Abstract
In industrial scale recombinant protein production it is often of interest to be able to translocate the product to reduce downstream costs, and heterologous proteins may require the oxidative environment outside of the cytoplasm for correct folding. High-level expression combined with translocation to the periplasm is often toxic to the host, and expression systems that can be used to fine-tune the production levels are therefore important. We previously constructed vector pJB658, which harbors the broad-host-range RK2 minireplicon and the inducible Pm/xylS promoter system, and we here explore the potential of this unique system to manipulate the expression and translocation of a host-toxic single-chain antibody variable fragment with affinity for hapten 2-phenyloxazol-5-one (phOx) (scFv-phOx). Fine-tuning of scFv-phOx levels was achieved by varying the concentrations of inducers and the vector copy number and also different signal sequences. Our data show that periplasmic accumulation of scFv-phOx leads to cell lysis, and we demonstrate the importance of controlled and high expression rates to achieve high product yields. By optimizing such parameters we show that soluble scFv-phOx could be produced to a high volumetric yield (1.2 g/liter) in high-cell-density cultures of Escherichia coli.
The application range of antibodies in medicine and biotechnology is broad, and there has been great progress in the design and selection of new variants with novel affinities. In particular, there has been a growing interest in the development of antibody fragments comprising the VH and VL domains connected to each other as a single chain (scFv) (4). The small size of scFv proteins (about 250 amino acids) compared to native antibodies confers certain therapeutic advantages because of their shorter half-life (rapid blood clearance) and faster tissue penetration. scFv molecules can be used as selective carriers for delivering radionucleids, toxins, or cytotoxic drugs to malignant cell populations, as well as providing valuable tools for studying antibody-antigen interactions in detail (14). In addition, this feature makes it possible to construct and screen large scFv libraries by using phage display approaches (for a review, see reference 23).
For medical applications scFvs are needed in large amounts, and the ability to produce high yields in Escherichia coli has gained considerable interest (14). Native scFv proteins have two disulfide bonds and require oxidative conditions to fold correctly. Although expression of native scFv proteins without disulfide bridge formation has been reported (9), cytoplasmic production in bacteria typically results in aggregation of scFv polypeptides into insoluble inclusion bodies (14). Therefore, in E. coli it is usually desirable to express scFvs as fusion proteins targeted for translocation to the oxidative periplasm to obtain functional products (3, 26). Various vector systems for recombinant scFv expression in E. coli have been reported (11, 15, 19), but the experiments were typically performed in shake-flask cultures with product yields of 10 to 30 mg/liter. Plasmid-based gene expression under high-cell-density cultivation may dramatically promote problems related to plasmid instability and lysis of recombinant cells (16, 28). Therefore, to obtain industrially relevant yields of soluble scFv, the expression tools should preferentially allow tight control of the expression level and the plasmids must be kept stable under high-cell-density cultivation.
We previously developed broad-host-range expression vectors based on the minimal replication elements oriV and trfA of the natural RK2 plasmid (5, 6). The trfA gene encodes a replication initiation protein also exerting negative control on replication from oriV (10, 12), and inducible expression of recombinant genes is governed by the flexible Pm/xylS promoter/regulator system. Relevant characteristics of these vectors include adjustable vector copy number, the Pm promoter is very strong and active in many hosts, several cheap and harmless (like benzoic acid) inducers can be used and they act in a dose-dependent manner, and inducer uptake is based on passive diffusion which further simplifies the use of the system across species barriers. With one such vector, pJB658, we have demonstrated regulated high and low level recombinant gene expression in several gram-negative bacterial species (6, 7, 29).
In the present study we utilize the unique properties of pJB658 to express a host-toxic antibody fragment, scFv-phOx, in high-cell-density cultivation of E. coli. By optimizing the vector copy number, induction conditions and choice of signal sequence, we produce recombinant scFv-phOx to very high volumetric yield (2.3 g/liter) of which 60% is represented as soluble and active product.
MATERIALS AND METHODS
Strains, plasmids, and DNA manipulations.
