Abstract
Objective
Breast cancer is the most common type of cancer and the leading cause of cancer related deaths in women in Turkey, as elsewhere around the world. However, detailed and systematic demographics, data on clinical and pathological characteristics, and treatment were largely unavailable in Turkey until now. This paper is intended to provide an analysis of clinical and pathological data on women registered in the National Breast Cancer Database (Ulusal Meme Kanseri Veri Tabanı [UMKVT]), established within Turkish Federation of Breast Diseases Societies (TMHDF) and available for use in Turkey since 2005.
Materials and Methods
Clinical and pathological data on breast cancer patients registered online in the database from May 01, 2005 to May 01, 2011 were investigated. Parameters examined in patients included age, menopausal status, distribution of clinical and pathological stage, histological type, tumor diameter, histological grades, regional lymphatic stage, estrogen (ER), progesterone (PR), HER-2 receptors and molecular subtypes. Analysis results of these parameters were compared with literature data and discussed.
Results
A total of 13,240 patients with breast cancer since April 07, 1992 were included in the study, and 99% of them were female. Female breast cancer patients whose requisite parameters had been completely entered in the database were included in the analysis. The mean age was 51.6 years (±12.6; range 12–97), 17% of them were younger than 40 years of age, and 45% were premenopausal. According to an analysis of age groups at diagnosis, the frequency of cancer peaked at the 45 – 49 age group with 16.7%, declining to 7.6% in the 65–69 age group, and then rose again. Most of the patients (78.7%) had invasive ductal, 7.8% were invasive lobular cancers, 9.8% were invasive mixed cancers (invasive ductal + invasive lobular), and 4% were other histological types (e.g. inflammatory, intracystic papillary, mucinous, etc.), respectively. Half of them (50%) had grade III histology. According to an analysis of pathological stages of all breast cancers (stage 0 – IV), 5% were stage 0, 27% were stage I, 44% were stage II, 21% were stage III, and 3% were stage IV breast cancer, respectively. The mean tumor diameter was 2.5 cm (±1.6; range 0.1–20 cm). The rates of lymphatic stages were pN0 50%, pN1 28%, pN2 15%, and pN3 7%, respectively. ER, PR, and HER-2 receptors were positive in 70%, 59%, and 23% of patients. A subtype analysis of tumors showed that 62% were type luminal A. This was followed by subtypes luminal B (15%), triple negative (15%), and HER-2 positive (8.5%).
Conclusion
As a conclusion patients with breast cancer in our breast cancer registry program were younger, and had more advanced disease, and worse prognostic factors than patients in developed countries.
Keywords: Turkey, breast cancer, stages, pathology, molecular subtypes, hormone receptors, prognostic factors
Introduction
One of the most important points in cancer is keeping accurate and complete records. The development of national health policies, the preparation of strategic plans, and the use of limited resources cannot be prioritized or be decided upon, unless reliable data is obtained and statistically evaluated.
The oldest and modern cancer registry program has been established in Hamburg in 1929 (1). In this program, it is indicated that not only medical and scientific issues, but also public health and economic aspects should be taken into consideration for cancer control. A population-based cancer registry program was initiated in the US, in 1935 (2). The SEER Program (The Surveillance, Epidemiology, and End Results) is affiliated to the NCI (National Cancer Institute), collecting and publishing cancer related data on approximately 28% of the US population. The Danish Cancer Registry is a program established in 1942 by the Danish Cancer Society, covering not only a city but also the entire country’s population with excellent function (2).
The World Health Organization (WHO) founded a dedicated cancer research center the IARC (International Agency for Research on Cancer) in 1965, and the International Association of Cancer Registries (IACR) in 1966 (3). This organization, in collaboration with IARC, is intended to help creating cancer registries and evaluate cancer incidence and treatment outcomes. Currently, there are approximately 200 population-based registry programs throughout the world and they are all monitored.
In our country, studies on cancer registry have started quite late (4, 5). Cancer has been accepted as a notifiable disease in 1982, and in 1983, the KSDB (Kanserle Savaş Dairesi Başkanlığı-Cancer Control Department) was established to keep and oversee records. One of the main tasks of the KSDB, which is responsible for cancer control, is to collect reliable and accurate data in a cancer registry that is of high quality.
On March 13, 1993, Cancer Monitoring and Control Center [Kanser İzlem ve Denetim Merkezi (KİDEM)] was established within the Izmir Provincial Health Directorate, and was assigned to coordinate study projects. KİDEM was accepted as a member of WHO, IARC and the IACR in 1995, and the European Network of Cancer Registries (ENCR) in 1997 (4). KSDB has included 12 cities after Izmir (Edirne, Trabzon, Samsun, Erzurum, Eskişehir, Ankara, Antalya, Izmir, Kayseri, Ankara, Adana and Bursa) in the active cancer registry program. Currently, Kocaeli (Dilovası area ranks first in cancer-related deaths) and Van (as a representative of the eastern region) have been added and the number reached to 14 cities. The cancer registry program continues to be implemented in these regions.
One of the ongoing major projects led by the Turkish Federation of Breast Diseases Societites [Türkiye Meme Hastalıkları Dernekleri Federasyonu (TMHDF)] is National Breast Cancer Database [Ulusal Meme Kanseri Veri Tabanı (UMKVT)]. The Federation Board decided to embark on this project in December 2004, and a professional software company was assigned for writing and implementation of the program. Data recording into the software program started in May 1, 2005 and as of August 2013, data on more than 20,000 patients were registered.
This study aimed to evaluate the clinical and histopathologic features of our patients registered into the program, identify the standard prognostic factors and compare them with data from other developed or developing countries.
Materials and Methods
The database is designed as computer software containing 576 parameters and has been implemented on May 1, 2005. It is composed of sections on identity, medical history, clinical data, histological diagnosis, surgical treatment, pathology, adjuvant treatment and follow-up. In this article, the data of 13,240 patients who were registered from May 1 2005 to May 1 2011 was analyzed.
