Abstract
Endoscopic oncoplastic breast surgery represents a minimal invasive approach with the aim of both removing cancer safely and also restoring the breast image. It has less noticeable scar, excellent cosmetic outcomes, high patient satisfaction rate and recently reported relatively long term safety. Operative techniques for both endoscopic breast conserving surgery and endoscopic nipple/areola/skin sparing mastectomy have been described in detail. Two different working planes in which one of them is subcutaneous and the other one is sub-mammary planes are being used during the surgery. Surgical techniqe needs some instruments such as endoscopic retractor, light guided specific mammary retractor, wound protector and bipolar scissor. Endoscopic breast retractors provide magnified visualization and extensive posterior dissection facility. Tunneling method and hydrodissection simplify the technique in the subcutaneous field. Oncoplastic reconstruction techniques are also applied after the tumor resection by endoscopic method. Complication rates of endoscopic breast surgery are similar to open breast surgery rates. Quite succesful local recurrence, distant metastasis and overall survival rates have been declared. However it looks reasonable to wait for the results with longer follow-up before having a judgement about oncologic efficiency and safety of the endoscopic breast cancer surgery.
Keywords: Breast cancer, endoscopic surgical procedures, breast conserving surgery, video-assisted surgery, subcutaneous mastectomy
Indication and Patient Selection
Breast-Conserving Surgery (EAO-BCS) is performed for T1–T2 tumors. The skin, pectoral muscle and chest wall invasions are contraindications. It cannot be performed in cases of multifocal tumors (1, 2). The exclusion criteria include tumor close to skin, thoracic deformity, hemorrhagic diathesis, elderly age, poor health condition and patient’s reluctance towards this method (3). The other limitations of breast-conserving surgery are applicable for this, as well. Some studies cited clinically positive axilla as a contraindication (1–7). There are also studies limiting the technique to cases that would have less than 20% of volume loss 1, 8). If the potential loss of volume in the breast is estimated at 20–40%, volume replacement techniques may be more appropriate in place of volume displacement method (1, 9–11).
EAO-BCS is rather targeted at Cup A and Cup B breasts. Also, the location of tumor is important, too. For tumors located in the inner or lower part of the breast, the spaces to emerge following excision have to be filled in via volume displacements through periareolar and axillary incision (1, 8). Oil necrosis may be frequent especially when reconstruction is performed with wide-ranging mobilization and volume displacement in old patients with low breast density (1, 8, 11, 12).
Mastectomy (EASM)
Skin-sparing mastectomy (EA-SSM) and nipple-/areola-sparing mastectomy (EA-NSM) can be performed with the assistance of endoscopy. Both techniques can be employed in breast cancer, ductal carcinoma in situ, risk-reducing mastectomy, large Phyllodes tumor and benign breast diseases that would require mastectomy. When mastectomy is required, EA-NSM is preferred with priority. The patient selection criteria are the same as in open surgery.
For endoscopically-assisted subcutaneous mastectomy (EASM), the skin, pectoral muscle and chest wall invasions are contraindications. Tumor that is close to skin, inflammatory cancer, thoracic deformity, hemorrhagic diathesis, elderly age, poor health condition and the patient’s reluctance towards the method are accepted as exclusion criteria. Large (Cup C or above) breast and breasts that are too flabby are not eligible for EASM. As for EA-NSM, the other exclusion criteria reported in the literature are as follows: tumor larger than 3 cm, tumor that is more than 2 cm near the tumor, bleeding nipple, tumor near the area under the nipple, Paget’s disease and large central tumor. Furthermore, clinically positive axilla, local recurrence and tumor with negative estrogen and progesterone receptors with a high potential for distant metastasis are accepted as exclusion criteria in many clinics. No consensuses are present for patients that would receive preoperative or post-operative radiotherapy. The results indicate that this technique can be performed in such patients (13–15).
Marking
Breast-Conserving Surgery
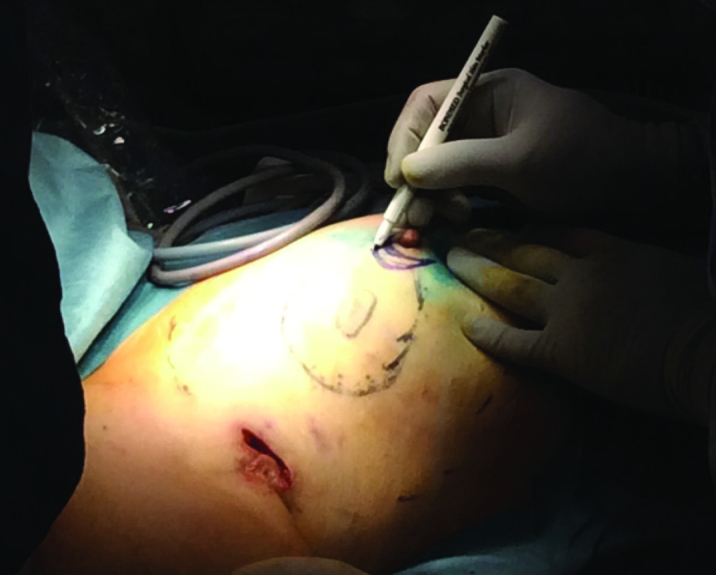
The projections of tumors or lesions on the skin are marked before surgery under ultrasound guidance (Figure 1). The excision margins are determined at 1–2 cm away from the tumor margins (1, 3, 6–9, 16). At the beginning of surgery, all-round color stain injections are performed at the excision margins (1, 4, 8, 11) (Figure 2). Since the blue stain is generally used for marking sentinel lymph nodes, the marking should be done using a stain that is not mixed with the blue stain in EAO-BCS. The stains used according to the literature include Gentiana Violet (4, 16), Diagnogreen (17), Indigo Carmine (3, 7) and Pyoctanin (8, 18). Furthermore, these stains are used by being mixed with gel (1% Lidocaine Gel or Xylocaine Gel) at a ratio of 1/1 in order to prevent the injected stain from being absorbed and spreading.
Figure 1.
