Abstract
A new personal bioaerosol sampler has recently been developed and evaluated for sampling of viable airborne bacteria and fungi under controlled laboratory conditions and in the field. The operational principle of the device is based on the passage of air through porous medium immersed in liquid. This process leads to the formation of bubbles within the filter as the carrier gas passes through and thus provides effective mechanisms for aerosol removal. As demonstrated in previous studies, the culturability of sampled bacterium and fungi remained high for the entire 8-h sampling period. The present study is the first step of the evaluation of the new sampler for monitoring of viable airborne viruses. It focuses on the investigation of the inactivation rate of viruses in the bubbling process during 4 h of continuous operation. Four microbes were used in this study, influenza, measles, mumps, and vaccinia viruses. It was found that the use of distilled water as the collection fluid was associated with a relatively high decay rate. A significant improvement was achieved by utilizing virus maintenance fluid prepared by using Hank's solution with appropriate additives. The survival rates of the influenza, measles, and mumps viruses were increased by 1.4 log, 0.83 log, and 0.82 log, respectively, after the first hour of operation compared to bubbling through the sterile water. The same trend was observed throughout the entire 4-h experiment. There was no significant difference observed only for the robust vaccinia virus.
The development of reliable methods and techniques for continuous sampling of airborne bioagents including aerosolized fungi, bacteria, and viruses is in high demand. Most of the currently available bioaerosol sampling devices are based on dry filtration, impaction onto agar, or impingement into liquid (3, 12, 13, 16-18). Due to strong desiccation effects and corresponding low microbial recovery rates associated with dry filtration, this method is not recommended for elongated viable bioaerosol monitoring procedures and can be employed mainly for total microorganism enumeration by microscopic research methods (8).
The selectiveness of methods based on the direct collection of airborne microbes on agar makes these methods of limited use for comprehensive monitoring of ambient air; some species may not be culturable, as different microorganisms require different types of nutrients. Also, for unknown concentrations of bacteria in the air, the agar plate may become overloaded, which reduces the accuracy of the subsequent colony count or makes counting impossible (18, 19).
Direct collection of viable aerosol particles in liquid has always been a preferred method of monitoring, as it allows the application various analytical procedures to obtain the most comprehensive information both qualitatively and quantitatively (11, 15, 20, 21). After collection, liquid can be serially diluted and cultured on various agars to identify the nature and amount of cultivable microorganisms. It can also be used for endotoxin determinations as well as for immunologic, genetic, and viral analyses (5, 18, 22). Various impingers are most commonly used for such collections; however, achieving sufficient physical collection efficiency requires a very high sampling velocity (up to 300 m/s), which usually results in violent bubbling of the collection fluid. Due to high-speed impingement and violent bubbling, conventional impingers such as the AGI-30 (Ace Glass Inc., Vineland, N.J.) may lose a considerable amount of the collection fluid within very short sampling periods (up to 2 h) (16). Some problems associated with impingers have been addressed with the recently developed swirling aerosol collector (14, 15, 21), which is commercially available as the BioSampler (SKC Inc., Eighty Four, Pa.). The BioSampler utilizes a viscous, nonevaporative collection fluid for long-term sampling at an airflow rate of 12.5 liters/min.
The majority of the above-mentioned bioaerosol monitoring devices designed to collect both viable and nonviable biological particles are not adaptable for personal monitoring (4, 7, 12) and, moreover, for the monitoring of viruses. Some of the devices are very resistant (impingers and BioSampler) and have a pressure drop of up to 50,000 Pa for the operational airflow rate of 10 to 50 liters/min, thus requiring stationary pumping equipment (17). Other monitoring devices have considerable sizes and weights that also restrict their use as personal samplers. Due to the limitations of stationary bioaerosol samplers, it is currently common practice to assess personal exposure to bioaerosols by using small filter cassettes originally designed for monitoring nonbiological aerosols. However, the above-mentioned desiccation effect, caused by large volumes of air passing through the filter during the sampling of bioaerosols, may dramatically diminish the viability of microorganisms, especially of the sensitive ones.