Data Entrance and Clearance
The centers that were linked to TMHDF were asked to enter information to the central database in either a prospective (online) or retrospective (offline) manner, the entered data was reviewed, and duplications, incompatible and non-eligible data were excluded from the analysis.
In this study, patients’ gender, age, clinical and pathologic stage, tumor size, histological type and grade, pathologic stage, estrogen receptor (ER), progesterone receptor (PR), and HER-2 receptors and breast cancer subtype distributions were analyzed. ER / PR value of ≥1% was considered positive, and for HER-2, a 3 + result or in suspected cases a positive SISH or CISH were accepted as positive.
Invasive cancer histological types were classified according to the World Health Organization’s proposed classification, the staging according to the American Joint Committee on Cancer (AJCC) TNM 2002 version, and the histological grade according to the modified Scarff Bloom-Richardson classification (6, 7). Another classification was performed separately as Hormone receptor (HoR) positive (at least one of ER or PR positive) or HoR negative (both ER and PR negative) patients.
Molecular subtypes were divided into 4 groups as luminal A (ER or PR positive + HER-2 negative), luminal B (ER or PR positive, HER 2 +), triple negative (ER, PR and HER-2 negative) and HER-2-positive (HER-2 group, ER and PR negative, HER-2 +) and were analyzed accordingly (8).
Statistical Analysis
The mean, median, mode, minimum, maximum and standard deviation were calculated for continuous variables. Kolmogorov-Smirnov test was performed to evaluate the distribution of variables. Mann-Whitney U test was used to compare the average of two independent groups, and Kruskal-Wallis test was applied to compare the average of more than two independent groups. When required, continuous variables were re-assessed in groups according to a standard cut-off point. The relationship between categorical variables was evaluated by chi-square test. The level of significance was accepted as 0.05 in Pearson’s chi-square test.
Results
A total of 13,420 breast cancer cases were recorded between 1 May 2005 and 1 May 2011 from 24 different health units (Table 1). After data cleaning, 11,542 cases with valid data were detected. Only patients with sufficient data for each parameter were included.
Table 1.
Centers providing data input and number of registered breast cancer patients
| Center providing the data | Number of patients | % | Date of first data entry |
|---|---|---|---|
| 1. Ege University | 4076 | 30.8 | 07.05.2005 |
| 2. IU Istanbul MF | 3775 | 28.5 | 29.03.2007 |
| 3. Uludağ University | 1423 | 10.7 | 28.06.2005 |
| 4. MAMER Surgery Center | 1308 | 9.8 | 18.06.2009 |
| 5. Ankara Dışkapı TH | 611 | 4.6 | 12.04.2007 |
| 6. Vahit Özmen, M.D. | 530 | 4.0 | 09.07.2009 |
| 7. Kocaeli University | 300 | 2.3 | 22.03.2006 |
| 8. Savaş Koçak, M.D. | 267 | 2.0 | 10.04.2006 |
| 9. IU Cerrahpaşa MF | 236 | 1.8 | 25.06.2009 |
| 10. Marmara University MF | 167 | 1.3 | 28.10.2005 |
| 11. A.Menderes University MF | 164 | 1.2 | 13.05.2009 |
| 12. Ankara University MF | 163 | 1.2 | 21.02.2007 |
| 13. Dicle University MF | 66 | 0.5 | 18.06.2009 |
| 14. Cumhuriyet University MF | 60 | 0.4 | 31.10.2005 |
| 15. Maltepe University MF | 39 | 0.3 | 29.04.2009 |
| 16. Southeastern Anatolia BS | 24 | 0.2 | 10.03.2008 |
| 17. Çukurova University MF | 13 | 0.1 | 16.04.2009 |
| 18. Others | 18 | 0.12 | 14.12.2007 |
| TOTAL | 13.420 | 100 |
IU: Istanbul University; MF: Medical Faculty; MAMER: Breast Diseases Research Center; TH: Teaching Hospital; BS: Breast Society
11,385 of the patients (99%) were female, with a mean age of 51.6 years (±12.6; 12–97). 48% of them were younger than 50 years of age, and 17% were under the age of 40. It is found that, in our country, breast cancer incidence significantly increased up to the age of 50 and this increase reached its peak in the age group of 45–49 years (17%), and then gradually decreased down to 7.6% in the age of 65 to 69 years, with another increase after 70 years of age to 10% (Chart 1). 45% of the patients were pre-menopausal.
The histologic types of invasive breast cancer were as follows: 79% of invasive ductal cancers (IDC), 7.4% of invasive lobular cancers (ILC), 9.8% of mixed types of cancer (IMC, ILC + IDC), and the remaining 3.8% of other types (Table 2). While 52% of the patients with IDC were pN0, 41% of patients diagnosed as ILC and IMC were found to be pN0 (p=0.0001). The rate of patients with early-stage (stage I, II) disease was 76.5 % in IDC patients, while this proportion was 68.5% in cases with ILC and IMC (p=0.0001).
Table 2.
