti-hormonal therapy within the 12 months before the MRI was performed. Clinical indications for breast MRI were either for problem solving after inconclusive breast ultrasound or mammography or for planning of the surgical approach preoperatively.
It is a routine daily practice for our breast diseases imaging department to conduct a questionnaire survey of the patients who are admitting for any breast examination. Patients are asked about their menopausal status, history of hormonal therapy, and presence of history of any previous breast cancer. Data about those information were collected from this archive.
Breast MRI technique and MRI interpretation
Breast MRI examinations were performed using two different 1.5 T MRI systems (Achieva; Philips Healthcare, Best, the Netherlands and Siemens Magnetom Symphony Quantum, Erlangen, Germany). All patients were examined in the prone position on a dedicated bilateral breast coil. Routine MRI protocol including axial T1-weighted turbo spin echo sequence and axial T1-weighted fat-suppressed dynamic contrast-enhanced 3D spoiled gradient echo sequence were obtained from all the patients (Table 1). A standard dosage of 0.1 mmoL/kg contrast agent was administered using an automatic injector with a flow rate of 2 mL/s following 20 ml of saline flush. One pre-contrast and five post-contrast image series for every one minute were obtained. After image acquisition, subtracted images were obtained by subtracting pre-contrast images from contrast-enhanced ones on a pixel-by-pixel basis.
Table 1.
Parameters of the MRI examinations
| Scanner 11 | Scanner 22 | |||
|---|---|---|---|---|
|
| ||||
| Parameters | T1-weighted TSE3 | T1- weighted 3D SGE4 | T1-weighted TSE3 | T1-weighted 3D SGE4 |
| TR/TE (ms) | 412 / 10 | 6.9 / 3.4 | 510 / 11 | 4.6 / 1.4 |
| Slice thickness (mm) | 3 | 1 | 4 | 1 |
| FOV (mm) | 340 | 340 | 320 | 320 |
| Matrix size (mm) | 340 × 270 | 340 × 337 | 288 × 384 | 336 × 448 |
| Flip Angle | 90 | 12 | 90 | 10 |
| NEX | 2 | 1 | 2 | 1 |
Achieva; Philips Healthcare, Best, the Netherlands
Siemens Magnetom Symphony Quantum, Erlangen, Germany
Axial T1-weighted turbo spin echo sequence
Axial T1-weighted fat-suppressed 3D spoiled gradient echo sequence
TR/TE: repetition time / echo time; FOV: field of view; NEX: number of excitation; MRI: magnetic resonance imaging
A radiologist with six years of experience in breast imaging interpreted the MRI examinations. The FGT proportion was determined by using axial T1-weighted MR images. BPE was determined by using the first post-contrast T1-weighted subtracted images. The radiologist was blinded to the hormone receptor expression and molecular subtypes of the tumors, age, and menopausal status of the patients.
The MRI examinations were read according to the lexicon of the American College of Radiology Breast Imaging and Reporting Data System (BIRADS) (12). Axial T1-weighted images were evaluated to determine the FGT proportion. Breast compositions were separated into four categories based on a visual assessment of the FGT proportion of the breast: BIRADS a: almost entirely fat; BIRADS b: scattered FGT; BIRADS c: heterogeneous FGT; BIRADS d: extreme FGT (Figure 1). BPE of the breast parenchyma was visually assessed and graded as minimal, mild, moderate, or marked (Figure 2).
Figure 1. a–d.
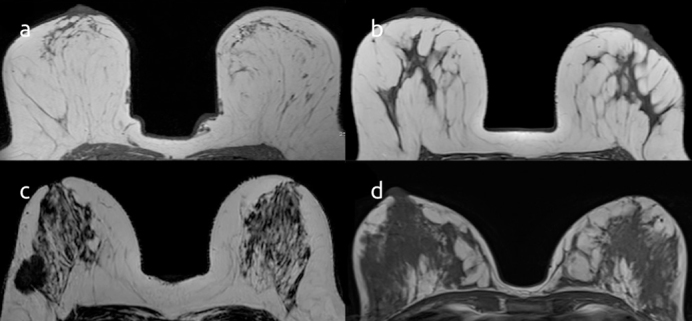
Axial T1-weighted images demonstrate the classification of the breasts according to FGT proportion; BIRADS a: almost entirely fat (a); BIRADS b: scattered FGT (b); BIRADS c: heterogeneous FGT (c); BIRADS d: extreme FGT (d)
Figure 2. a–d.