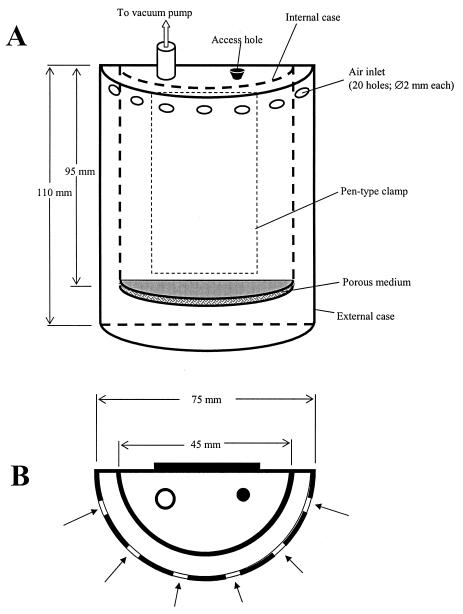
Based on an engineering control method which was previously applied to the removal of particles from gas carriers (1, 2), a new personal bioaerosol sampler was developed (4). A schematic diagram of the device is shown in Fig. 1. The prototype consists of two coaxial half-cylindrical cases, a 45-mm-diameter internal case with porous medium placed at the bottom and a 75-mm-diameter external case. Both are hermetically connected to the back and top sides of the device, while the external case is hermetically connected to the bottom of the sampler. The prototype sampler (height, 140 mm; diameter, 75 mm; wall thickness, 2 mm) was made out of plastic (polyvinyl chloride). A polypropylene fibrous medium with a fiber diameter of 12 μm, a packing density of 16%, and a thickness of 6 mm was placed at a distance of 15 mm from the bottom of the device and sealed to the bottom of the internal case to prevent the air bypass. A mist eliminator was installed below the air exit tube to baffle relatively large droplets that may have been generated by the bubbling process. A pen-type clamp, used for pinning the device to the user's lapel, was glued to the back wall of the sampler.
FIG. 1.
Schematic diagram of the personal sampler (A, side view; B, top view).
Before sampling, 50 ml of collection fluid was added to the sampler. The air enters the device through air inlet holes and moves down along the gap between the two cases. The peripheral arrangement of air entrance holes ensures representative sampling for the range of wind directions within 180°. The dimensions of the external and internal cases as well as the size and number of the air inlet holes were chosen to prevent particle deposition on the wall of the internal case after the particles enter the sampler through the air entrance holes but before they reach the collection fluid (4). Such prevention of internal losses is particularly important, as viable microorganisms that have settled on the dry wall could be damaged or killed by the desiccation. After reaching the bottom of the sampler and coming into contact with the collection fluid, the air turns by 180° and passes through the porous medium submerged into the fluid. Particles, including viable airborne microorganisms, are collected by the wet medium, and effluent air then leaves the device through a 12-mm-diameter pipe connected to a vacuum pump. A battery-operated portable sampling pump (224-PCXR8; SKC Inc.) widely used for personal aerosol sampling was utilized to run the sampler at 4 liters/min for up to 8 h.
The performance characteristics of the new sampler were evaluated for an 8-h continuous sampling of airborne Pseudomonas fluorescens and Bacillus subtilis var. niger (spores) bacteria and Aspergillus versicolor fungal spores (4). The viability of sampled microorganisms resulting from the laboratory-generated data was shown to remain high after the long-term sampling; the recovery rate of stress-sensitive gram-negative P. fluorescens bacteria was 61% ± 20%, and the rates were 95% ± 9% and 97% ± 6% for stress-resistant B. subtilis bacteria and A. versicolor fungal spores, respectively. The field evaluation, in which six identical personal samplers were tested simultaneously on a simplified human manikin in an office environment, demonstrated that the viable microbial concentration data obtained during 2-, 4- and 8-h sampling periods were not significantly affected by the sampling time. Intersample variation did not exceed 30%. The estimation of the detection limits has indicated that the sampler is capable of monitoring microbial exposure in environments with bacterial concentrations above 15 CFU/m3 and fungal concentrations above 5 CFU/m3. Thus, the new sampling method was found suitable for the personal monitoring of airborne bacteria and fungal spores.