Demographic Characteristics of patients
| Patient and Tumor Characteristics | Number (%) |
|---|---|
| Number of patients | 11.542 (100%) |
| Male | 157 (1%) |
| Female | 11.385 (99%) |
| Median age | 51.6 (12–97 age) |
| <40 age number of patients | 1.950 (17%) |
| ≥40 age number of patients | 9.435 (83%) |
| Number of patients, menopausal status known | 5.471 (100%) |
| Number of premenopausal patients | 2.440 (45%) |
| Number of menopausal patients | 3.031 (55%) |
| Number of patients, histopathology known | 4.510 (100%) |
| Ductal carcinoma in situ (DCIS%) | 223 (4.9%) |
| Invasive cancer | 4.287 (95.1%) |
| Histopathologic type | |
| Invasive ductal cancer | 3.387 (79%) |
| Invasive lobular cancer | 317 (7.4%) |
| Mixed type | 425 (9.8%) |
| Others | 18 (3.8%) |
| Number of patients, histologic grade known | 6.336 (100%) |
| I | 317 (5%) |
| II | 2.851 (45%) |
| III | 3.168 (50%) |
| Resceptor Positivity | |
| ER | 2383/3442 (69%) |
| PR | 1867/3199 (58%) |
| HoR | 2522/3328 (75.8%) |
| HER-2 | 391/1703 (23%) |
| Pathologic Stage at Diagnosis | |
| Stage 0 | 184/3780 (4.9%) |
| Stage I | 1007/3780 (26.6%) |
| Stage II | 1696/3780 (44.9%) |
| Stage III | 787/3780 (20.8%) |
| Stage IV | 106/3780 (2.8%) |
ER: Estrogen; PR: progesterone; DCIS: ductal carcinoma in situ; HoR: hormone receptor
The clinical stages were as: Stage 0 (Ductal Carcinoma in Situ (DCIS)) 3%, stage I 26%, stage II 54%, stage III 14% and stage IV %3. Stage III breast cancer rate in women under 40 years of age was 19%, while this rate was 12.7% in those between 60–69 years of age (p<0.001). The incidence of Stage III disease decreased with increasing age, with an increase after 70 years. Early stage breast cancer rate was lower in pre-menopausal patients than in menopausal patients, but this difference was not significant (p>0.05, Table 2).
The patients were divided into two groups according to age, as ≥40 and <40 years of age and pathologic tumor size distribution were examined in these groups. The rate of T1 tumors was 43% in women under the age of 40, while this rate was 50% in women aged ≥40 years (p=0.0001). Accordingly, T2 and T3 tumors were significantly higher in women <40 years of age (p=0.0001). pT1 was detected in 47% of premenopausal women, and in 49% of postmenopausal women (p=0.059, Table 3).
Table 3.
Characteristics according to age groups (<40 years x≥40 years)
| Patient characteristic | Total Number | <40 years (%) | ≥40 years (%) | p value |
|---|---|---|---|---|
| Number of patients | 11.385 | 17% | 83% | |
| Pathologic T1 (<2 cm) | 3.167 | 43% | 50% | p=0.0001 |
| Pathologic N0 | 2.599 | 44% | 51% | p=0.001 |
| Pathologic Stage 0, 1, 2 | 2.841 | 71.5% | 77.5% | p=0.005 |
| Histologic Grade 3 | 3.212 | 60% | 48% | p=0.0001 |
| Receptor positivity | 3.442 | 61% | 71% | p=0.0001 |
| ER | ||||
| PR | 3.199 | 57% | 59% | p>0.05 |
| HoR | 3.328 | 77% | 71% | p=0.005 |
| HER-2 | 1.703 | 26.5% | 22.2% | p<0.05 |
| Molecular subtype | 1.692 | 17% | 83% | |
| Luminal A | 1.054 | 56% | 64% | p<0.05 |
| Luminal B | 247 | 19% | 14% | p<0.05 |
| HER-2 Positive | 144 | 8% | 8% | NS |
| Triple Negative | 247 | 17% | 14% | NS |
ER: Estrogen; PR: progesterone; DCIS: ductal carcinoma in situ; HoR: hormone receptor
Pathological lymphatic stages were found as 50% pN0, 28% pN1, 15% pN2, and 7% pN3. pN0 patients were mostly in the ≥70 years group. It was observed that as age at diagnosis increased regional lymphatic involvement decreased therefore resulting in decreased lymphatic stage (p=0.0001). While 44% of women diagnosed with invasive cancer under the age of 40 were pN0, 51% of women aged ≥40 years were staged as pN0 (p=0.001). 47% of premenopausal women, and 53% of menopausal women were pN0 (p=0.018).
pN0 rate in pathologic T1 patients was 61%, whereas this rate was significantly lower in pT2–3 tumors (42% vs. 18%, respectively) (p=0.0001). The rates of pathologic stage were: 4.9% Stage 0, 27% stage I, 45% stage II, 21% stage III, 3% stage IV. With increasing age at diagnosis, pathologic stage is decreased, and this difference was statistically significant (p=0.011).
The patients aged ≥40 years and <40 years of age were divided into two groups and were classified according to pathologic stage in these groups (Table 3). Early stage (stage 0, I, II) breast cancer in women under the age of 40 was 71.5%, while the rate of advanced stage (ever III, IV) was 28.5%. In cases over 40 years of age early and advanced stage disease rates were 77.5% and 22.5%, respectively (p=0.005).
The histological grades (HG) were found as HG I 5%, HG II 45%, and HG III 50%. HG III tumors were detected in half of the cases, whereas HG decreased with advancing age (p=0.0001). Sixty percent of tumors detected in patients younger than 40 years, while this rate was 48% in patients ≥ 40 years (p=0.0001). In patients with pT1 44.5% were HG III, as tumor size increased the rate of HG III increased, up to 57% in T2, and 61% in pT3 (p=0.0001).
51% of pN0 cases, and 71% of pN3 patients were HG III (p=0.0001). Lymphatic involvement was seen in only 30% of HG I patients. As HG increased, regional lymphatic involvement rate increased significantly (p=0.0001).
In 69% of patients, ER was positive. This rate decreased to 61% in patients <40 years of age, and increased to 71% in patients ≥40 years (p=0.0001). ER was positive in 66% of pre-menopausal, and 73% of menopausal women (p=0.0001).
Progesterone receptor positivity rate was 58%, when patients were divided into two groups of ≥40 years and <40 years of age; 57% of patients <40 years and 59% of patients ≥40 years were positive (p>0.05, Table 3).
Hormone receptor (HoR) positivity (at least one positive hormone receptor) was found as 76%. HoR positivity was significantly higher in patients over 40 years of age as compared to those <40 years (77% vs 71%, respectively, p=0.005). HoR was positive in 79% of pT1 cases. However, HoR positivity rate was reduced as tumor diameter increased (p=0.0001). HoR positivity was 77% in patients with pN0, and 69 % in pN3 (p<0.029). HoR was positive in 94% of HGI patients. As HG increased HoR positivity rate was reduced (p=0.0001).