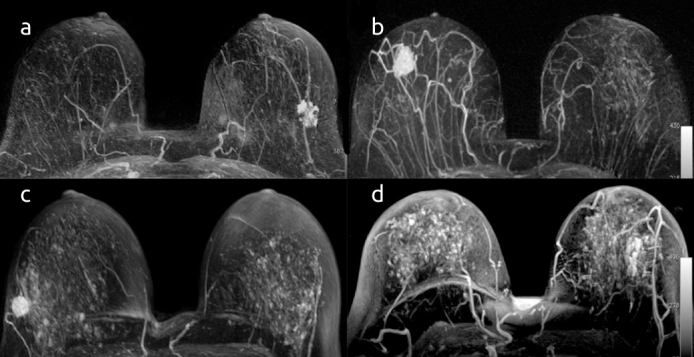
Axial fat-suppressed T1-weighted dynamic, contrast-enhanced MR images show different breast tissues with minimal (a), mild (b), moderate (c), and marked (d) BPE
Pathologic data collection, hormone receptor expression, and molecular subtypes of breast cancer
Pathology results of the cases were reviewed to identify ER, PR, and HER2 status. ER and PR positivity were determined via immunohistochemical analysis. Tumors were classified ER or PR positive if at least 1% positive staining was observed (13). The evaluation of HER2 status was based on the guidelines of the American Society of Clinical Oncology College of American Pathologists (ASCO/CAP) and was classified as negative, undetermined, or positive. Silver in situ hybridization (SISH) was performed on undetermined cases with an automatized system (Ventana Benchmark XT, Ventana Medical Systems, Illkirch, France) (14).
Lesions were classified into four subtypes according to immunocytochemical characteristics: Luminal A (ER+ and/or PR+, plus HER2−), Luminal B (ER+ and/or PR+, plus HER2+), HER2 enriched (ER− and PR−, plus HER2+) and Triple Negative (ER− and PR−, plus HER2−) (15).
Statistical analysis
For statistical analysis, ACR breast composition categories a and b (almost entirely fatty and scattered FGT) were combined and identified as fatty breast tissue, categories c and d (heterogeneous FGT and extreme FGT) were combined and identified as high fibroglandular breast tissue. Breasts with minimal or mild BPE were classified as low BPE breasts; breasts with moderate or marked BPE were classified as high BPE breasts. Statistical analyses were performed with Statistical Package for the Social Sciences 18.0 (SPSS Inc.; Chicago, IL, USA) for windows. The frequencies were compared, using the Pearson Chi-square, Continuity Correction Chi-square, and Fisher Exact test. One proportion test (z test) was used to compare MRI imaging features and molecular subtypes of breast cancer. A p value less than 0.05 was considered as statistically significant.
Results
MRI features, immunohistochemical characteristics, and molecular subtypes of the cases are summarized in Table 2. Among 75 patients with invasive breast cancer, 49 women were premenopausal (65%) and 26 women were postmenopausal (35%). Mean age of the study population was 46.7 years (range: 27–77). Among 75 patients with invasive breast cancer, molecular subtypes of the lesions were 40 for Luminal A (53.3%), 20 for Luminal B (26.7%), 8 for HER2 enriched (10.7%), and 7 for Triple Negative (9.3%). Thirty-five patients had fatty breast tissue (breast composition categories a and b), and 40 patients had high fibroglandular breast tissue (breast composition categories c and d). In terms of BPE: 45 cases were low BPE breasts (minimal + mild) and 30 cases were high BPE breasts (moderate + marked).
Table 2.