The strains and plasmids used in this study are listed in Table 1. Standard DNA manipulations were performed as described elsewhere (25).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | General cloning host | BRL |
| RV308 | Production strain | ATCC 31608 |
| Plasmids | ||
| pHOG21-phOx | ColE1 replicon harboring scFv-phOx fused to pelB, c-myc, and his6, Apr | 15 |
| pHKK | ColE1 replicon with lacP/O promoter-operator for recombinant transcription, hok-sok stabilization elements, Apr | 13 |
| pBR322 | ColE1 replicon, copy number of 30 per genome, Apr Tcr | NEB |
| pBR322Δamp | pBR322 with deleted bla gene, Tcr | This study |
| pJB658 | RK2-based expression vector harboring Pm/xylS regulatory promoter system for expression of cloned genes, Apr | 1 |
| pJB655cop271Cluc | Harbors trfA with cop-271 mutation | 2 |
| pJB655cop251Mluc | Harbors trfA with cop-251 mutation | 2 |
| pJBphOx | pJB658 with pelB-scFv-phOx-c-myc-his6 fusion gene downstream of Pm, harbors hok-sok suicide system (see text) | This study |
| pJBphOx-271 | pJBphOx with 1,853-bp ClaI/XmaI region substituted with corresponding region from pJB655cop271Cluc | This study |
| pJBphOx-251 | pJBphOx with 1,853-bp ClaI/XmaI region substituted with corresponding region from pJB655cop251Mluc | This study |
| pJB-251E | 8,350-bp pJBphOx-251 NdeI/NotI fragment blunt-ended and religated | This study |
| pJBphOx-271ompA | Similar to pJBphOx-271 but with pelB substituted with ompA (see text) | This study |
| pJBphOx-271CSP | Similar to pJBphOx-271 but with pelB substituted with consensus signal peptide (see text) | This study |
| pJBphOx-271ΔxylS | pJBphOx-271 was digested with AgeI/HpaI, cohesive ends of the large fragment were blunt-ended and religated (xylS) | This study |
| pJBphOx-251ΔxylS | pJBphOx-271 was digested with AgeI/HpaI, cohesive ends of the large fragment were blunt-ended and religated (xylS) | This study |
Some of the plasmids are described in the text. Ap, ampicillin; Tc, tetracycline.
Construction of vector pJBphOx.
The scFv-2-phenyloxazol-5-one (phOx) coding sequence fused to c-myc was PCR amplified from vector pHOG21-phOx with the following primer pair: scFv-F1, 5′-TTACTCGCGGCCCAGCCGGCCATGGCGCAGGTGCAGCTGGTGCAGTCT-3′, and scFv-R1, 5′-GTGATCGGCCCCCGAGGCCTTTAGGTCTTCTTCTGAGATCAGCTTTTGTTC-3′.
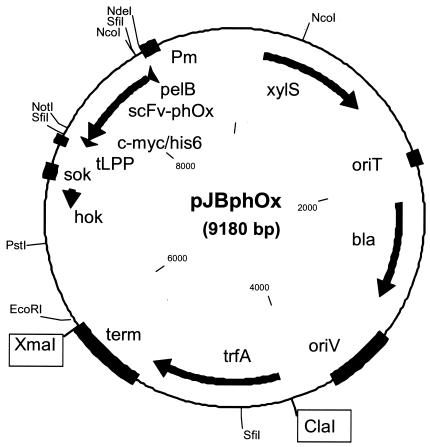
The PCR was performed on the Perkin Elmer GeneAmp PCR system 2400. The resulting 843-bp PCR product was end digested with SfiI (recognition sites are italic in the primer sequences) and cloned between the two SfiI sites of plasmid pHKK. From this construct, the 2,420-bp NdeI/EcoRI fragment was excised and cloned into the corresponding sites of plasmid pJB658, yielding vector pJBphOx (Fig. 1).
FIG. 1.