In 23% of patients, HER-2 expression was positive according to results of immunohistochemical analysis (FISH or SISH test). This rate was higher in young (<40 years) patients and pre-menopausal women, although the difference was not significant (p>0.05). HER-2 positivity was detected in 24.5% of patients with IDC histopathology, and 14% in ILC and IMC cases (p=0.0001). ER positivity rate was 70% in all patients, 68% in patients diagnosed with IDC, and 78% in those diagnosed with ILC-IMC (p=0.0001).
HER-2 positivity did not show any significant difference according to tumor size, however it significantly increased as lymphatic involvement and HG increased. HER-2 positivity rate was 20% in pN0 and 26.5% in pN+ patients (p=0.002); and 10% in HG1 and 28% in HGIII cases (p=0.0001).
Molecular subtype distribution among cases was as follows: Luminal A 62%, Luminal B 15%, HER-2 Group 8.5% and Triple Negative 15%. As patient age increased the likelihood of the tumor being Luminal A molecular subtype also increased (p=0.006).
On analysis of the distribution of molecular subtypes according to age, it was found that 64% of Luminal A subtype was detected in patients ≥40 age. However, Luminal B and triple negative group (TNG) tumors were found at higher rates in patients below 40 years of age (p=0.044). Out of all the pT1 cases, 66% were Luminal A, 15% were Luminal B, 6% were HER-2 positive and 12% were in the TNG group. As tumor size increased the rate of patients with Luminal A and B molecular subtypes decreased while rate of HER-2 positivity and TNG patients increased (p=0.0001).
Out of all the pN0 patients, 64% was in Luminal A, 13.5% in Luminal B, 6% in HER-2 positive and 17% in TNG group. When the molecular subtype variables were independently evaluated, it was determined that with increasing lymphatic involvement stage the incidence of Luminal A type tumors decreased, while HER-2 positive tumor rates increased. These findings were also statistically significant (p=0.001). However, no significant relationship between lymphatic involvement and Luminal B and TNG subtypes was revealed.
Among HGI cases, 87% were Luminal A, 10% were Luminal B, 3% were in the TNG molecular subtype. With increase in HG, Luminal A subtype incidence decreased while the rates of Luminal B, HER-2 positive and TNG tumors increased (p=0.0001).
Discussion and Conclusions
Breast cancer incidence displays a rapid increase in Turkey. Breast cancer incidence had been previously calculated as 24.1/100,000 in 1993, and it is estimated that by 2010 the same rate raised to 50/100,000. These results show a two-fold increase in breast cancer incidence in Turkey over the last 20 years (9–12).
In the USA, 6.6% of women diagnosed with breast cancer are under the age of 40, while 33% are above 65 years (13). The median age is 61 years, with 25% premenopausal patients (13, 14). In our study, the rate of younger patients is high: 17% of all cases were ≤40, 18% were above 65 years with a median age of 51 years. In other words, the median age was 10 years younger in our patients than those in the USA. Furthermore, premenopausal breast cancer cases constituted 45% of our cases.
The incidence of young aged (≤40) breast cancer was shown to be high in Asian and African countries reaching up to 30% (15). This is due to the general population’ being younger in Turkey and other developing countries, and a higher young/old population ratio. According to the data acquired from the Turkish Institution for Statistics (Türkiye İstatistik Kurumu [TÜİK]), women under 40 years of age represent 68% of total female population in Turkey (16). In the USA, the rate of women under 40 years of age is only 45% (14). This difference shows a relative over-population in the younger subgroup and a relative increase in breast cancer in the younger age.
The identification of advanced staged breast cancer in younger women at the time of diagnosis is thought to be related to lack of screening programs among this subpopulation and the relatively higher rate of false negativity due to higher density of the breast tissue (17). The rates of clinical stage I and III breast cancer in patients under 40 years of age is 21% and 19%, while these rates are 29 and 13% in the 50–59 aged subgroup, respectively. Another surprising result was detected in patients above 70 years of age. In this group, the rate of clinical stage I disease at first presentation was higher (26%) than that of the group under 40 years, but lower than that of the 50–59 years of age group. The general disregard of diseases in the advanced age group and their usually painless manifestation results in delay in diagnosis. There is a general misbelief among our population that a painless mass is harmless.
Similarly, the mean tumor size was 2.8 cm among those under 40 years of age, while it decreased to 2.4 cm in the 40–69 year aged subgroup. Evaluation of age groups according to pathological tumor stages revealed the following distribution: pathological stage I breast cancer was detected in 22% of patients under 40 years and in 30% of those aged 50–59, and pathological stage III cancer was detected in 26 and 19% of the same age groups, respectively.
In addition to the data above, in younger breast cancer patients the rate of axilla positivity and HG were higher, ER and PR positivity were lower and HER-2 positivity was also higher (17–20). In our database, in patients under 40 years of age pN0 was 44%, HGIII rate was 60%, ER positivity was 61%, PR positivity was 57%, and HER-2 positivity was 26.5%, while in the more advanced aged group pN0 was 60%, HG3 rate was 44%, ER positivity was 71%, PR positivity was 59%, and HER-2 positivity was 23%.
The relatively dense breast tissue in premenopausal patients results in the diagnosis of the disease at a more advanced stage (21, 22). Clinical stage I and pN0 state breast cancer rates in premenopausal patients were 24.5 and 47% while these rates were 27.2 and 53% in menopausal patients, respectively. In this particular group, ER positivity was lower (66% vs. 73%) and PR positivity was a slightly higher (61% vs. 58%) as compared to the menopausal group. The comparison between the two groups concerning molecular subtypes, Luminal A and B breast cancer rates were similar, while HER-2 positivity (10% vs. 7%) and triple negative (16% vs. 13%) breast cancer rates were higher in the premenopausal group.