Demographic data, MRI features, hormone receptor expression and molecular subtypes of breast cancer
| Characteristics | Number (%) |
|---|---|
| Cases (Number) | 75 |
| Premenopausal | 49 (65.3) |
| Postmenopausal | 26 (34.6) |
| Breast Density on MRI | |
| BIRADS a | 16 (21.3) |
| BIRADS b | 19 (25.3) |
| BIRADS c | 19 (25.3) |
| BIRADS d | 21 (28.0) |
| Background Parenchymal Enhancement | |
| Minimal | 34 (45.3) |
| Mild | 11 (14.7) |
| Moderate | 16 (21.3) |
| Severe | 14 (18.7) |
| Pathology | |
| Invasive Ductal Carcinoma | 62 (82.7) |
| Ductal Carcinoma in Situ | 4 (5.3) |
| Invasive Lobulary Carcinoma | 4 (5.3) |
| Mucinous Carcinoma | 3 (4) |
| Tubulolobulary Carcinoma | 1 (1.3) |
| Papillary Carcinoma | 1 (1.3) |
| Estrogen Receptor | |
| Positive | 60 (20) |
| Negative | 15 (80) |
| Progesterone Receptor | |
| Positive | 53 (70.7) |
| Negative | 22 (29.3) |
| HER2 | |
| Positive | 28 (37.3) |
| Negative | 47 (62.7) |
| Molecular Subtypes | |
| Luminal A | 40 (53.3) |
| Luminal B | 20 (26.7) |
| HER2 Enriched | 8 (10.7) |
| Triple Negative | 7 (9.3) |
BIRADS: Breast Imaging and Reporting Data System; HER2: Human Epidermal Growth Factor 2-neu; MRI: magnetic resonance imaging
The association of BPE with ER and PR positivity was found to be statistically significant (p=0.018 and p=0.013 respectively; Table 3). High BPE breasts tended to have increased rate of ER and PR positive tumors. Of the 30 high BPE cases, 28 (93.3%) were ER positive and 26 (86.7%) were PR positive. We performed subgroup analysis for premenopausal and postmenopausal women separately and the association was again found to be statistically significant for premenopausal women (p=0.011 and p=0.021).
Table 3.
Association of hormone receptor expression with FGT proportion and BPE
| Estrogen Receptor | Progesterone Receptor | HER2 Status | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| ER− (%) | ER+ (%) | p | PR− (%) | PR+ (%) | p | HER2− (%) | HER2+ (%) | p | ||
| All Patients | FGT proportion | 0.009 | 0.256 | 0.453 | ||||||
| Fatty | 12 (34.3) | 23 (65.7) | 13 (37.1) | 22 (62.9) | 24 (68.6) | 11 (31.4) | ||||
| High Fibroglandular | 3 (7.5) | 37 (92.5) | 9 (22.5) | 31 (77.5) | 23 (57.5) | 17 (42.5) | ||||
| BPE | 0.018 | 0.013 | 0.922 | |||||||
| Low BPE | 13 (28.9) | 32 (71.1) | 18 (40) | 27 (60) | 28 (62.2) | 17 (37.8) | ||||
| High BPE | 2 (6.7) | 28 (93.3) | 4 (13.3) | 26 (86.7) | 19 (63.3) | 11 (36.7) | ||||
| Premenopausal Patients | FGT proportion | 0.005 | 0.050 | 0.974 | ||||||
| Fatty | 7 (41.2) | 10 (58.8) | 8 (47.1) | 9 (52.9) | 12 (70.6) | 5 (29.4) | ||||
| High Fibroglandular | 2 (6.3) | 30 (93.8) | 6 (18.8) | 26 (81.3) | 21 (65.6) | 11 (34.4) | ||||
| BPE | 0.011 | 0.021 | 1.000 | |||||||
| Low BPE | 8 (33.3) | 16 (66.7) | 11 (45.8) | 13 (54.2) | 16 (66.7) | 8 (33.3) | ||||
| High BPE | 1 (4) | 24 (96) | 3 (12) | 22 (88) | 17 (68) | 8 (32) | ||||
| Postmenopausal Patients | FGT Proportion | 0.628 | 0.668 | 0.090 | ||||||
| Fatty | 5 (27.8) | 13 (72.2) | 5 (27.8) | 13 (72.2) | 12 (66.7) | 6 (33.3) | ||||
| High Fibroglandular | 1 (12.5) | 7 (87.5) | 3 (37.5) | 5 (62.5) | 2 (25) | 6 (75) | ||||
| BPE | 1.000 | 1.000 | 0.635 | |||||||
| Low BPE | 5 (23.8) | 16 (76.2) | 7 (33.3) | 14 (66.7) | 12 (57.1) | 9 (42.9) | ||||
| High BPE | 1 (20) | 4 (80) | 1 (20) | 4 (80) | 2 (40) | 3 (60) | ||||
ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor 2-neu; BPE: background parenchymal enhancement
In terms of FGT proportion, we found significant differences with ER positivity (p=0.009) whereas no significant difference with PR positivity was found (p=0.256). Thirty-seven of 40 women (92.5%) with high fibroglandular breasts had ER positive tumors. The analysis again was found to be statistically significant for premenopausal women (p=0.005). There was no correlation between HER2 status and any of the imaging features (p=0.453 and p=0.922).