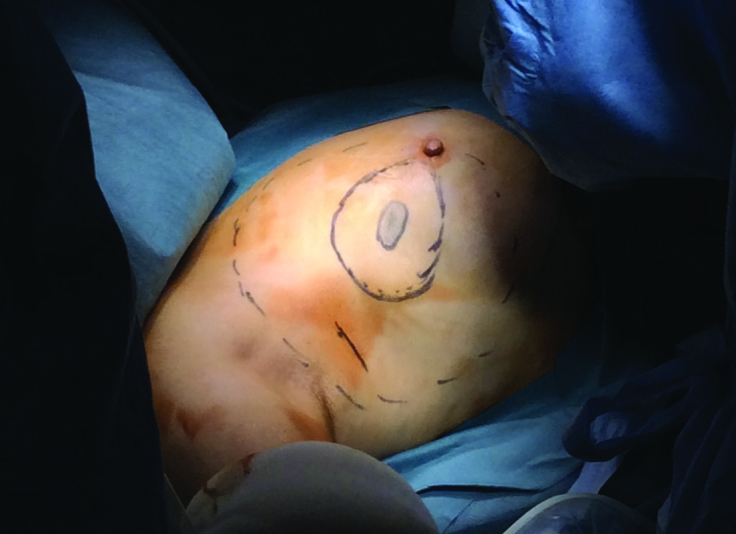
Marking and drawing on the breast. The tumor margins (innermost), excision margins (middle ring), margins of dissection and mobilization to be performed in the anterior and posterior sites (outermost) and the lymph node incision in the axilla are shown
Figure 2.
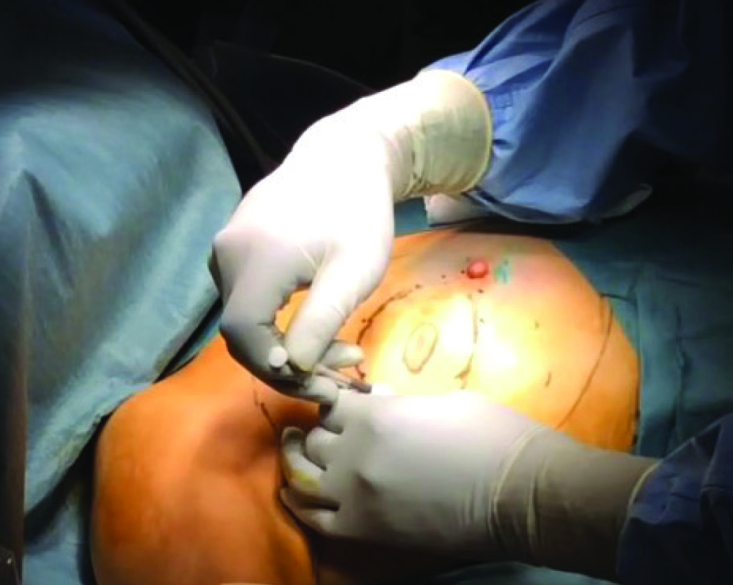
Subcutaneous stain injections at several points on the excision margins are seen
Mastectomy
The projections of tumors or lesions on the skin are marked by drawings prior to surgery. The all-round margins of breast tissue are also included in marking. Furthermore, the marking of internal thoracic and artery branches on the breast in the parasternal area would be beneficial for the preservation of blood flow during dissection (1, 8, 19).
Incision
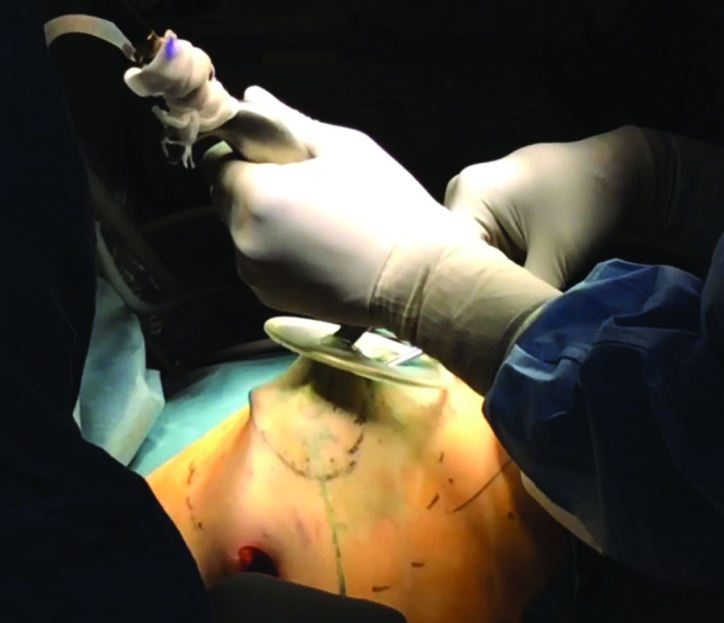
Axillary and periareolar incisions are the most frequently used incisions in both mastectomy and breast-conserving surgery. The incision used in axilla is a generally an incision that is 2 cm, which is made for sentinel lymph node, and it is used for dissection performed in the posterior part of the breast (1–4, 6–9, 11, 16, 17, 20). To create a skin flap, a periareolar incision is used. In addition to this incision, an additional skin excision in the shape of half moon is used to enlarge the incision, thereby facilitating the removal of excised tissue through here. The site for periareolar incision is determined on the basis of the location of tumor in the breast. The incision is kept at a size that is approximately 2/5 of the perimeter of areola (2, 3, 8, 11). Since work is done through a small incision with EAO-BCS, the skin around the incision during the procedure may be injured. To prevent this, a wound protector (Alexis; Hakko Co; Johnson & Johnson) is used (1, 5–8, 11, 16) (Figure 3).
Figure 3.