The extensive application of population based screening programs enables frequent detection of in situ breast cancers. Before the introduction of mammography into routine screening, DCIS was only diagnosed when it became palpable and accounted for only 1% of all breast cancers (23). Currently, DCIS is generally diagnosed as non-palpable lesions and constitutes around 20–25% of newly diagnosed breast cancer cases (23). Due to lack of fully organized population based screening in our country, in our database DCIS represents around 5% of all breast cancers. It is expected that the rate of detection of DCIS will soon rise due to the newly implemented fully organized population based mammographic screening program in Bahçeşehir, once gains wider spread and popularity. Indeed, in our program that screened 6500 women between 2009–2012, 21% of patients diagnosed with breast cancer had DCIS and 61% of them were stage I patients (24). Moreover, the fact that 48% of breast cancer cases detected in this prospective clinical study were in the 40–49 years age group, the KSDB (Cancer Control Department) adjusted the existing age limit for screening from 50–69 years to 40–69 years of age as of 2012.
“Invasive ductal carcinoma” is the most common histological type of breast cancer, constituting 49 to 75% of invasive breast cancer according to various studies (25–28). According to our study, histological types of breast cancer were as follows: 79% IDC, 7% ILC, 10% IMC and 4% other rare forms. Positive expression of ER and PR was higher in ILC’s than in IDC cases (25–28). It is thought that hormone replacement therapy results in increase of tumors of especially ILC histology due to this increase in expression of hormone receptors (29). Similar to the mentioned studies, in our database, rates of ER positivity in ILC and IMC (78%) were significantly higher than in IDC (68%) (p=0.0001).
The pN0 rates in our patients with newly diagnosed IDC were 52%, while it was 41% for those with ILC and IMC, similar to the literature (30). The pathological stage of cases in our study was also more advanced in ILC+IMC cases. The rate of stage I and II breast cancer was 76.5% for IDC and 68% for ILC+IMC.
It is known that HER-2 positivity that is present in 20–30% of invasive breast cancers is associated with decrease in overall and disease-free survival along with reduction in chemotherapy response rates (31). Various studies report ILC cases to be ER/PR positive, HER-2 negative, bcl-2 positive and p53 negative (32). Similar to the aforementioned reports in our study, among all cases with HER-2 expression, the rate of tumors with IDC histopathology was found to be approximately 9-fold higher than tumors with ILC and IMC histopathology (p=0.0001). HER-2 positivity was seen in 24.5% of patients with IDC, and in 14% of cases with ILC and IMC (p=0.0001).
In developed countries, the mean tumor size is around 10 mm, and the incidence of non-palpable breast cancer is 50% (33). According to our database, the mean tumor size was 25 mm, and the tumor was ≤1 cm in 9.5%, ≤2 cm in 48%, between 2–5 cm in 46%, and >5 cm in 6% of all patients. pT1 tumors were detected in 43% of women under 40 years and in 50% above 40 years of age. With increasing tumor size, axillary lymph node involvement incidence was also increasing. Nemoto et al. (34) reported the rate of pN0 patients according to tumor size as 75% in tumors of 0.6–1.0 cm size, 66% in 1.1–2.0 cm size, 50% in 3.1–4.0 cm size and 35.5% in those >5 cm. In our study, the pN0 rates in patients with pT1, 2 and 3 tumors were 61%, 42% and 18%, respectively. There was a parallel correlation between tumor size and HG, as tumor size increased the HG increased. HGIII rate was 44% in pT1 patients, and 61% in pT3 patients.
Studies focused on the correlation of tumor size and hormone receptor revealed that there is a negative correlation between tumor size and hormone receptor expression (35, 36). Similarly, in our patients, hormone receptor positivity decreased as tumor size increased. The rate of at least one receptor positivity was 79% in patients with tumor size ≤ 2 cm, whereas this rate was 73% in pT2, and 68% in pT3 patients.
A few studies assessing the relationship between tumor size and HER-2 expression reported that HER-2 positivity rate was increasing with growing tumor size (35, 36). In a study by Kong et al. (35), high levels of serum HER-2 was found to be correlated with tumor size of ≥2 cm, age (<35), menopausal status, stage III breast cancer, lymph node involvement and ER/PR negativity in univariate analysis, and multivariate analysis showed that as HER-2 serum levels increased overall and disease-free survival was decreased. Our study partially supports these data. HER-2 positivity was determined as 21.5% in pT1 and elevated to 25% in pT2 cancers.
It is known that in developing countries breast cancer is seen in younger ages, is diagnosed in more advanced stages, and the rate of HGIII and triple negative cancers are higher (9, 10, 15, 36, 37). In our National Breast Cancer Database, the rate of HGI was 5%, HGII 45%, and HGIII approximately 50%. Thus, in half of the patients HG rates were as high as African/American race (37). The distribution of cases according to age was similar to the general trend presented in the literature; younger patients had a higher HG (13, 38–40). The rate of HGI in patients less than 40 years of age were half the rate in the group of 60–69 years, and the rate of HGIII (60%) was 16% higher than the rate in the group of 60–69 years. If patients are stratified as age <40 and ≥40 years, the rate of HGI was 2.6% and HGIII was 60% in patients younger than 40, and 5% and 48% respectively in patients aged ≥40 years.
Various studies show that there is a direct correlation between HG and HER-2 positivity; as HG increases HER-2 positivity significantly increases (41–43). In a clinical study by Hoff et al. (43), HER-2 positivity rate in HGI patients was found to be <1%. In addition, in our study, out of all the HER-2 positive patients only 2% were HGI, 28% were HGII and 70% were HGIII.
It is determined that rates of HR positivity of breast cancer patients in developed countries are higher than the rates of patients in developing countries. Indeed, in a USA based evaluation of 360,933 cases, ER positivity was found in 79% of Caucasian, 72% of Asian and 63% of African patients (37). Progesterone receptor positivity was also similar; 68% in Caucasian, 62% in Asian and 53% in African descent patients. In 70% of our patients, ER was positive while PR positivity was 58%, which is lower than the rate in Caucasians, similar to the rate in Asian descent and higher than the rate in African descent patients.