Considering the associations of the molecular subtypes of breast cancer and FGT proportion and BPE, we found significant differences between molecular subtypes and FGT proportion for the whole study population (p=0.014), but no significant difference was found between molecular subtypes and BPE (p=0.087) (Table 4). When we re-performed a subgroup analysis for premenopausal women and postmenopausal women separately, in the premenopausal group both FGT proportion and BPE were found to be associated with molecular subtypes (p=0.025 and p=0.042). All of the six triple negative tumors were seen in low BPE breasts. Luminal A and luminal B subtypes tended to be seen in high fibroglandular breasts. In the postmenopausal group, no significant differences between molecular subtypes and any of the imaging features were found (p=0.860 and p=0.055).
Table 4.
Association of molecular subtypes of breast cancer with BPE and FGT proportion
| BPE | FGT Proportion | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Molecular subtype | Low (%) | High (%) | p | Fatty (%) | High Fibroglandular (%) | p |
| All Patients | 0.087 | 0.014 | ||||
| Luminal A | 21 (52.5) | 19 (47.5) | 0.875 | 18 (45.0) | 22 (55.0) | 0.636 |
| Luminal B | 11 (55.0) | 9 (45.0) | 0.824 | 5 (25.0) | 15 (75.0) | 0.041 |
| HER2 Enriched | 6 (75.0) | 2 (25.0) | 0.289 | 6 (75.0) | 2 (25.0) | 0.289 |
| Triple Negative | 7 (100) | 0 (0) | 0.016 | 6 (85.7) | 1 (14.3) | 0.125 |
| Premenopausal Patients | 0.042 | 0.025 | ||||
| Luminal A | 10 (37.0) | 17 (63.0) | 0.248 | 7 (25.9) | 20 (74.1) | 0.019 |
| Luminal B | 6 (46.2) | 7 (53.8) | 1.000 | 3 (23.1) | 10 (76.9) | 0.092 |
| HER2 Enriched | 2 (66.7) | 1 (33.3) | 1.000 | 2 (66.7) | 1 (33.3) | 1.000 |
| Triple Negative | 6 (100.0) | 0 (0) | 0.031 | 5 (83.3) | 1 (16.7) | 0.219 |
| Postmenopausal Patients | 0.860 | 0.055 | ||||
| Luminal A | 11 (84.6) | 2 (15.4) | 0.022 | 11 (84.6) | 2 (15.4) | 0.022 |
| Luminal B | 5 (71.4) | 2 (28.6) | 0.453 | 2 (28.6) | 5 (71.4) | 0.453 |
| HER2 Enriched | 4 (80.0) | 1 (20.0) | 0.375 | 4 (80.0) | 1 (20.0) | 0.375 |
| Triple Negative | 1 (100.0) | 0 (0.00) | 1.000 | 1 (100.0) | 0 (0) | 1.000 |
BPE: background parenchymal enhancement; HER2: human epidermal growth factor 2-neu
Fibroglandular tissue proportion was associated with BPE (p<0.001; Table 5). Among 35 cases with fatty breasts, 31 (88.6%) showed minimal or mild BPE. Twenty-six of 40 women with high fibroglandular breasts (65%) demonstrated moderate or marked BPE. When we re-performed the statistical analysis for premenopausal and postmenopausal group separately, we found significant correlation in each group again (p=0.002 and p=0.020, respectively).
Table 5.