Physical map of the expression plasmid pJBphOx. Plasmid pJBphOx is based on the broad-host-range plasmid pJB658 (Table 1) and harbors the regulatory Pm/xylS promoter system for expression of scFv-phOx. Relevant genetic elements: tLPP, transcription terminator; term, bidirectional transcription terminator; bla, β-lactamase; oriT, origin for conjugative transfer; hok-sok, suicide system. For remaining elements, see the text.
Construction of the ompA and consensus signal peptide signal sequences.
The DNA molecules encoding OmpA and consensus signal peptide were made synthetically by annealing of the following single-stranded oligonucleotide pairs: ompA-F, 5′-TATGAAAAAAACTGCTATCGCTATCGCTGTTGCTCTGGCTGGTTTCGCTACTGTTGCTCAGGCGGCGGC-3′, and ompA-R, 5′-CATGGCCGCCGCCTGAGCAACAGTAGCGAAACCAGCCAGAGCAACAGCGATAGCGATAGCAGTTTTTTTCA-3′, and CSP-F, 5′-TATGAAAAAAAAATTATTGGCGTTAGCCTTGTTAGCGTTATTGTTTAACGGCGCGCAGGC-3′, and CSP-R, 5′-CATGGCCTGCGCGCCGTTAAACAATAACGCTAACAAGGCTAACGCCAATAATTTTTTTTTCA-3′.
Each pair of oligonucleotides were annealed as described previously (30) and the NdeI (2 bp) and NcoI (4 bp) sticky ends formed at the ends of the resulting double-stranded DNA fragments are in italic. The double-stranded DNA molecules obtained could be directly cloned into the NdeI/NcoI sites of any plasmid.
Media, feeding solutions, and preparation of inoculum to high-cell-density cultivation fermentations.
For routine cloning experiments and estimation of plasmid copy number, E. coli cells were grown at 37°C in liquid Luria broth (LB) or on solid Luria agar (LA) plates (25). The defined preculture medium and the main culture medium used were composed as described by others (13), whereas feeding solution 1 was slightly modified and contained glucose (480 g/kg solution) and MgSO4 · 7H2O (15.8 g/kg solution). All media, unless otherwise stated in the text, were supplemented with ampicillin (0.1 g/liter), and tetracycline (12 mg/liter) was used when appropriate.
The fermentation inoculum was prepared as follows. E. coli was grown overnight at 30°C on LA plates, and single colonies were transferred to 100 ml of LB medium in shake flasks (500 ml baffled) and incubated for 7 h at 30°C (200 rpm; orbital movement amplitude, 2.5 cm). Cells were diluted 200-fold into 100 ml of prewarmed preculture medium, and growth was continued at 30°C for 16 h to an optical density at 600 nm (OD600) of 4 to 5. From these cultures, cells were used to inoculate 0.75 liter of prewarmed main culture medium in the fermentor (3-liter Applikon) to an initial OD600 of 0.05. Inducer solution (0.5 M) was prepared by dissolving m-toluate in ethanol.
High-cell-density cultivation.
All fermentations were performed at 30°C, and the pH was maintained at 6.8 by addition of 12.5% (vol/vol) NH3. Antifoam (Adecanol LG-109; Asahi Denka Kogyo, Japan) was added from the start (40 μl/liter) and thereafter when needed. The dissolved oxygen was maintained at 20% saturation by automatic adjustments of the stirrer speed or by controlled feeding of feeding solution 1. The airflow rate was initially 0.25 liter/liter of medium per min and thereafter increased to 1.1 liter/liter of medium per min throughout the batch phase (see below). Inducer (m-toluate) was added to the fermentors when cell growth reached an OD600 of 90.
The fermentations were divided into three different phases as follows. (i) Batch phase. Glucose (22.5 g/liter) was added to the freshly inoculated main culture medium, and cell growth was continued until all the sugar had been consumed. (ii) Exponential feeding phase. Growth was controlled to a specific growth rate (μ) of 0.2 h−1 by feeding with feeding solution 1. Initially the feed rate of feeding solution 1 was set to 17 g/liter/h and thereafter increased exponentially. This exponential feeding was continued until the stirrer speed reached 1,900 rpm, which is close to the maximal speed of the reactor (2,100 rpm). (iii) Induction phase. Bacterial growth was controlled by automatic adjustments of the feeding solution 1 feeding rate (flexible feeding control) so that the dissolved oxygen and stirring speed were maintained at 20% saturation and 1,900 rpm, respectively. Throughout the fermentations, pH, dissolved oxygen, feeding rate, airflow, and the molar fraction of CO2 in the exhausted gas were monitored and recorded.