Luminal A, B, HER-2 and TNG molecular subtypes in our database were 62%, 15%, 8% and 15%, respectively. When these rates were compared with western rates, the HER-2 and TNG molecular sub-types are found to be lower in our patients (44). In our younger patients (≤40 years), Luminal A, B, HER-2 and TNG breast cancer rates were 56%, 18.5%, 8% and 17%, respectively. In the older subpopulation (50–59 years of age), these rates were 63%, 15%, 10% and 12%, respectively. These results reveal that molecular subtypes indicating worse prognosis were significantly higher in younger patients. This difference was more prominent in the group aged >70 years, with HER-2 positivity rate of 7.5% and TNG rate of 8.8%.
Evaluation of HG level according to molecular subtypes, show that triple negative and HER-2 positive breast cancers have higher levels of HG (44, 45). In a clinical study, the rate of HG3 according to molecular subtypes was reported as 76% in TNG breast cancer, 67% in HER-2 positive group, 15% in Luminal A and 47.5% in Luminal B patients (45). According to our database 87% of patients with HGI were Luminal A, 10% were Luminal B, and 3% were TNG subtype, while none of the patients with HGI revealed to be HER-2 positive. The rate of breast cancer patients with HG3 were; 83.5% in the triple negative group, 82% in HER-2 positive group, 43% in Luminal A and 61% in Luminal B. Although the list of HGIII rates were similar to the results of Spitale et al. (45), it was observed that our HGIII rates in all molecular subtype groups were higher than the rates in developed countries. These findings, as was emphasized earlier, support the statement that breast cancer has higher histological grade and worse prognosis in developing countries.
In another study evaluating molecular subtypes, Luminal A group was shown to have a smaller tumor size, and less multifocality, lymph node involvement and lymphovascular invasion (45). Spitale et al. (45) compared molecular subtypes among 1214 patients, and found that the mean tumor size was 19.6 mm in Luminal A and B, 22.6 mm in HER-2 positive group and 26 mm in TN group, with the differences showing statistical significance. In our patients, the rate of pT1 was 51% in Luminal A, 50% in Luminal B, 41% in TNG and 37.5% in HER-2 group. As tumor size increases the rate of Luminal A and B decreases, while HER-2 and TNG rates increase. In pT3 tumors, HER-2 and TNG molecular subtypes showed a nearly 100% increase, which is parallel to the findings of other studies stating that patients with smaller size tumors have a better prognosis (44, 45). The pN0 rate in our patients were 55% in Luminal A, 49.7% in Luminal B, 39.6% in HER-2+ and 59.7% in triple negative group. This finding shows that the risk of local spread is higher in the HER-2 positive group as compared to TNG.
Triple Negative (TN) breast cancer incidence is higher in premenopausal women (43–45). In a study, 37% of TN breast cancers were detected in premenopausal women, and 13% of HER-2 positive patients and 23% of Luminal A cases were premenopausal (45). In our study, no statistically significant difference was found between Luminal A, B and HER-2 positive groups and menopausal status, although TN cancer rate was found to be 16.3% in premenopausal and 13.2% in menopausal women.
The tumor proliferation is high in the HER-2+ molecular subgroup, 75% of these patients is high grade, and more than 40% display p53 gene mutation (44, 45). It represents nearly 5–10% of all breast cancers. In our study, 8.5% of the patient population was HER-2 positive. The greater tumor size and higher axillary involvement rate results in worse prognosis in this group (44, 45). In our study population, the increase in tumor size increased the number of patients in HER-2 positive group. 6.4% of pT1 patients, 10% of pT2 patients and 11.2% of pT3 patients were found to be in this group. Similarly, in HER-2 group 6% of cases were pN0 whereas the rate of pN3 cases was three-fold higher (18%).
Approximately 7–30% of patients are in the triple negative molecular subtype, and the rate of TNG is higher in younger (<40 years of age), premenopausal and Asian/African descent women (43–45). The rate of patients in the TNG group was 14.6% in our patients and this rate was lower than the rate in Asian and African descent races but higher than the rate in some developed countries. The rate of TN breast cancer patients was 17.4% in the younger subgroup (≤40 years) while the same rate was less than half of this rate in women over 70 years of age (8.6%).
According to evaluation of our database, it can be concluded that our patients are younger, have more advanced stage breast cancers and worse prognostic factors than those patients in developed countries.
Figure 1.
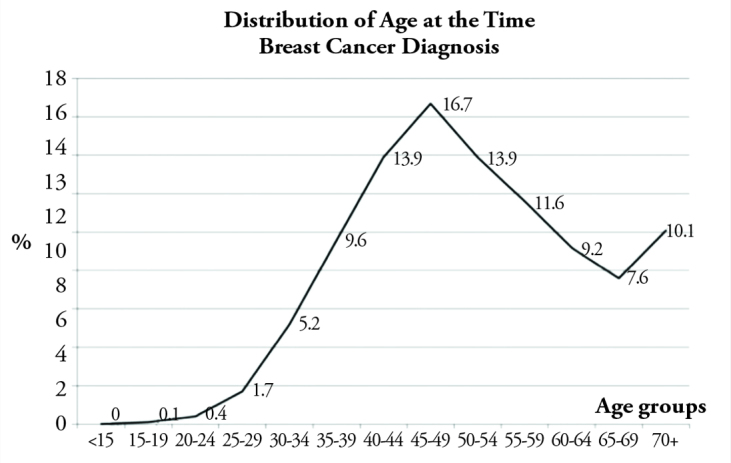
Breast cancer frequency according to age at diagnosis (%)
Acknowledgements
We would like to thank Dr. Nilüfer Özaydın for the statistical analysis of the data, and Dr. Bahadır Güllüoğlu, Sarah Özmen and Dr. Tolga Özmen for participation in preparation and writing of this manuscript.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study.
Conflict of Interest: No conflict of interest was declared by the author.