Association of BPE and FGT proportion
| FGT Proportion | BPE | ||
|---|---|---|---|
|
| |||
| Low BPE (%) | High BPE (%) | p | |
| All Patients | <0.001 | ||
| Fatty | 31 (88.6) | 4 (11.4) | |
| High Fibroglandular | 14 (35.0) | 26 (65.0) | |
| Premenopausal Patients | 0.002 | ||
| Fatty | 14 (82.4) | 3 (17.6) | |
| High Fibroglandular | 10 (31.3) | 22 (68.8) | |
| Postmenopausal Patients | 0.020 | ||
| Fatty | 17 (94.4) | 1 (5.6) | |
| High Fibroglandular | 4 (50.0) | 4 (50.0) | |
Discussion
Breast tissue consists of two components: fat and FGT. Radiologically, the ratio of FGT to fat tissue is an issue of interest because it strongly increases the risk of having breast cancer. BPE, which is the enhancement of normal FGT on breast MRI, was also shown to be a risk factor for breast cancer (11). BPE may vary depending on which phase of the menstrual cycle the MRI is performed, because the FGT is very sensitive to hormonal influence. Progesterone with its mitogenic activity induces FGT proliferation (16). Estrogen may induce vasodilation and may increase vascular permeability by its histamine-like effect (16). The combination of these two hormone effects may result in increased FGT proportion and high BPE.
In accordance with previously published studies, breast density and BPE were reported to be associated with tumor characteristics. In a cohort study, high breast density was shown to increase the risk of ER and PR positive tumors (17). Another study reported that mammographic density was strongly related to ER positivity (18). According to a recently published study, breast density was associated with ER and PR positivity whereas BPE was independent of tumor characteristics for Asian patients (19). Our study demonstrated a significant association between BPE and ER and PR positivity. Women with high BPE tended to have ER and PR positive tumors. When we repeated our analysis for premenopausal women, we again found a significant correlation with a lower p value for ER. Our results also demonstrated that high fibroglandular breasts were more likely to have ER-positive tumors. This may suggest that estrogen has a stronger influence than progesterone on the pathogenesis of breast cancer.
Previously published studies have found that postmenopausal hormone therapy, nulliparity, and late-onset pregnancies were associated with increased mammographic density (20–23). These hormone-related factors were also found to be associated with ER+ /PR+ tumors (24, 25). In our study, high fibroglandular breast tissues were more common among luminal A and luminal B tumors. As luminal subtypes of the breast cancers are also ER and/or PR positive tumors, the pathway that increases FGT proportion may also include the mechanism of developing the luminal subtype of breast cancer. Similar to FGT proportion, it may be hypothesized that increased BPE may increase the risk of developing hormone receptor-positive tumors. Our findings demonstrating that ER and PR positivity associated with BPE confirmed this hypothesis, but when the question of molecular subtypes arises, our results demonstrated no significant association between BPE and molecular subtypes. Separating the groups for premenopausal and postmenopausal patients and re-performing the analysis revealed that there were significant differences between BPE and molecular subtypes in premenopausal women. This finding again may be due to the hormonally active state of premenopausal patients. But as there was no association in the whole study group, BPE may not have a significant influence on determining the molecular subtype. More studies are needed to confirm these results.
We found a significant association between FGT proportion and BPE. Significant correlation was present both in postmenopausal and premenopausal groups with a lower p value in premenopausal patients. This finding may be due to the hormonally active state of premenopausal women. It is recommended to perform breast MRI within the second week of the menstrual cycle to reduce the enhancement of normal breast parenchyma (26, 27). However, some experts’ opinion is to disregard the phase of the cycle if the imaging is performed for the staging of a known malignancy (28). Previous studies demonstrated no correlation between breast density and BPE when the breast MRI of the patient has been adjusted for the menstrual cycle (16, 29). However, there is one publication that reported significant correlation of the breast density and BPE when the MRI examination was performed without adjusting for the menstrual phase (30). Our study confirms their findings. As our study population also composed of patients with known malignancy or patients with high suspicion of malignancy, we also didn’t consider the patients’ phase of the menstrual cycle when the imaging was performed.
Our study has several limitations. It was a single center and single reader study. It was also a retrospective study, which may cause selection bias. Interpretation of BPE and FGT proportion were subjective entities, which were determined according to the visual assessment of enhancing glandular tissue and FGT proportion. Moreover, we have studied a population of a small size (75 cases) especially for the subgroups like triple negative, therefore the statistical significance of these findings might be inconclusive.
Conclusion
We conclude that, in women with invasive breast cancer, both high fibroglandular breasts and high BPE breasts tended to have ER positive tumors.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study.
Informed Consent: Owing to the retrospective nature of the study, the requirement for informed consent was waived.