Determination of the fraction of plasmid-free cells.
Cell samples were collected from each high-cell-density cultivation culture and diluted in LB medium to approximately 1,000 CFU per ml and grown on LA plates without antibiotic selection at 30°C overnight. From these plates 192 single colonies were picked with the Genetic QpixII colony picker and individually transferred to 96-well plates loaded with 140 μl of LB medium supplemented with ampicillin (100 μg/ml). In addition, each colony was similarly transferred to 96-well plates without antibiotic selection. The well plates were incubated at 30°C overnight, and cell growth in individual wells was analyzed by visual inspection. Plasmid-free cells were detected based on the inability to grow in the selective medium (observed as clear medium).
Preparation of cell samples for protein product analyses by ELISA and Western blotting.
Cell samples (1 ml) were harvested by centrifugation (6,000 × g, 10 min) and the supernatants (medium) were collected. Periplasmic extraction was performed essentially as described elsewhere (21) by resuspension of the pellets in 0.5 ml of 50 mM Tris-HCl buffer, pH 8.0, addition of 0.5 ml of sucrose solution (40% sucrose containing 2 mM EDTA in 50 mM Tris-HCl buffer, pH 8.0), 1 mg of lysozyme per ml, and 125 U of benzonase (Merck) per ml. After incubation for 1 h at room temperature with shaking, the suspensions were centrifuged (16,000 × g, 8 min) and the supernatant (periplasm) and pellet fractions were collected.
Analysis of soluble scFv-phOx was performed by enzyme-linked immunosorbent assay (ELISA). Maxisorb plates (Nunc) were coated with phOx-bovine serum albumin (10 μg/ml) in Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 7.5, 150 mM NaCl) overnight at 4°C. All further incubations were performed for 1 h at room temperature. The wells were washed three times with TBS and blocked with Blotto (1% skim milk powder and 0.02% antifoam A in TBS). Serial dilutions of samples in Blotto were then added to the wells, and after washing three times with TBST (TBS containing 0.05% Tween), anti-c-Myc (Invitrogen) (1:5,000 in Blotto) was added. The wells were then washed three times with TBST, and rabbit anti-mouse immunoglobulin-peroxidase (Dako) (1:1,000 in Blotto) was added. Finally, the wells were washed three times with TBST and once with TBS, the substrate 2,2′-ethylbenzthiasolinesulfonic acid (ABTS) (Bio-Rad) was added, and the absorbance was read at 405 nm after 10 min.
Analysis of insoluble scFv-phOx in the pellet fractions was performed by Western blot. The pellets were added to 1 ml of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) running buffer (Bio-Rad) and 1-mm glass beads (KeboLab) and resuspended with vortexing for 1 to 2 min, and dilution series were made and prepared by boiling in denaturing sample buffer. The samples were separated by SDS-12.5% PAGE and transferred to a nitrocellulose membrane, and the membrane was blocked with Blotto. Recombinant scFv-phOx was detected with the same antibodies and washing buffers as described for the ELISA (above) except that tetramethylbenzidine (Pharmingen) was used as the substrate. Comparison of band intensities with standard scFv-phOx of known concentrations was performed and used to estimate the yields of insoluble scFv-phOx. The scFv-phOx standard used in both the ELISA and Western blot experiments had been purified by Ni-Sepharose (Amersham Bioscience) according to the supplier's recommendations, and the concentrations of scFv-phOx were calculated from the absorbance at 280 nm.
RESULTS AND DISCUSSION
Construction and testing of vector pJBphOx for the production of soluble scFv-phOx in high-cell-density cultivation of E. coli.