The present study was designed to investigate the rate of inactivation of viruses in the bubbling processes. As bubbling through porous medium submerged in a liquid has been utilized for the collection of microorganisms, the decay of collected viruses in the bubbling process is becoming a crucial issue influencing a general possibility of using the personal sampler for such microorganisms. In this paper, time-related natural decay of four different viruses in the bubbling processes during 4 h of continuous operation was investigated, and the results are evaluated for further development of the unique possibility of utilizing the personal sampler for representative monitoring of viable airborne viruses.
MATERIALS AND METHODS
As was found previously (2), the physical efficiency of the device for removal of nonbiological particles from the air carrier is higher than 96% for particles smaller than 0.5 μm. Therefore, if a sampler is utilized for required elongated operating periods (up to 4 h), the main challenge is not the actual physical removal of airborne viruses from the ambient air but the minimization of the natural decay of collected microorganisms in the bubbling process during the remaining sampling time. To investigate this issue, this study focuses on the decay of viruses in the course of bubbling in order to determine whether the performance of the new sampling method remains sufficient during extended sampling periods (typical for personal bioaerosol exposure monitoring).
Microorganisms.
Four common viruses causing infections transmitted via aerosol, mumps, measles, influenza, and vaccinia, were employed for the tests. The reason for such selection was based on the fact that the above-mentioned microorganisms represent two groups that are very resistant (vaccinia) and very sensitive (mumps, measles, and influenza) to external physical and physiological stresses.
Mumps and measles.
Measles virus strain Edmonston was received from the American Type Culture Collection (ATCC VR-24). Mumps virus strain Enders was also received from the American Type Culture Collection (ATCC VR-106).
Influenza.
Influenza virus A/Aichi/2/68 (H3N2) was obtained from the Moscow Ivanovsky Institute of Virology. The virus was passaged 12 times in mice and twice in embryonated chicken eggs (ECE). Allantoic fluid produced by the cultivation of virus in 9- to 11-day-old ECE which had a viral concentration of 108 to 109 50% embryonic infecting doses (EID50) per ml was used in these experiments.
Vaccinia.
Vaccinia virus strain LIVP (C0355 K0602) was obtained from the Moscow Ivanovsky Institute of Virology in 1986. The virus was passaged 10 times in ECE. The virus-containing material with the concentration of 107 PFU/ml was obtained by culturing on 4647 cells (kidney cells of Cercopithecus aethiops embryo) with triple freezing-defrosting of infected cell culture in the maintenance medium modified Eagle medium (catalog number 11-100-22; ICN Biomedicals, Inc., Aurora, Ohio). Before its use in experiments, the virus-containing medium was kept at a temperature of −70°C.
Experimental procedures.
The experiments were undertaken in the PC3 protected facility with HEPA filters installed in the pipeline connecting the sampler and vacuum pump to prevent the contamination of equipment. Two types of collection liquid, sterile water and specially prepared virus maintenance absorbing fluid, were used in this study. The absorbing fluid consisted of Hank's solution (6) containing a 2% volume of inactivated bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. To avoid extensive foam formation which could unfavorably interfere with the physical bubbling process, the antifoaming agent Antifoam A (Sigma Chemical Company, St Louis, Mo.) was added to the collection medium. Fifty milliliters of freshly prepared concentrated suspension of each virus was placed into the sampler. Three devices operating in parallel were employed for each experimental run. All samplers charged with viral suspension operated continuously aspirating HEPA-filtered air at 4 liters/min (standard sampling flow rate) during 4 h. The experiments were undertaken at room conditions with a temperature of 24°C and relative humidity of around 48%. Such temperature and relative humidity provided “low-evaporation” conditions and allowed samplers to run for up to 4 h with minimal (less than 5%) evaporative losses of viral suspension. One milliliter of suspension was collected from each sampler after 0, 1, 2, and 4 h of operation. The standard plaque assay technique was used for determination of virus concentrations of mumps, measles, and vaccinia viruses in the collected suspension samples. First, the viral suspension was diluted in maintenance medium containing antibiotics. Eight 10-fold serial dilutions were then made, and 100 μl was added to confluent Vero cell monolayers in 24-well cluster plates (Costar, Pleasanton, Calif.). The virus was allowed to adsorb for 1 h at 37°C in a humidified incubator in a 5% CO2 atmosphere. The cluster plates were rocked every 10 to 15 min, fluid was aspirated after 1 h, and 2 ml of overlay 1% agar (Difco) on RPMI 1640 (10) medium (containing 2% fetal calf serum and antibiotics) was added. Cells were incubated for 6 days at 37°C in a humidified incubator in a 5% CO2 atmosphere, at which time cell monolayers were stained with neutral red and plaques were enumerated (9). The results were calculated as the PFU/milliliter of suspension.