Peer-review: Externally peer-reviewed
Author Contributions: Consept - M.O., A.V.P., Y.S., L.T., A.K.P.; Design - M.O., A.V.P., Y.S., L.T., A.K.P.; Supervision - M.O., A.V.P., Y.S., L.T., A.K.P.; Data Collection and/or Processing - M.O., A.V.P.; Analysis and/or Interpretation - M.O., A.V.P., L.T.; Literature Review - M.O., A.V.P.; Writer - M.O., A.V.P., Y.S., L.T., A.K.P.; Critical Review - M.O., A.V.P., Y.S., L.T., A.K.P.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. 2013. [accessed 11.07.16]. http://seer.cancer.gov/statfacts/html/breast.html.
- 2.Trop I, LeBlanc SM, David J, Lalonde L, Tran-Thanh D, Labelle M, ElKhoury MM. Molecular classification of infiltrating breast cancer: toward personalized therapy. Radiographics. 2014;34:1178–1195. doi: 10.1148/rg.345130049. https://doi.org/10.1148/rg.345130049. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ panel members. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. AnnOncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. https://doi.org/10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Tr. 2008;109:123–39. doi: 10.1007/s10549-007-9632-6. https://doi.org/10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phipps AI, Buist DS, Malone KE, Barlow WE, Porter PL, Kerlikowske K, Li CI. Reproductive history and risk of three breast cancer subtypes defined by three biomarkers. Cancer Cause Control. 2011;22:399–405. doi: 10.1007/s10552-010-9709-0. https://doi.org/10.1007/s10552-010-9709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aebi S, Sun Z, Braun D, Price KN, Castiglione-Gertsch M, Rabaglio M, Gelber RD, Crivellari D, Lindtner J, Snyder R, Karlsson P, Simoncini E, Gusterson BA, Viale G, Regan MM, Coates AS, Goldhirsch A. Differential efficacy of three cycles of CMF followed by tamoxifen inpatients with ER-positive and ER-negative tumors: long-term follow up on IBCSG Trial IX. Ann Oncol. 2011;22:1981–1987. doi: 10.1093/annonc/mdq754. https://doi.org/10.1093/annonc/mdq754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen PL, Taghian AG, Katz MS, Niemierko A, Raad RFA, Boon WL, Bellon JR, Wong JS, Smith BL, Harris JR. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. https://doi.org/10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 8.Wo JY, Taghian AG, Nguyen PL, Raad RA, Sreedhara M, Bellon JR, Wong JS, Gadd MA, Smith BL, Harris JR. The association between biological subtype and isolated regional nodal failure after breast-conserving therapy. Int J Radiat Oncol. 2010;77:188–196. doi: 10.1016/j.ijrobp.2009.04.059. https://doi.org/10.1016/j.ijrobp.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 9.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. https://doi.org/10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 10.Saftlas AF, Hoover RN, Brinton LA, Szklo M, Olson DR, Salane M, Wolfe JN. Mammographic densities and risk of breast cancer. Cancer. 1991;67:2833–2838. doi: 10.1002/1097-0142(19910601)67:11<2833::aid-cncr2820671121>3.0.co;2-u. https://doi.org/10.1002/1097-0142. [DOI] [PubMed] [Google Scholar]
- 11.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology. 2011;260:50–60. doi: 10.1148/radiol.11102156. https://doi.org/10.1148/radiol.11102156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris EA, Comstock CE, Lee CH. ACR BI-RADS Magnetic Resonance Imaging. In: Reston VA, editor. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. American College of Radiology; 2013. [Google Scholar]
- 13.Hammond ME, Hayes DF, Dowsett M, Wolff AC, Mangu BB, Temin S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–197. doi: 10.1200/JOP.777003. https://doi.org/10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2013;138:241–256. doi: 10.5858/arpa.2013-0953-SA. https://doi.org/10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazurowski MA, Zhang J, Grimm LJ, Yoon SC, Silber JI. Radiogenomic analysis of breast cancer: luminal B molecular subtype is associated with enhancement dynamics at MR imaging. Radiology. 2014;273:365–372. doi: 10.1148/radiol.14132641. https://doi.org/10.1148/radiol.14132641. [DOI] [PubMed] [Google Scholar]