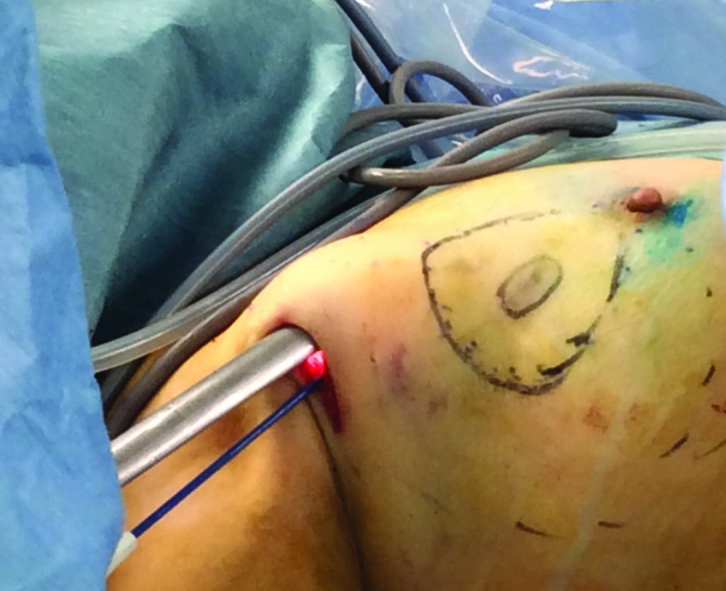
The dissection performed using special lighted breast retractor in deep sites while the skin flap is prepared in the posterior site is observed. Wound protective material is also used for areola dissection
Although there are recommendations in the literature for mastectomy such as long (5–10 cm), single axillary incision and lateral breast incision (17, 19, 21, 22), these are not currently used at an extensive rate. A comparative series demonstrated that 5.5 cm axillary incision had superiority over open skin conserving mastectomy (22). Periareolar incision is performed laterally on the breast. This ensures facilitation in the placement of implant or expander and in the creation of a pocket.
Another technique used in EASM is the endoscopic technique where trocars are also used. With this technique, the working area is created via insufflation over a single-port entry on a single axillary incision of 4–6 cm, and then an anterior site dissection is performed followed by a posterior site dissection (23, 24). In this series of 10 cases, the average operation time was reported as 250 minutes, rate of partial nipple necrosis corrected with medical treatment as 30%, hematoma as 10% and infection as 10%.
Posterior Dissection
Dissection in the retromammary space is performed between the posterior face of the breast and the pectoral muscle (Figure 4). Retractors with optical systems (Vein Harvest, Ultra Retractor, Vein Retractor) are also used for blunt dissection while bipolar scissors or electro-cautery is used for coagulation (1, 5, 8, 11, 16, 17) (Figure 5, 6). Techniques for creating the work area using pre-peritoneal dissection balloon (17, 21) or insufflation (22) were used in the past as part of posterior dissection; however, they are not preferred today.
Figure 4.
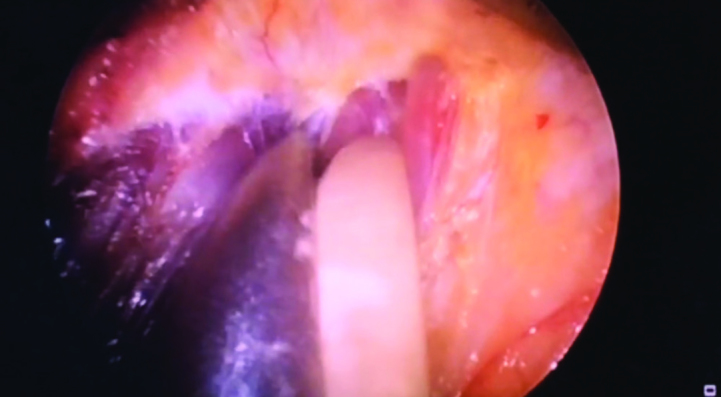
Endoscope (retractor) is inserted through the axillary incision and the posterior site is dissected with electro-cautery as seen. Here, endoscope is also used as a retractor
Figure 5.
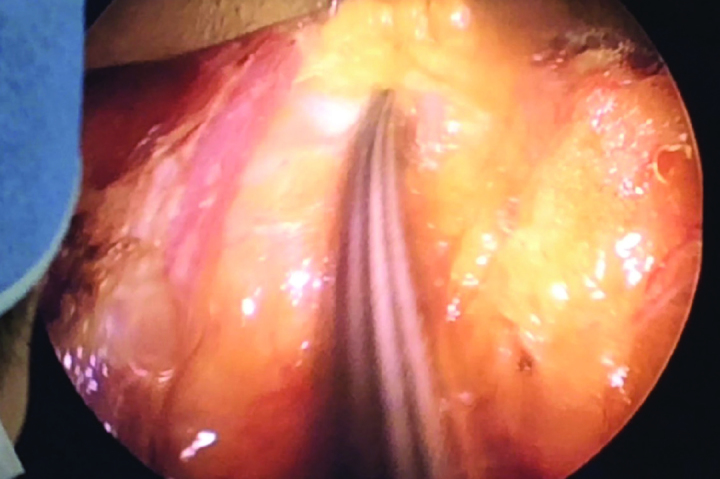
Dissection in the posterior site is seen in the monitor. Sharp dissection is performed with a pair of bipolar scissors in the plane between the breast tissue and pectoral muscle under imaging guidance and the breast tissue is mobilized
Figure 6.
Dissection using a pair of bipolar scissors under endoscopic guidance in the posterior site is seen in the monitor. The breast tissue is mobilized in the space between the breast tissue and pectoral muscle and at the edge of the pectoral muscle in the outer lateral side
In cases of Breast-Conserving Surgery, mobilization is performed in such a way as to cover an area further beyond the tumor margins in order to facilitate especially volume displacements. In cases that will undergo mastectomy, the dissection site is consistent with the anatomic margins of the breast.