The titration on 10-day-old chicken embryo procedure (6) was used to identify the concentration of the influenza virus in the collecting liquid. Tenfold dilutions of the viral suspension collected after 0, 1, 2, and 4 h of the sampler operation were inoculated into the embryonated eggs' amniotic sacs following incubation of the eggs for 72 h. The results of titration were expressed as the EID50/milliliter of suspension.
RESULTS
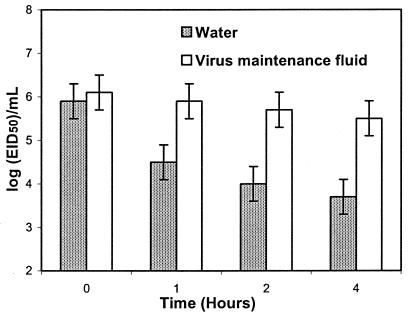
The viral concentrations in the collecting liquids at 0, 1, 2, and 4 h of continuous sampler operation are shown in Fig. 2 to 5. Each graph contains the results for collection of liquids, with error bars representing standard deviations of data from at least three experimental runs. Note that the scale of the y axis in Fig. 5 is different from the remaining graphs, representing a much smaller variation in the magnitude of vaccinia virus concentration. Also, taking into account the different analytical procedures employed, the results for the influenza virus are shown as the log EID50/milliliter, while data for all other viruses are shown as log PFU/milliliter.
FIG. 2.
Time-related concentration of influenza virus in the collection fluid. Error bars represent standard deviations of at least three experimental runs.
FIG. 5.
Time-related concentration of vaccinia virus in the collection fluid. Error bars represent standard deviations of at least three experimental runs.
As shown in Fig. 2, the concentration of influenza virus after the first operating hour was decreased by 1.4 log in sterile water and only 0.2 log in the maintenance liquid. After 2 h of operation, the corresponding results were 1.9 log and 0.4 log, respectively, and finally, the concentrations decreased by about 2.2 log in water compared to just above 0.6 log in maintenance fluid after a 4-h run. These numbers look very encouraging, as the number of surviving microorganisms is sufficient for their reliable quantitative determination by the microbiological analytical procedures described above, even after 4 h of continuous operation. Similar results can also be observed in Fig. 3 and 4, representing the decay of the mumps and measles viruses, respectively. Both are more viable in the absorbing fluid than in sterile water. In contrast, as shown in Fig. 5, representing the results of experimental runs employing the vaccinia virus, the difference between the two collection liquids is minimal and did not exceed 0.3 log in the worst-case scenario. The standard deviation of all runs is relatively low (does not exceed 0.5 log), which demonstrates a good repeatability of the results. Also, the viral inactivation results obtained for both liquids were statistically compared by the analysis of variance single-factor test, and the results are presented in Table 1.
FIG. 3.
Time-related concentration of measles virus in the collection fluid. Error bars represent standard deviations of at least three experimental runs.
FIG. 4.
Time-related concentration of mumps virus in the collection fluid. Error bars represent standard deviations of at least three experimental runs.
TABLE 1.