- 16.Hegenscheid K, Schmidt CO, Seipel R, Laqua R, Ohlinger R, Kühn JP, Hosten N, Puls R. Normal breast parenchyma: contrast enhancement kinetics at dynamic MR mammography—influence of anthropometric measures and menopausal status. Radiology. 2013;266:72–80. doi: 10.1148/radiol.12112590. https://doi.org/10.1148/radiol.12112590. [DOI] [PubMed] [Google Scholar]
- 17.Conroy SM, Pagano I, Kolonel LN, Maskarinec G. Mammographic density and hormone receptor expression in breast cancer: the Multiethnic Cohort Study. Cancer Epidemiol. 2011;35:448–452. doi: 10.1016/j.canep.2010.11.011. https://doi.org/10.1016/j.canep.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding J, Warren R, Girling A, Thompson D, Easton D. Mammographic density, estrogen receptor status and other breast cancer tumor characteristics. Breast J. 2010;16:279–289. doi: 10.1111/j.1524-4741.2010.00907.x. https://doi.org/10.1111/j.1524-4741.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim MY, Choi N, Yang JH, Yoo YB, Park KS. Background parenchymal enhancement on breast MRI and mammographic breast density: correlation with tumour characteristics. Clin Radiol. 2015;70:706–710. doi: 10.1016/j.crad.2015.02.017. https://doi.org/10.1016/j.crad.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer I. 2003;95:30–37. doi: 10.1093/jnci/95.1.30. https://doi.org/10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 21.Titus-Ernstoff L, Tosteson AN, Kasales C, Weiss J, Goodrich M, Hatch EE, Carney PA. Breast cancer risk factors in relation to breast density(United States) Cancer Causes Control. 2006;17:1281–1290. doi: 10.1007/s10552-006-0071-1. https://doi.org/10.1007/s10552-006-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aiello EJ, Buist DS, White E. Do breast cancer risk factors modify the association between hormone therapy and mammographic breast density? (United States) Cancer Causes Control. 2006;17:1227–1235. doi: 10.1007/s10552-006-0073-z. https://doi.org/10.1007/s10552-006-0073-z. [DOI] [PubMed] [Google Scholar]
- 23.El-Bastawissi AY, White E, Mandelson MT, Taplin SH. Reproductive and hormonal factors associated with mammographic breast density by age (United States) Cancer Causes Control. 2000;11:955–963. doi: 10.1023/a:1026514032085. https://doi.org/10.1023/A:1026514032085. [DOI] [PubMed] [Google Scholar]
- 24.Potter JD, Cerhan JR, Sellers TA, McGovern PG, Drinkard C, Kushi LR, Folsom AR. Progesterone and estrogen receptors and mammary neoplasia in the Iowa Women’s Health Study: how many kinds of breast cancer are there? Cancer Epidem Biomar. 1995;4:319–326. [PubMed] [Google Scholar]
- 25.Cotterchio M, Kreiger N, Theis B, Sloan M, Bahl S. Hormonal factors and the risk of breast cancer according to estrogen-and progesterone-receptor subgroup. Cancer Epidem Biomar. 2003;12:1053–1060. [PubMed] [Google Scholar]
- 26.Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, Schild HH. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203:137–144. doi: 10.1148/radiology.203.1.9122382. https://doi.org/10.1148/radiology.203.1.9122382. [DOI] [PubMed] [Google Scholar]
- 27.Müller-Schimpfle M, Ohmenhäuser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology. 1997;203:145–149. doi: 10.1148/radiology.203.1.9122383. https://doi.org/10.1148/radiology.203.1.9122383. [DOI] [PubMed] [Google Scholar]
- 28.Morris EA. Diagnostic breast MR imaging: current status and future directions. Radiol Clin N Am. 2007;45:863–880. doi: 10.1016/j.rcl.2007.07.002. https://doi.org/10.1016/j.rcl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Cubuk R, Tasali N, Narin B, Keskiner F, Celik L, Guney S. Correlation between breast density in mammography and background enhancement in MR mammography. La radiologia medica. 2010;115:434–441. doi: 10.1007/s11547-010-0513-4. https://doi.org/10.1007/s11547-010-0513-4. [DOI] [PubMed] [Google Scholar]
- 30.Uematsu T, Kasami M, Watanabe J. Should breast MRI be performed with adjustment for the phase in patients’ menstrual cycle? Correlation between mammographic density, age, and background enhancement on breast MRI without adjusting for the phase in patients’ menstrual cycle. Eur J Radiol. 2012;81:1539–1542. doi: 10.1016/j.ejrad.2011.04.059. https://doi.org/10.1016/j.ejrad.2011.04.059. [DOI] [PubMed] [Google Scholar]