Anterior Dissection (Creation of a Skin Flap)
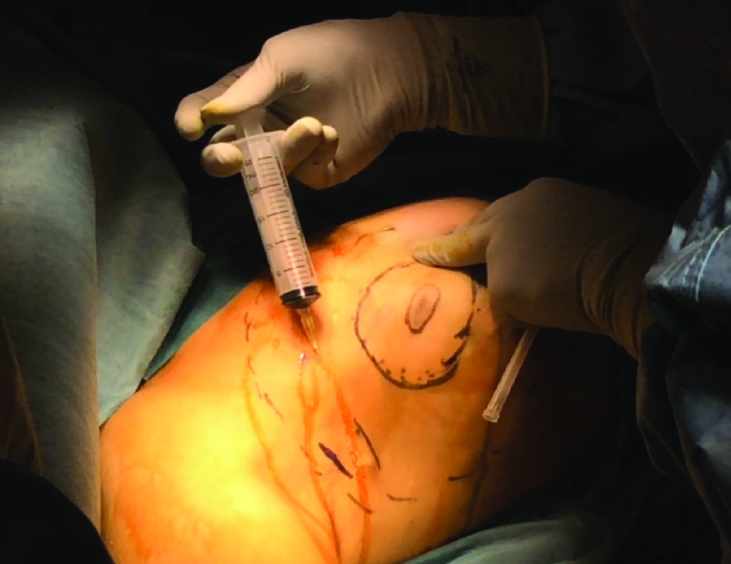
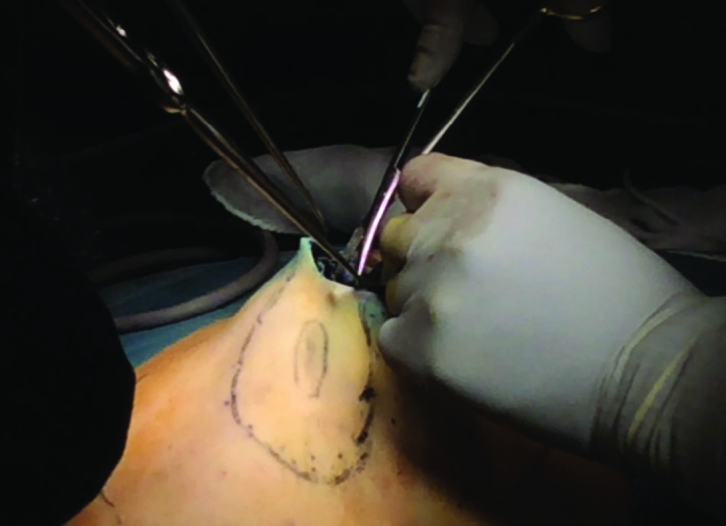
This dissection is performed between the breast and skin and periareolar incision is used (Figure 7). Before the dissection, injections of physiological saline solution with Epinephrine at a ratio of 1/1,000,000 (approximately 150 cc) are administered in this plane (Figure 8). This technique is termed the “Tumescent Technique” or “Hydrodissection” and it not only facilitates dissection, but also ensures that they are performed with less bleeding (8). Following hydro-dissection, the dissection is completed by using an optical system and bipolar scissors between the subcutaneous plane and the breast tissue (8) (Figure 9). Attention should be shown during dissection to make sure that the flap is not too thin. Very thin flaps increase the potential for ischemia and necrosis in the skin (8). For dissection, “harmonic scalpel” or electro-cautery may also be used (1, 2, 7, 9, 16, 17) (Figure 10). The flap is gently retracted using a special lighted breast retractor (Mamma Retractor-Four Medics, Tokyo; Cold Light Retractor-Komagowa, Spain; Oral Retractor-TISE) in order to facilitate the dissection.
Figure 7.
The periareolar incision line is drawn before anterior site dissection is started. The incision entrance will have been slightly enlarged with a skin incision in a half moon shape
Figure 8.
Before starting dissection in the anterior site, injections of physiological saline solution with Epinephrine at a ratio of 1/1.000.000 are performed in the previously marked mobilization area. This procedure would ensure a dissection that is not only easy, but also causes relatively less bleeding
Figure 9.
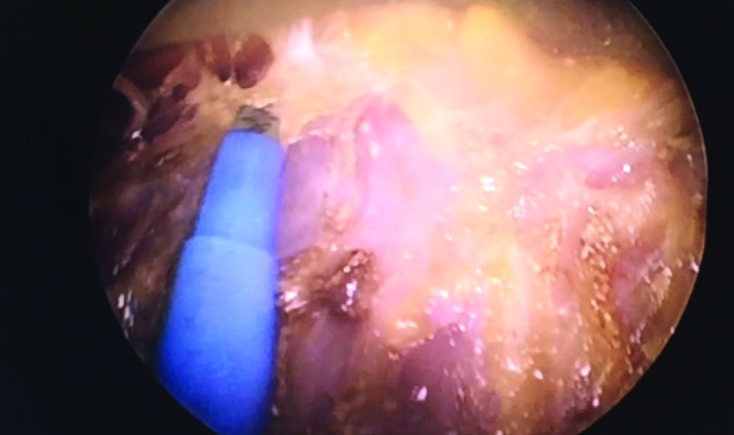
Working through the periareolar incision, the skin traction is ensured, advancement in the subcutaneous plane is made and the breast tissue is separated from the skin
Figure 10.
After the completion of the mobilization of skin flap in the anterior site, the tumor tissue has been excised in line with the margins and hemostasis control is achieved in the tumor bed as accompanied by endoscopy. At the same time, breast tissue with enough mobilization for the volume displacement procedure is prepared
Subcutaneous dissection is made easier by the tunnel method. With this technique, a multiple tunnel is opened using scissors on the subcutaneous plane in a radial way towards the periphery from the nipple (7). Then, the septa between tunnels are cut. One of the different methods employed as part of the tunnel method is the creation of tunnels using bladeless trocars (Optiview, Bladeless Trocar, Endopath, Visiport Plus) (1, 3–6, 8, 11, 13, 16, 17, 20).
One of the techniques for subcutaneous dissection, which is recommended in the literature, yet has not been widely adopted in practice, is the use of traction sutures placed on the skin to facilitate dissection (10, 11, 16) or the use of needles stuck on the skin in order to delineate the excision margins (16).
Specimen Excision and Reconstruction
Breast-Conserving Surgery
The tissue that has been excised is removed through the periareolar incision. Some surgeons use “Endo-catch” in order to remove the specimen (1, 16). The cavity is marked using clips (1, 4, 8). All the oncoplastic techniques used in reconstruction can be employed here, as well. The most commonly used techniques include the volume displacement, volume replacement and filling techniques.
Volume Displacement
This is the most commonly used technique. The breast tissue that is mobilized by being removed over the pectoral muscle posteriorly and from the skin anteriorly is pulled from both sides towards the cavity in the middle under the guidance of imaging and stitched together using sutures. If plication develops on the skin following volume displacement, skin mobilization is performed on a wider area (1–4, 6, 8, 19).