Statistical significance of the differences in viral survival rates in sterile water and maintenance medium as analyzed by a standard single-factor analysis of variance test (α = 0.05)a
| Virus | Statistical significance at time of device operation (h) (P value)
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Influenza | No (0.807) | Yes (0.029) | Yes (0.014) | Yes (0.011) |
| Measles | No (0.829) | Yes (0.041) | Yes (0.012) | Yes (0.031) |
| Mumps | No (0.91) | Yes (0.032) | Yes (0.01) | Yes (0.0066) |
| Vaccinia | No (0.81) | No (0.76) | No (0.64) | No (0.17) |
The difference is significant if P is <0.05. No indicates no statistical significance; Yes indicates statistical significance.
DISCUSSION
The results obtained in the above-described experiments look very promising for the further development of equipment and protocols for personal sampling of viable airborne viruses. As has been demonstrated, the natural decay of microorganisms can be minimized to allow 4 h of continuous operation of the device. This issue is crucial for personal bioaerosol monitors which are required to be capable of providing elongated sampling time intervals to detect possible bioaerosol concentration bursts occurring in various areas of monitoring, including bioterrorism, military operations, hospitals, agricultural activities, and others.
Four microorganisms representing both stress-sensitive (influenza, mumps, and measles) and robust (vaccinia) viruses were chosen for this research. All of the microorganisms were found to be sufficiently survivable in the bubbling processes even during 4 h of continuous device operation. It was found that the decay rate of all stress-sensitive viruses employed in this research was relatively high during bubbling through sterile water and exceeded 2.5 log after 4 h of device operation. The robust vaccinia virus did not show noticeable decay for the entire operational procedure. However, the inactivation rate could be significantly decreased by using alternative fluids. The virus maintenance fluid allowed the improvement of this parameter by more than 1.2 log for all stress-sensitive microorganisms. Since the virus maintenance fluid did not contribute to alterations in the physical bubbling process (no extra foam was generated, the physical efficiency of particle collection was the same as that of sterile water, and no increase in hydrodynamic resistance of the device was noticed), it is recommended for use as the most suitable fluid for the virus collection procedure. Also, the results of statistical analysis show that for all three stress-sensitive microorganisms, the difference in survival rates between bubbling in maintenance liquid and bubbling in sterile water is statistically significant. The robust vaccinia virus demonstrated no such difference throughout the entire device operation.
Finally, it should be noted that the research procedure used in this project considers viruses that are exposed to the bubbling regime throughout the entire operation of the sampler. In reality, the microorganisms could be collected by the device throughout the whole sampling time, which would minimize exposure for the microorganisms that arrive later and correspondingly provide a higher recovery rate than the theoretical one.
Acknowledgments
The project was supported by grant 1R21 RR 17026-01A1 from the National Center for Research Resources.
REFERENCES
- 1.Agranovski, I., T. Myojo, and R. Braddock. 1999. Removal of aerosols by bubbling through porous media. Aerosol Sci. Technol. 31:249-257. [Google Scholar]
- 2.Agranovski, I., T. Myojo, and R. Braddock. 2001. Comparative study of the performance of nine filters utilized in filtration of aerosols by bubbling. Aerosol Sci. Technol. 35:852-859. [Google Scholar]
- 3.Agranovski, I., V. Agranovski, T. Reponen, K. Willeke, and S. Grinshpun. 2002. Collection of airborne microorganisms into liquid by bubbling through porous medium. Aerosol Sci. Technol. 36:502-509. [Google Scholar]
- 4.Agranovski, I., V. Agranovski, S. Grinshpun, T. Reponen, and K. Willeke. 2002. Development and evaluation of a new personal sampler for viable airborne microorganisms. Atmos. Environ. 36:889-898. [Google Scholar]
- 5.Alvarez, J. A., M. P. Buttner, and L. D. Stetzenbach. 1995. PCR for bioaerosol monitoring: sensitivity and environmental interference. Appl. Environ. Microbiol. 61:3639-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett, T., and S. C. Inglis. 1985. Growth, purification and titration of influenza viruses, p 119-150. In B. W. J. Mahy (ed.), Virology: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 7.Burge, H. A., and W. R. Solomon. 1987. Sampling and analysis of biological aerosols. Atmos. Environ. 21:451-456. [Google Scholar]
- 8.Cox, C. S., and C. M. Wathes (ed.). 1995. Bioaerosols handbook. CRC Lewis Publishers, Boca Raton, Fla.