As a model protein in our study we chose scFv-phOx, which recognizes the hapten 2-phenyloxazol-5-one (phOx) (17). This important hapten can be easily conjugated to proteins and peptides and the clinical application of scFv-phOx for selective targeting of imaging and therapeutic agents in vivo has been demonstrated (8). In a previous report (15) the expression of 16.5 mg of soluble scFv-phOx/liter was obtained in E. coli shake-flask cultures with the pelB signal sequence. To our knowledge, no reports describe scFv-phOx expression under high-cell-density cultivation. The DNA fragment of plasmid pHOG21-phOx including the scFv-phOx gene fused to the c-myc coding region was cloned in frame between the pelB and the His6 coding sequences of plasmid pHKK (Table 1). From this construct the region encoding the pelB-scFv-phOx-c-myc-his6 fusion gene, as well as hok-sok, was excised and cloned downstream of the Pm promoter of pJB658, yielding plasmid pJBphOx (Fig. 1). In this way, scFv-phOx is expressed as a fusion protein targeted for translocation (governed by PelB) and with C-terminal tags (c-myc-his6) for facilitated detection and purification.
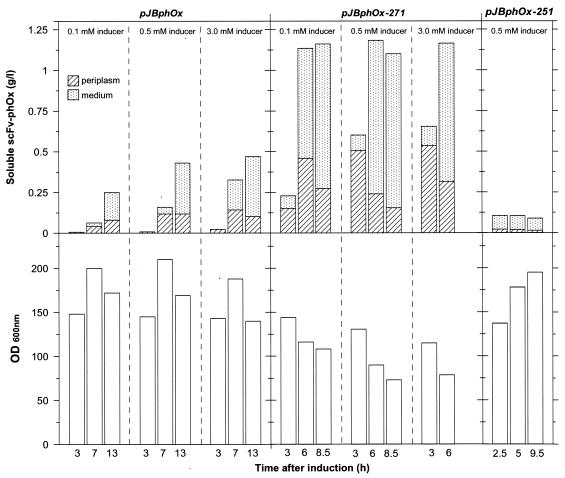
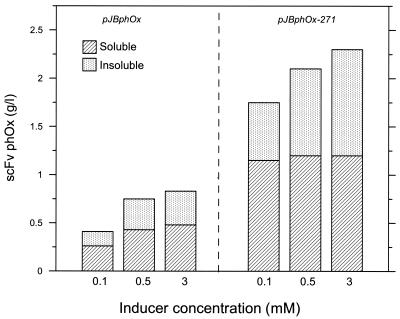
Three parallel fermentors with strain RV308 (pJBphOx) were run and induced at an OD600 of 90 (about 30 g dry weight per liter) by adding 0.1, 0.5, and 3.0 mM m-toluate. The production rate of soluble scFv-phOx increased as more inducer was added, and the highest yield was reached 13 h after induction in all cases (Fig. 2). At 3 mM inducer, 0.48 g/liter of soluble scFv-phOx was produced, which is comparable to the results obtained with similar proteins (13). Note that at 13 h most of the soluble product was localized in the growth medium. This may be due to toxic accumulation of scFv-phOx in the periplasm, leading to lysis of the producing cells (see OD600 values in Fig. 2). Representative samples were analyzed for insoluble product, and the results of these experiments (Fig. 3) showed that insoluble scFv-phOx yield also increased as more inducer was added, and it constitutes about 35 to 40% of the total scFv-phOx (soluble and insoluble) expressed. It seems probable that these insoluble fractions largely represent intracellular (not translocated) scFv-phOx proteins, while the formation of insoluble inclusion bodies in the periplasm could not be ruled out.
FIG. 2.
Volumetric yield of soluble scFv-phOx (top) and cell densities (bottom) at different induction levels (m-toluate) in fermentations with E. coli RV308 strains harboring pJBphOx (low copy number) and its derivatives pJBphOx-271 (medium copy number) and pJBphOx-251 (high copy number). In all cases inducer was added at an OD600 of 90. The fermentation with RV308(pJBphOx-271) with 3 mM inducer was terminated 6 h after induction due to foaming and control difficulties caused by cell lysis.