Volume Replacement
If the excised tissue is 30% of the total breast or more or the cavity that emerged cannot be closed with volume displacement, the latissimus dorsi flap or lateral thoracic adipose tissue flap mobilized with endoscopic technique can be brought to the cavity by working through the axillary incision (1, 9).
Cavity Filling
The site of the excised breast tissue is filled in with a new synthetic material. These techniques do not have a proven success. The materials that have been tested include absorbable synthetic mesh (Vicryl Mesh, Johnson&Johnson) wrapped in an adhesion barrier (Interceed, Johnson&Johnson) (4, 16, 17, 25). A study reported excessive fluid buildup in almost all the cases and an infection rate of 11%. This method is not recommended in patients with collagen disease or on steroids (17). Furthermore, “Oxidized Cellulose” (Surgicel, Johnson&Johnson) was also tested in order to wrap the mesh used for filling in the cavity (1, 5, 16, 17, 25). The mesh here causes the growth of granulation, reactive fluid and fibrosis tissue while “oxidized cellulose” prevents the mesh from adhering to the skin (25).
Mastectomy
For reconstruction after EASM, the methods that are employed in the open technique are used. Reconstruction with implant is performed with priority. A technique with dual or single procedure is preferred depending on the case or the surgeon. During the EASM procedure, mastectomy is completed and then work is done through the periareolar incision. An endoscopic retractor is used to start dissection of the pectoralis major muscle from its lateral margin with the aid of imaging, the area under the muscle is entered with sharp dissection and a pocket for implant is created using an expander. An implant or expander is inserted in the pocket through the axillary incision (11, 13, 21).
Operation Time
In general, longer times are reported for the endoscopic surgeries of the breast (11). The operation times are closely related with the reconstruction technique that is used. The endoscopic procedures performed at the beginning bring about an additional 30–40 minutes on average to the conventional surgical times (7, 11, 12, 22). However, this time is associated with the learning curve. Operation times equal to those of open surgery when the learning process was completed have been reported.
Cost
The increased cost is associated with the materials that are used. Single-use instruments increase the cost. Re-usable instruments reduce the average cost. A study reported that the essential setup cost of the system to be able to start endoscopic breast surgery in addition to open surgery was $10,000 (3). In another study where a rough cost analysis was performed, the cost of endoscopic lumpectomy was reported as $1150 and open lumpectomy as $500 (11, 22).
Cosmetic Results
Generally speaking, reasonable aesthetic results are reported with EAO-BCS. Two studies reported better results as compared to open surgery (1, 2, 8, 17, 18). A significant difference is achieved especially in terms of scars (1, 8, 18). For a comprehensive and objective assessment, a 4-point-scoring aesthetic evaluation should be made and the quality of life should also be questioned. The assessment methods appropriate for this include the “Breast Cancer-Specific Quality of Life Questionnaire” (EORTC-QLQ-BR23) or “Patient Satisfaction Rate” (FACT-B) by EORTC (European Organization for Research and Treatment) (1, 18).
In the “5-item” system, which is commonly used in cosmetic assessment (ABNSW), the important assessment parameters include asymmetry, breast shape, nipple shape, skin condition and wound scar. The scoring is done using a 4-points scoring system (excellent=3, good=2, moderate=1, poor=0). A score of 11 points in total or above is considered as good or excellent breast aesthetics (1, 5, 7, 8).
The Japanese Breast Cancer Society, on the other hand, uses an 8-item classification. The themes used include the breast size, breast shape, breast scar, breast hardness, nipple and areola size, nipple and areola shape, nipple and areola color, nipple and areola position and inframammary groove condition. Every theme is scored according to a 3-point system (good: 2 points, moderate: 1 point, poor: 0 points). In total, a score of 11–12 points is considered an excellent cosmetic result, 8–10 points good, 5–7 points moderate and 0–4 points a poor cosmetic result (1, 8).
The results of endoscopic-assisted breast surgeries are generally reported as minimal scar and excellent cosmetic results. Kitamura reported an excellent result of 85% with the endoscopic technique and of 60% with the open technique in a study comparing this technique with open surgery (11, 22). As for the questionnaire studies related to patient satisfaction, it is seen that the majority of patients receiving EOSM are pleased with the result.
Complications
The complications reported with endoscopic breast surgery are generally the same in type and equal in rate with open surgery. Fan reported in his comparative series that the complication rates were equal for endoscopic and open technique and that they varied according to the surgical technique and type of reconstruction performed (11, 12). The most frequently encountered complication is the development of seroma (21). Superficial or deep skin burns and ecchymoses due to the inadequate protection of skin are also often encountered (7, 9, 11–13, 26–28). The wound site infection rates range between 1% and 9% and they are not higher than in open breast surgery (11, 13). The infection rates are higher for mastectomy and implant procedures. The requirement to remove the prosthesis due to infection develops in approximately 10% cases where implants were used (21). When the insufflation technique is used to create a surgical working area, subcutaneous emphysema is often seen in the breast and surrounding tissues (11, 27, 28). Furthermore, asymmetry, deformity and skin plication may develop in the breast and nipples depending on the reconstruction technique and procedure. There are no studies comparing endoscopic breast surgery with open surgery in relation to post-operative pain and the use of analgesics (11).
Breast-Conserving Surgery
Especially in elderly patients and patients with low breast density, oil necroses may be seen when reconstruction is performed with wide-ranging mobilization and volume displacement using the endoscopic technique (1, 8, 12, 25). The complication rate for the EAO-BCS is approximately 10% on average. Skin and nipple necroses are rather rare in EAO-BCS.
Mastectomy
In terms of severe complications, skin and nipple necroses may be encountered. Especially the “tumescent technique” has resulted in increased skin ischemia and necroses (8).
Nipple Necrosis
It is one of the serious complications developing with EA-NSM. The rates reported in the literature are in the range of 0–20% (13). Full or partial necrosis may develop. The ratio of complete necrosis is below 10%. The reason why different rates are seen in the literature is that the tissue left in the tissue with EA-NSM is variable. No standard tissue thicknesses exist on which consensus has been achieved. Leaving a tissue with a thickness of 5 mm on an area with a width of 2 cm has been recommended (13). Another factor influencing the rate of complications is the use of cautery in dissection. For the dissection of the area below the nipple, the use of scissors rather than cautery is recommended.