- 9.Fujinami, R. S., and M. B. A. Oldstone. 1981. Failure to cleave measles virus fusion protein in lymphoid cells: a possible mechanism for viral persistence in lymphocytes. J. Exp. Med. 154:1489-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould, E. A., and J. C. S Clegg. 1985. Growth, titration and purification of alfa-viruses and flaviviruses, p 43-78. In B. W. J. Mahy (ed.), Virology: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 11.Henningson, E. W., I. Fangmark, E. Larsson, and L. E. Wikstrom. 1988. Collection efficiency of liquid samplers for microbiological aerosols. J. Aerosol Sci. 19:911-914. [Google Scholar]
- 12.Jensen, P. A., W. F. Todd, G. N. Davis, and P. V. Scarpino. 1992. Evaluation of eight bioaerosol samplers challenged with aerosols of free bacteria. Am. Ind. Hyg. Assoc. J. 53:660-667. [DOI] [PubMed] [Google Scholar]
- 13.Juozaitis, A., K. Willeke, S. A. Grinshpun, and J. Donnelly. 1994. Impaction onto a glass slide or agar versus impingement into a liquid for the collection and recovery of airborne microorganisms. Appl. Environ. Microbiol. 60:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin, X., T. A. Reponen, K. Willeke, Z. Wang, S. A. Grinshpun, and M. Trunov. 2000. Survival of airborne microorganisms during swirling aerosol collection. Aerosol Sci. Technol. 32:184-196. [Google Scholar]
- 15.Lin, X., T. A. Reponen, K. Willeke, S. A. Grinshpun, K. K. Foarde, and D. S Ensor. 1999. Long-term sampling of airborne bacteria and fungi into a nonevaporating liquid. Atmos. Environ. 33:4291-4298. [Google Scholar]
- 16.Lin, X., K. Willeke, V. Ulevicius, and S. A. Grinshpun. 1997. Effect of sampling time on the collection efficiency of all-glass impingers. Am. Ind. Hyg. Assoc. J. 58:480-488. [Google Scholar]
- 17.Macher, J. M., M. A. Chatigny, and H. A. Burge. 1995. Sampling airborne microorganisms and aeroallergens, p. 279-321. In B. S. Cohen and S.V. Hering (ed.), Air sampling instruments for evaluation of atmospheric contaminants. ACGIH, Cincinnati, Ohio.
- 18.Nevalainen, A., K. Willeke, F. Liebhaber, J. Pastuszka, H. Burge, and E. Henningson. 1993. Bioaerosol sampling, p. 471-492. In K. Willeke and P. A. Baron (ed.), Aerosol measurement: principles, techniques and applications. Van Nostrand Reinhold, New York, N.Y.
- 19.Stewart, S. L., S. A. Grinshpun, K. Willeke, S. Terzieva, V. Ulevicius, and J. Donnelly. 1995. Effect of impact stress on microbial recovery on an agar surface. Appl. Environ. Microbiol. 61:1232-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter, M. V., B. Marthi, V. P. Fieland, and L. M. Ganio. 1990. Effect of aerosolization on subsequent bacterial survival. Appl. Environ. Microbiol. 56:3468-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willeke, K., X. Lin, and S. A. Grinshpun. 1998. Improved aerosol collection by combined impaction and centrifugal motion. Aerosol Sci. Technol. 28:439-456. [Google Scholar]
- 22.Williams, R. H., E. Ward, and H. A. McCartney. 2001. Methods for integrated air sampling and DNA analysis for detection of airborne fungal spores. Appl. Environ. Microbiol. 67:2453-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]