FIG. 3.
Total volumetric yields of scFv-phOx (soluble and insoluble) at different induction levels in fermentations obtained with RV308 strains harboring pJBphOx (low copy number) and pJBphOx-271 (medium copy number). Strains harboring pJBphOx-251 (high copy number) were not included in this experiment due to massive loss of this plasmid prior to induction (see the text).
Manipulations with pJBphOx copy number and effects on recombinant scFv-phOx production levels.
Plasmid pJB658 has a copy number of five to seven per genome (6) and from the results described above, we predicted that an elevated scFv-phOx gene dosage may accelerate its expression rate and thereby possibly lead to even higher levels of soluble product before cell lysis. To test this hypothesis, the 1,853-bp ClaI/XmaI fragment (including the trfA gene; see Fig. 1) was substituted by the corresponding fragment of plasmids pJB655cop271Cluc and pJB655cop251Mluc (Table 1). The expected copy numbers of the resulting derivatives, pJBphOx-271 and pJBphOx-251, should be about three- to fourfold and eightfold higher than that of the parental plasmid pJB658, respectively (6, 10, 12).
To experimentally verify these copy numbers, we took advantage of the ability to cotransform our recombinant strains with an additional replicon, pBR322, with a known copy number (about 30 per genome in E. coli). The 752-bp EcoRI-PstI region of pBR322 including the 5′ end of the β-lactamase gene was deleted to yield pBR322Δbla (Table 1), and this plasmid was introduced into E. coli strains harboring pJB658, pJBphOx, pJBphOx-271, and pJBphOx-251. The resulting recombinant strains were grown overnight, and dilution series of the plasmids isolated from these cultures were analyzed by gel electrophoresis. Comparison of the band intensities was used to estimate the copy number of each derivative, and the data obtained (not shown) were in good agreement with the corresponding expected values as indicated above.
Strain RV308(pJBphOx-271) was tested for production of scFv-phOx as described above, and the volumetric yield of soluble scFv-phOx obtained was more than twofold higher (1.2 g/liter) than with pJBphOx (Fig. 2), which is in agreement with the hypothesis above. Inducer concentrations above 0.1 mM had no positive effects on the final volumetric yield of soluble scFv-phOx, whereas the production rate in the first 3 h was higher with 0.5 mM. Presumably, a higher production rate leads to an accelerated release of soluble product into the culture medium (Fig. 2), and the relative fraction of released scFv-phOx increased with incubation time after induction. The maximum cell densities obtained with this strain (OD600 between 115 and 144) were lower than those obtained with cells harboring pJBphOx (OD600 of about 200) and decreased throughout the induction phase (Fig. 2). These observations support the assumption that high recombinant expression levels lead to rapid toxic accumulation of soluble scFv-phOx in the periplasm, resulting in cell lysis. In spite of this, a faster production rate allowed more total product to be made.
Analysis of RV308(pJBphOx-271) cells for insoluble product demonstrated that about twofold more insoluble scFv-phOx was produced with 3 mM compared to 0.1 mM inducer, even though the amount of soluble product did not increase (Fig. 3). This probably means that translocation is a limiting parameter for further improved production of soluble scFv-phOx under the present conditions, leading to a total production of about 2.3 g/liter of scFv-phOx.