An important factor in nipple necroses that develop following EA-NSM is the incision performed. Radial or lateral incisions should be preferred rather than medial incisions (13). For transareolar incisions, nipple necrosis develops at the highest frequency with a rate of 80%. Partial or full necrosis develops at a rate of 17% in periareolar incisions and a rate of 4–8% in radial or inframammary incisions (15).
One of the factors effective in the development of nipple necrosis following EA-NSM is the “coring” technique which is performed in certain centers. The tissue under the nipple is completely excised by being cored with oncological concerns and only the nipple skin is left behind. The rates of necrosis in cases where the nipple is cored amounts to 40% and nearly 24% of them are complete necrosis (13).
The most important factors influencing nipple necrosis are the ones that pertain to the patient. Patients who have diabetes, vascular disease and smoke have higher rates of nipple necrosis. Nipple necroses secondary to perfusion disturbance in large (Cup C and above) and overly flappy breasts also have higher rates (14, 24). Most of the nipple necroses, especially partial ones, improve with medical treatment and do not require excision (21).
Loss of Sensation in the Nipple/Areola
The loss of sensation in the skin, nipple and areola following endoscopic breast-conversing surgery is rare. It is reported to generally improve in a period of 6 months to 1 year in patients receiving mastectomy.
Loss of Blood
In the initial periods when endoscopic technique entered into use, Kitamura reported that the endoscopic mastectomy group had more bleeding in his study where he reported the early results (11, 22). In the consequent years, 3 different studies were performed in relation to intraoperative bleeding and postoperative drainage with the EASM technique and no differences were found between endoscopic and open methods (1, 8, 11, 12). A study where reconstruction was made using the filling method following EAO-BCS found that bleeding was lower than with the open method (1, 17).
Oncological Results
Breast-Conserving Surgery
Rates that are equal to those of open surgery are reported with respect to local recurrence, distant metastasis and overall survival while certain studies report better oncological results. However, the average follow-up durations are between 12 and 40 months and this is a rather short period to make a clear decision about oncological results (1, 3, 4, 6–9, 11, 26).
Local Recurrence
There are 6 studies assessing local recurrence following EAO-BCS. The studies where local recurrence is cited in the range of 0–4% have average follow-up durations of 12–38 months (1, 3, 4, 6–9, 11). Nakajima specified tumor size as a risk factor for local recurrence in EAO-BCS with local recurrence rates of 3.7% for T1 tumors and 5.1% for T2 tumors in his series (1, 9, 11). To date, local recurrence on periareolar or axillary incision in any of the EAO-BCS cases has not been reported.
Distant Metastasis
Three studies related to the development of distant metastasis in patients who received EAO-BCS have been reported (1, 4, 8, 9). In the study with an average follow-up period of 40 months, it was reported that the distant metastasis rate was associated with the tumor diameter (1, 9, 26). In a study with 244 cases, no differences were found in terms of distant metastasis among patients undergoing EAO-BCS and open surgery (11, 26). Another study citing a metastasis rate of 10%, distant metastasis was attributed to the high axillary involvement ratio (41%) and high tumor load was blamed (9, 11)
Overall Survival
Five studies related to overall survival in patients undergoing EAO-BCS have been reported. The follow-up periods of the studies are short, but the results look excellent (1, 3, 4, 7–9, 11). A study citing data sorted by the tumor diameter reported an overall survival rate of 97.3% for T1 tumors and 95.7% for T1 tumors (1, 9, 11). Another study demonstrated that there were no differences in terms of survival among State I and Stage II patients who underwent EAO-BCS (1, 26).
Mastectomy
As in the open nipple-conserving mastectomy technique, discussions on the oncological risk of breast tissue left behind the nipple with EAO-BCS are also ongoing (13). The collection and examination of biopsy from the tissue under the nipple during EAO-BCS constitutes a method that is rather widely implemented. However, suspicion of tumor or marginal positivity at the nipple are identified at rates amounting to 9% in the paraffin wax cross-sections in post-operative period in spite of this procedure, which may require these cases to undergo nipple excision in the aftermath (21). There are also studies recommending radiotherapy during or after surgery for the breast tissue remaining behind the tipple (11, 13, 29).
Local Recurrence
Eight of the published papers cited the local recurrence rates (2, 11, 19, 20, 27, 29–32). No recurrences were reported in studies with an average follow-up period of 2 years on average and in non-prospective studies (11, 13, 21). It is obvious that studies with longer follow-up periods are required in the light of studies indicating that recurrence is increased especially after the 3rd year (9, 11). A non-randomized study compared EASM and open, skin-conserving mastectomy cases in terms of local recurrence and it was reported that none of the cases had recurrence (7, 11). Another study compared EASM and open breast-conserving surgery and no was demonstrated with the rate being 1.9% for open breast-conserving surgery and 8% for EASM (11, 12).
Distant Metastasis
Three studies in the literature cited distant metastasis rates (11–13, 22) and a rate in the range of 4.5–10% with the longest follow-up period being 38 months was reported. No differences in terms of distant metastasis were identified among EASM and open skin-conserving mastectomy patients in 2 studies with 143 patients in total (11, 12, 22).
Overall Survival
One of the studies where EASM was performed and overall survival was reported, no significant differences were identified between EASM and open skin-conserving mastectomy. In other studies, a survival rate of 100% was reported for EASM with an average follow-up period of 12 months to 4 years (11, 12, 16, 21, 22).
Advantages
The most important advantages of endoscopically assisted breast surgery are “less scar”, “better cosmetic” and “more patient satisfaction.”
Disadvantages
Longer Operation Time
The reason behind is that work is done on a more limited surgical site. Furthermore, an influencing factor is that it is a new technique and requires training. The learning period also influences the learning period. The “Tumescent Technique” reduced the operation time (1, 8).
Additional Cost
EASM technique requires a new group of instruments and materials. The single-use instruments used in other laparoscopic surgeries have not yet been approved for endoscopic breast surgery. This deficiency creates a cost- and legislation-related problem in the implementation of this technique. For the solution, simple and re-usable instruments should be developed for the field of endoscopic breast surgery. In five studies reported in the literature, re-usable endoscopic retractors were used (1, 3, 7–9, 11, 20).