Peer-review: Externally peer-reviewed.
Informed Consent: N/A.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ozmen V. Sirketi. İstanbul: Yelken Basim Yayin Sanayi ve Ticaret Ltd; 2013. Breast Cancer in Turkey. [Google Scholar]
- 2.Wagner G. History of cancer registration. 1991. Available from: http://www.iarc.fr/en/publications/pdfs-online/epi/sp95/sp95-chap2.pdf. [PubMed]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. http://dx.doi.org/10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Eser SY. Cancer registry and cancer data centers. In: Tuncer Murat., editor. Cancer Control in Turkey. Ankara: Onur Matbaacılık; 2008. [Google Scholar]
- 5.Ozmen V. Breast cancer in Turkey and in the world. J Breast Health. 2008;4:6–12. [Google Scholar]
- 6.Dillon DA, Guidi AJ, Schnitt SJ. Pathology of invasive breast cancer. In: Harris JR, Lipmann ME, Morrow M, Osborne CK, editors. Diseases of the Breast. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 7.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th edition. chapter 32. Springer; Berlin, Germany: 2010. Available from: URL: http://www.scribd.com/doc/41422083/Complete-AJCC-Cancer-Staging-Manual-7e-Text. [Google Scholar]
- 8.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. http://dx.doi.org/10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozmen V, Anderson BO. The challenge of breast cancer in low- and middle-income countries—implementing the breast health global initiative guidelines. US Oncology. 2008:76–79. [Google Scholar]
- 10.http://globocan.iarc.fr/factsheets/cancers/all.asp
- 11.http://seer.cancer.gov
- 12.Fidaner C, Eser SY, Parkin DM. Incidence in Izmir in 1993–1994; First results from Izmir cancer registry. Eur J Cancer. 2001;37:83–92. doi: 10.1016/s0959-8049(00)00355-5. http://dx.doi.org/10.1016/S0959-8049(00)00355-5. [DOI] [PubMed] [Google Scholar]
- 13.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast Cancer Before Age 40 Years. Semin Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. http://dx.doi.org/10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Cancer Society. Breast Cancer Facts & Figures 2009–2010. Available from: http://www.cancer.org/acs/groups/content/@nho/documents/document/f861009final90809pdf.pdf.
- 15.Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PSY. Spectrum of Breast Cancer in Asian Women. World J Surg. 2007;31:1031–1040. doi: 10.1007/s00268-005-0585-9. http://dx.doi.org/10.1007/s00268-005-0585-9. [DOI] [PubMed] [Google Scholar]
- 16.www.tuik.gov.tr
- 17.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. http://dx.doi.org/10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 18.Liukkonen S, Leidenius M, Saarto T, Sjöström-Mattson J. Breast cancer in very young women. Eur J Surg Oncol. 2011;37:1030–1037. doi: 10.1016/j.ejso.2011.08.133. http://dx.doi.org/10.1016/j.ejso.2011.08.133. [DOI] [PubMed] [Google Scholar]
- 19.Neuschatz AC, DiPetrillo T, Safaii H, Price LL, Schmidt-Ullrich RK, Wazer DE. Long-term follow-up of a prospective policy of margin-directed radiation dose escalation in breast-conserving therapy. Cancer. 2003;97:30–39. doi: 10.1002/cncr.10981. http://dx.doi.org/10.1002/cncr.10981. [DOI] [PubMed] [Google Scholar]
- 20.Anders CK, Acharya CR, Hsu DS, Broadwater G, Garman K, Foekens JA, Zhang Y, Wang Y, Marcom K, Marks JR, Mukherjee S, Nevins JR, Blackwell KL, Potti A. Age-specific differences in oncogenic pathway deregulation seen in human breast tumors. PLoS One. 2008;3:e1373. doi: 10.1371/journal.pone.0001373. http://dx.doi.org/10.1371/journal.pone.0001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de la Rochefordiere A, Asselain B, Campana F, Scholl SM, Fenton J, Vilcoq JR, Durand JC, Pouillart P, Magdelenat H, Fourquet A. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043. doi: 10.1016/0140-6736(93)92407-k. http://dx.doi.org/10.1016/0140-6736(93)92407-K. [DOI] [PubMed] [Google Scholar]
- 22.Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S, Abner A, Recht A, Vicini F, Harris JR. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I and II breast cancer. J Clin Oncol. 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 23.Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast recurrence: A systematic review. J Cancer. 2011;2:232–261. doi: 10.7150/jca.2.232. http://dx.doi.org/10.7150/jca.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozmen V, Ozkan-Gurdal S, Kayhan A, Ozaydin N, Cabioglu N, Ozcinar B, Aribal E. Successful results of a population-based organized mammography screening program in a developing country: The Turkish experience. San Antonio Breast Cancer Symposium; 2013; San Antonio, Tx, USA. Dec 10–14, 2013. [Google Scholar]
- 25.Ozmen V. Terzi Cem., editor. İnvaziv duktal karsinom. Türk Cerrahi Derneği Temel ve Klinik Cerrahi Kitabı. Available from: URL: http://www.tcdcerrahi.org/fulltext.php?id=340.