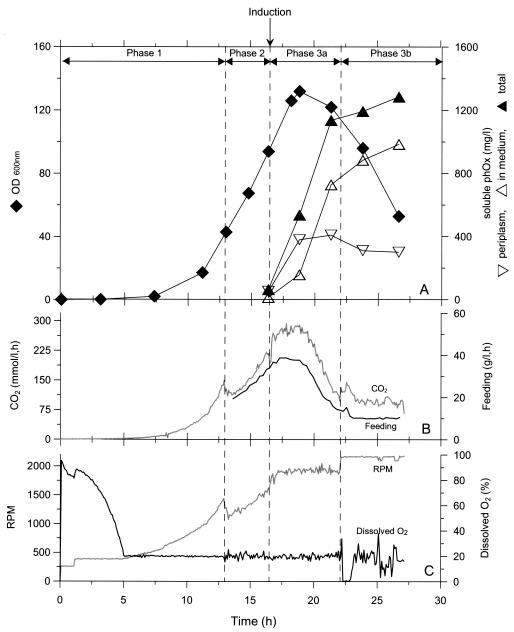
In order to analyze the production course of RV308(pJBphOx-271), the fermentation with this strain was repeated (five parallel fermentors) with an induction level of 0.5 mM, and a typical fermentation course is shown in Fig. 4. The volumetric yield of soluble scFv-phOx obtained in these experiments ranged from 1.1 to 1.3 g/liter, demonstrating good reproducibility. The production of scFv-phOx was initiated immediately after the induction, and 90% of the soluble scFv-phOx was produced within 6 h (phase 3a in Fig. 4A). In this period the cell density reached its maximal value and then gradually dropped. The drop in optical density was accompanied by a substantial leakage of scFv-phOx to the extracellular environment, consistent with cell lysis.
FIG. 4.
Fermentation course of recombinant RV308 (pJBphOx-271) under high-cell-density cultivation. Time courses of bacterial growth (OD600) and scFv-phOx production (A), exhausted CO2 and feeding rate (B), and stirrer speed and dissolved oxygen (C). Phases 1 and 2 are the batch and exponential feeding phase of the fermentation, respectively (see Materials and Methods). The time of induction (addition of 0.5 mM m-toluate) is marked with an arrow. Phases 3a and b represent the initial and prolonged induction phases, respectively. In phase 3a, the flexible feeding solution 1 control was applied. In phase 3b, constant feeding and stirrer rates were used.
In the induction phase (phase 3a, Fig. 4) the feeding rate and dissolved oxygen are maintained constant at the setpoint values by automatic adjustments of the feeding rate. The feeding rate is at its highest level at induction start and is thereafter automatically gradually reduced to one-third of the maximum rate during the next 6 h (phase 3a in Fig. 4B). The reduction of the feeding rate is a consequence of reduced oxygen transfer capacity in the fermentation broth caused by cell lysis. In order to push the system to a maximum level of production, the induction phase was prolonged beyond 6 h (phase 3b in Fig. 4). To control the foaming caused by cell lysis, substantial amounts of antifoam were added, leading to problems with the automatic control of the fermentor. The prolonged induction phase had little impact on the volumetric yield (additional 10%), and the optical density continued to drop throughout this period.
It could not be excluded that production yield of soluble scFv-phOx could be further improved by increasing the plasmid copy number beyond that of pJBphOx-271. Surprisingly, irrespective of the inducer concentration applied (data not shown), the maximum level of soluble scFv-phOx produced by strain RV308(pJBphOx-251) was only 0.15 g/liter (Fig. 2), which is even lower than that of cells harboring the parental vector. The reasons for this unexpected result could later be shown to correlate with extensive plasmid loss before induction (see below).
Effects of high plasmid copy numbers on plasmid stability and viability of recombinant cells.
It is generally known that the metabolic burden associated with elevated plasmid copy numbers and/or high recombinant gene expression levels may lead to impaired cell growth and concomitant plasmid loss from recombinant cells (1, 2). We analyzed the various fermentation cultures for plasmid-free cells, and the results of these experiments demonstrated that the low (pJBphOx) and medium (pJBphOx-271) copy number vectors were maintained throughout the fermentation. In contrast, vector pJBphOx-251 was found to be highly unstable, and about 90% of the cells originally harboring this vector were already plasmid-free prior to induction.
We speculated that this massive plasmid loss could be due to high leakage of scFv-phOx expression from Pm, and to test this we measured soluble scFv-phOx levels in the fermentor cultures prior to induction. Only traces (below 5 mg/liter) of recombinant protein were detected with the low-copy number plasmid pJBphOx, while significant levels of soluble scFv-phOx were detected with pJBphOx-271 (50 mg/ml) and pJBphOx-251 (120 mg/liter). Considering that the majority of the cells in the latter culture were plasmid-free under these conditions (see above), this result suggested that the leakage from Pm in strain RV308 (pJBphOx-251) is high.