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.F.; Design - G.S.; Supervision - G.S.; Funding - G.S.; Materials - E.F.; Data Collection and/or Processing - E.F.; Analysis and/or Interpretation - G.S.; Literature Review - G.S.; Writer - G.S.; Critical Review - G.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authours declared that this study has received no financial support.
References
- 1.Ozaki S, Ohara M. Endoscopy-assisted breast-conserving surgery for breast cancer patients. Gland Surgery. 2014;3:94–108. doi: 10.3978/j.issn.2227-684X.2013.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee EK, Kook SH, Park YL, Bae WG. Endoscopy assissted breast conserving surgery for early breast cancer. World J Surg. 2006;30:957–964. doi: 10.1007/s00268-005-0202-y. http://dx.doi.org/10.1007/s00268-005-0202-y. [DOI] [PubMed] [Google Scholar]
- 3.Saimura M, Mitsuyama D, Anan K, Koga K, Watanabe M, Ono M, Toyoshima S. Endoscopy assisted breast conserving surgery for early breast cancer. Asian Endosc Surg. 2013;6:203–208. doi: 10.1111/ases.12018. http://dx.doi.org/10.1111/ases.12018. [DOI] [PubMed] [Google Scholar]
- 4.Park HS, Lee JS, Lee JS, Park S, Kim SI, Park BW. The feasibility of endoscopic-assisted breast conservation surgery for patients with early breast cancer. J Breast Cancer. 2011;11:52–57. doi: 10.4048/jbc.2011.14.1.52. http://dx.doi.org/10.4048/jbc.2011.14.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong YI, Shin H. Endoscopy-assisted breast conserving surgery for breast cancer: A preliminery clinical experience. J Breast Cancer. 2010;13:138–146. http://dx.doi.org/10.4048/jbc.2010.13.2.138. [Google Scholar]
- 6.Tamaki Y, Sakita I, Miyoshi Y, Sekimoto M, Takiguchi S, Monden M, Noguchi S. Transareolar endoscopy-assisted partial mastectomy: a preliminary report of six cases. Surg Laparosc Endosc Percutan Tech. 2001;11:356–362. doi: 10.1097/00129689-200112000-00003. http://dx.doi.org/10.1097/00129689-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita K, Shimizu K. Endoscopic video-assissted breast surgery: Procedures and short term results. J Nippon Med Sch. 2006;73:193–202. doi: 10.1272/jnms.73.193. http://dx.doi.org/10.1272/jnms.73.193. [DOI] [PubMed] [Google Scholar]
- 8.Ozaki S, Ohara M, Shigematsu H, Sasada T, Emi A, Masumoto N, Kadoya T, Murakami S, Kataoka T, Fujii M, Arihiro K, Okada M. Technical feasibility and cosmetic advantage of hybrid endoscopy assisted breast conserving surgery for breast cancer patients. J Laparoendosc Adv Surg Tech A. 2013;23:91–99. doi: 10.1089/lap.2012.0224. http://dx.doi.org/10.1089/lap.2012.0224. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima H, Fujiwara I, Mizuta N, Sakaguchi K, Hachimine Y. Video assisted skin-sparing breast-conserving surgery for breast cancer and immediate reconstruction with autologous tissue. Ann Surg. 2009;249:91–96. doi: 10.1097/SLA.0b013e31818e3fa6. http://dx.doi.org/10.1097/SLA.0b013e31818e3fa6. [DOI] [PubMed] [Google Scholar]
- 10.Serra-Renom JM, Serra-Mestre JM, Martinez L, D’Andrea F. Endoscopic reconstruction of partial mastectomy defects using latissimus dorsi muscle flap without causing scars on the back. Aest Plast Surg. 2013;37:941–949. doi: 10.1007/s00266-013-0192-3. http://dx.doi.org/10.1007/s00266-013-0192-3. [DOI] [PubMed] [Google Scholar]
- 11.Leff DR, Vashist R, Yongue G, Keshtgar M, Yang GZ, Darzi A. Endoscopic breast surgery: where are we now and what might the future hold for video-assisted breast surgery? Breast Cancer Res Treat. 2011;125:607–625. doi: 10.1007/s10549-010-1258-4. http://dx.doi.org/10.1007/s10549-010-1258-4. [DOI] [PubMed] [Google Scholar]
- 12.Fan LJ, Jiang J, Yang XH, Zhang Y, Li XG, Chen XC, Zhong L. A prospective study comparing endoscopic subcutaneous mastectomy plus immediate reconstruction with implants and breast conserving surgery for breast cancer. Chin Med J (Engl) 2009;122:2945–2950. [PubMed] [Google Scholar]
- 13.Sakamoto N, Fukuma E, Higa K, Ozaki S, Sakamoto M, Abe S, Kurihara T, Tozaki M. Early results of an endoscopic nipple sparing mastectomy for breast cancer. Ann Surg Oncol. 2009;16:3406–3413. doi: 10.1245/s10434-009-0661-8. http://dx.doi.org/10.1245/s10434-009-0661-8. [DOI] [PubMed] [Google Scholar]
- 14.Gould DJ, Huntb KK, Liu J, Kuerer HM, Crosby MA, Babiera G, Kronowitz SJ. Impact of surgical techniques, biomaterials, and patient variables on rate of nipple necrosis after nippleüsparing mastectomy. Plast Reconstr Surg. 2013;132:1–14. doi: 10.1097/PRS.0b013e31829ace49. http://dx.doi.org/10.1097/PRS.0b013e31829ace49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endara M, Chen D, Verma K, Nahabedian MY, Spear SL. Breast reconstruction following nipple sparing mastectomy: A systematic review of the literature with pooled analysis. Plast Reconstr Surg. 2013;132:1043–1054. doi: 10.1097/PRS.0b013e3182a48b8a. http://dx.doi.org/10.1097/PRS.0b013e3182a48b8a. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita K, Shimizu K. Transaxillary retromammary route approach of video-assisted breast surgery for breast conserving surgery. Am J Surg. 2008;196:578–581. doi: 10.1016/j.amjsurg.2008.06.028. http://dx.doi.org/10.1016/j.amjsurg.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Takemoto N, Koyanagi A, Yamamoto H. Comparison between endoscope assissted oartial mastectomy with filling of dead space using absorbabl mesh and conventional method on cosmetic outcome in patients with stage I or II breast cancer. Surg laparos Endosc Percutan Tech. 2012;22:68–72. doi: 10.1097/SLE.0b013e3182414b25. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi H, Fujii T, Nakagawa D, Inoue Y, Akashi M, Toh U, Iwakuma N, Takahashi T, Takenaka M, Fukuma E, Dhirouzu K. Usefulness od endoscopic breast conserving surgery for breast cancer. Surg Today. 2014;44:2037–2044. doi: 10.1007/s00595-013-0767-2. http://dx.doi.org/10.1007/s00595-013-0767-2. [DOI] [PubMed] [Google Scholar]