- 26.Gurdal SO, Karanlik H, Cabioglu N, Ozcinar B, Yavuz E, Tuzlali S, Ozmen V. Positive or close margins in breast conserving surgery: Is reexcision always necessary? Eur J Surg Oncol. 2012;38:399–406. doi: 10.1016/j.ejso.2012.02.182. http://dx.doi.org/10.1016/j.ejso.2012.02.182. [DOI] [PubMed] [Google Scholar]
- 27.Ellis IO, Humphreys S, Michell M, Pinder SE, Wells CA, Zakhour HD UK National Coordinating Commmittee for Breast Screening Pathology; European Commission Working Group on Breast Screening Pathology. Best Practice No 179. Guidelines for breast needle core biopsy handling and reporting in breast screening assessment. J Clin Pathol. 2004;57:897–902. doi: 10.1136/jcp.2003.010983. http://dx.doi.org/10.1136/jcp.2003.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molino A, Giovannini M, Auriemma A, Fiorio E, Mercanti A, Mandara M, Caldara A, Micciolo R, Pavarana M, Cetto GL. Pathological, biological and clinical characteristics, and surgical management of elderly women with breast cancer. Crit Rev Oncol Hematol. 2006;59:226–233. doi: 10.1016/j.critrevonc.2006.01.007. http://dx.doi.org/10.1016/j.critrevonc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. http://dx.doi.org/10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 30.Vandorpe T, Smeets A, Van Calster B, Van Hoorde K, Leunen K, Amant F, Moerman P, Deraedt K, Brouckaert O, Van Huffel S, Wildiers H, Christiaens MR, Neven P. Lobular and non-lobular breast cancers differ regarding axillary lymph node metastasis: a cross-sectional study on 4,292 consecutive patients. Breast Cancer Res Treat. 2011;128:429–435. doi: 10.1007/s10549-011-1565-4. http://dx.doi.org/10.1007/s10549-011-1565-4. [DOI] [PubMed] [Google Scholar]
- 31.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. http://dx.doi.org/10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Dabbs DJ, Shuai Y, Niemeier LA, Bhargava R. Classical-type invasive lobular carcinoma with HER2 overexpression: clinical, histologic, and hormone receptor characteristics. Am J Clin Pathol. 2011;136:88–97. doi: 10.1309/AJCP7URIW0QETTAT. http://dx.doi.org/10.1309/AJCP7URIW0QETTAT. [DOI] [PubMed] [Google Scholar]
- 33.Kiderlen M, Bastiaannet E, Walsh PM, Keating NL, Schrodi S, Engel J, van de Water W, Ess SM, van Eycken L, Miranda A, de Munck L, van de Velde CJ, de Craen AJ, Liefers GJ. Surgical treatment of early stage breast cancer in elderly: an international comparison. Breast Cancer Res Treat. 2012;132:675–682. doi: 10.1007/s10549-011-1892-5. http://dx.doi.org/10.1007/s10549-011-1892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980;45:2917–2924. doi: 10.1002/1097-0142(19800615)45:12<2917::aid-cncr2820451203>3.0.co;2-m. http://onlinelibrary.wiley.com/doi/10.1002/1097-0142(19800615)45:12%3C2917::AID-CNCR2820451203%3E3.0.CO;2-M/abstract. [DOI] [PubMed] [Google Scholar]
- 35.García-Closas M, Brinton LA, Lissowska J, Chatterjee N, Peplonska B, Anderson WF, Szeszenia-Dabrowska N, Bardin-Mikolajczak A, Zatonski W, Blair A, Kalaylioglu Z, Rymkiewicz G, Mazepa-Sikora D, Kordek R, Lukaszek S, Sherman ME. Established breast cancer risk factors by clinically important tumour characteristics. Br J Cancer. 2006;95:123–129. doi: 10.1038/sj.bjc.6603207. http://dx.doi.org/10.1038/sj.bjc.6603207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mise I, Vucić M, Maricević I, Sokcević M, Curić-Jurić S. Histologic subtypes of invasive lobular carcinoma in correlation with tumor status and hormone receptors. Acta Clin Croat. 2010;49:275–281. [PubMed] [Google Scholar]
- 37.Kakarala M, Rozek L, Cote M, Liyanage S, Brenner DE. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S.--a SEER analysis. BMC Cancer. 2010;10:191. doi: 10.1186/1471-2407-10-191. http://dx.doi.org/10.1186/1471-2407-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albain KS, Allred DC, Clark GM. Breast cancer outcomes and predictors of outcome: are there age differentials? J Natl Cancer Inst Monogr. 1994;16:35–42. [PubMed] [Google Scholar]
- 39.Fornier MN, Modi S, Panageas KS, Norton L, Hudis C. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer. 2005;104:1575–1579. doi: 10.1002/cncr.21385. http://dx.doi.org/10.1002/cncr.21385. [DOI] [PubMed] [Google Scholar]
- 40.Warner E. Clinical practice. Breast-cancer screening. N Engl J Med. 2011;365:1025–1032. doi: 10.1056/NEJMcp1101540. http://dx.doi.org/10.1056/NEJMcp1101540. [DOI] [PubMed] [Google Scholar]
- 41.Ivkovic-Kapicl T, Knezevic-Usaj S, Djilas-Ivanovic D, Panjkovic M. Correlation of HER-2/neu protein overexpression with other prognostic and predictive factors in invasive ductal breast cancer. In Vivo. 2007;21:673–638. [PubMed] [Google Scholar]
- 42.Ferrero-Poüs M, Hacène K, Bouchet C, Le Doussal V, Tubiana-Hulin M, Spyratos F. Relationship between c-erbB-2 and other tumor characteristics in breast cancer prognosis. Clin Cancer Res. 2000;6:4745–4754. [PubMed] [Google Scholar]
- 43.Hoff ER, Tubbs RR, Myles JL, Procop GW. HER2/neu amplification in breast cancer: stratification by tumor type and grade. Am J Clin Pathol. 2002;117:916–921. doi: 10.1309/4NTU-N6K4-F8JF-EWRX. http://dx.doi.org/10.1309/4NTU-N6K4-F8JF-EWRX. [DOI] [PubMed] [Google Scholar]
- 44.Carey LA, Perou CM. Gene arrays, prognosis, and therapeutic interventions. In: Harris Jay R, Lippman Marc E, Morrow Monica, Kent Osborne C., editors. Diseases of the Breast. 4th ed. chapter 32. Lippincott & Wilkins, a Wolters Kluwer; Philadelphia, USA: 2010. pp. 458–472. [Google Scholar]
- 45.Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol. 2009;20:628–635. doi: 10.1093/annonc/mdn675. http://dx.doi.org/10.1093/annonc/mdn675. [DOI] [PubMed] [Google Scholar]