- 19.Ho WS, Ying SY, Chan ACW. Endoscopic assissted subcutaneous mastectomy and axillary dissection with immediate mammary prosthesis reconstruction for early breast cancer. Surg Endosc. 2002;16:302–306. doi: 10.1007/s004640000203. http://dx.doi.org/10.1007/s004640000203. [DOI] [PubMed] [Google Scholar]
- 20.Owaki T, Yoshinaka H, Ehi K, Kijima Y, Uenosono Y, Shirao K, Nakano S, Natsugoe S, Aikou T. Endoscopic quadrantectomy for breast cancer with sentinel lymph node navigation via a small axillary incision. Breast. 2005;14:57–60. doi: 10.1016/j.breast.2004.05.002. http://dx.doi.org/10.1016/j.breast.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Ito KI, Kanai T, Gomi K, Watanabe T, Ito T, Komatsu A, Fujita T, Amano J. Endoscopic assissted skin sparing mastectomy combined with sentinel node biopsy. ANZ J Surg. 2008;78:894–898. doi: 10.1111/j.1445-2197.2008.04687.x. http://dx.doi.org/10.1111/j.1445-2197.2008.04687.x. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura K, Ishida M, Inoue H, Kinoshita J, Hashizume M, Sugimachi K. Early results of an endoscope assissted subcutaneous mastectomy and reconstruction for breast cancer. Surgery. 2002;131:324–329. doi: 10.1067/msy.2002.120120. http://dx.doi.org/10.1067/msy.2002.120120. [DOI] [PubMed] [Google Scholar]
- 23.Tükenmez M, Ozden BC, Agcaoglu O, Kecer M, Ozmen V, Muslumanoglu M, Igcı A. Videoendoscopic single port nipple sparing mastectomy and immediate reconstruction. J Laparoendosc & Adv Surg Tech A. 2014;24:1–6. doi: 10.1089/lap.2013.0172. http://dx.doi.org/10.1089/lap.2013.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sosin M, Tousimia EA. Commentary on videoendoscopic single port nipple sparing mastectomy and immediate reconstruction. J Laparoendosc & Adv Surg Tech A. 2014;24:506–507. doi: 10.1089/lap.2014.0065. http://dx.doi.org/10.1089/lap.2014.0065. [DOI] [PubMed] [Google Scholar]
- 25.Sanuki J, Fukuma E, Wadamori K, Higa K, Sakamoto N, Tsunoda Y. Volume replacement with polyglycolic acid mes for correcting breast deformity after endoscopic conservative surgery. Clinical Breast Cancer. 2005;6:175. doi: 10.1016/s1526-8209(11)70718-2. http://dx.doi.org/10.1016/S1526-8209(11)70718-2. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima H, Fujiwara I, Mizuta N, Sakaguchi K, Hachimine Y, Magae J. Video-assisted skin sparing breast conserving surgery for breast cancer and immediate reconstruction with autologous tissue: clinical outcomes. Ann Surg Oncol. 2009;16:1982–1989. doi: 10.1245/s10434-009-0429-1. http://dx.doi.org/10.1245/s10434-009-0429-1. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura K, Hashizume M, Sugimachi K, Kataoka A, Ohno S, Kuwano H, Maehara Y. Early experience of endoscopic extirpation of benign breast tumors via an extra-mammary incision. Am J Surg. 1998;176:235–8. doi: 10.1016/s0002-9610(98)00143-3. http://dx.doi.org/10.1016/S0002-9610(98)00143-3. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura K, Inoue H, Ishida M, Kinoshita J, Hashizume M, Sugimachi K. Endoscopic extirpation of benign breast tumors using an extramammary approach. Am J Surg. 2001;181:211–4. doi: 10.1016/s0002-9610(01)00562-1. http://dx.doi.org/10.1016/S0002-9610(01)00562-1. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita K, Shimizu K. Video-assisted breast surgery and sentinel lymph node biopsy guided by three-dimensional computed tomographic lymphography. Surg Endosc. 2008;22:392–397. doi: 10.1007/s00464-007-9407-5. http://dx.doi.org/10.1007/s00464-007-9407-5. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita K, Shimizu K. Trans-axillary retro-mammary gland route approach of video-assisted breast surgery can perform breast conserving surgery for cancers even in inner side of the breast. Chin Med J (Engl) 2008;121:1960–1964. [PubMed] [Google Scholar]
- 31.Tamaki Y, Nakano Y, Sekimoto M, Sakita I, Tomita N, Ohue M, Komoike Y, Miyazaki M, Nakayama T, Kadota M, Monden M. Transaxillary endoscopic partial mastectomy for comparatively early-stage breast cancer. An early experience. Surg Laparosc Endosc. 1998;8:308–312. http://dx.doi.org/10.1097/00019509-199808000-00015. [PubMed] [Google Scholar]
- 32.Nakajima H, Sakaguchi K, Mizuta N, Hachimine T, Ohe S, Sawai K. Video-assisted total glandectomy and immediate reconstruction for breast cancer. Biomed Pharmacother. 2002;56:205s–208s. doi: 10.1016/s0753-3322(02)00281-0. http://dx.doi.org/10.1016/S0753-3322(02)00281-0. [DOI] [PubMed] [Google Scholar]