To rule out the possibility that the observed plasmid loss was somehow caused by the vector itself, the 830-bp region including the scFv-phOx-encoding gene was deleted from pJBphOx-251, yielding construct pJB-251E (Table 1). Recombinant strains harboring this plasmid were analyzed as described above, and the results showed that this derivative was 100% stable during the entire fermentation period, supporting the assumption that the instability of pJBphOx-251 is related solely to leaky expression of scFv-phOx.
Both data previously reported by us (6) and the results presented here suggest that uninduced expression from Pm increases much more than the increase in copy number, and it has been reported that high XylS levels may accelerate constitutive transcription from Pm (24). To investigate this, we deleted the 776-bp region including the 5′-terminal region of xylS and upstream sequences of the copy-up derivatives pJBphOx-271 and pJBphOx-251, yielding plasmids pJBphOx-271ΔxylS and pJBphOx-251ΔxylS, respectively (Table 1). Recombinant RV308 strains harboring these constructs produce only 5 and 30 mg/liter of soluble scFv-phOx, respectively, under noninduced conditions, which is far below the levels detected with the parental vectors (see above). Interestingly, no plasmid-free cells were detected in these cultures. Together, these results indicate that high basal expression from Pm present in a high-copy-number vector is mediated solely by the simultaneous presence of a correspondingly increased copy number of the xylS gene. By controlling the xylS expression level from a regulated promoter, the problems with plasmid loss at high copy numbers might be avoided, and one might possibly also envision improved yields of scFv-phOx or other protein products of interest.
Plasmid pJBphOx-271 is stably maintained under high-cell-density cultivation without antibiotic selection.
The numbers of generations obtained in the preculture medium and the main culture medium under the conditions used in the present study are 9 and 11 to 12, respectively. To further test the stability of our expression vectors, high-cell-density cultivation fermentation of RV308(pJBphOx-271) was performed without antibiotic selection. Moreover, to obtain conditions comparable to a large-scale fermentation, preculture medium cultivation was repeated twice before transfer to the main culture medium, resulting in approximately 30 generations for the three successive cultivations. As a control, a parallel experiment was run with the standard amount of ampicillin (0.1 g/ml) added to the media. The results of these experiments demonstrated that pJBphOx-271 was stably maintained during the entire fermentations and the production levels of soluble scFV-phOx were also similar (data not shown). The hok-sok suicide elements have been shown to significantly enhance the stability when present on other replicons (20), but their direct contribution to the stability observed in our constructs remains unknown. In any case, the option of running industrial processes without antibiotics in the production medium confers both economic and regulatory advantages.
Different signal sequences display considerable effects on the expression levels of recombinant scFv-phOx.
In E. coli it has been shown that the first codons at the 5′ end of a structural gene may have a severe impact on translation initiation (27). In our constructs it is likely that the 5′-terminally fused pelB sequence, which is of bacterial origin, contributes to effective expression of scFv-phOx in addition to governing translocation. Although the majority of the scFv-phOx expressed in our strains is presumably exported, we could not rule out that translocation is limiting for higher yields of soluble scFv-phOx under the conditions tested. In order to test this, we applied the two alternative signal sequences ompA and consensus signal peptide. The former has been used for effective translocation of various recombinant proteins in E. coli (22), whereas consensus signal peptide was designed here based on published sequence alignments of several bacterial signal peptides (18). Both signal sequences were made synthetically (see Materials and Methods) and used to substitute pelB in pJBphOx-271, yielding derivatives pJBphOx-271ompA and pJBphOx-271CSP (Table 1).
Recombinant strains harboring these plasmids produced soluble scFv-phOx at significantly lower yields (0.15 and 0.43 g/liter, respectively) than the strain harboring the parental vector. We proceeded to measure insoluble scFv-phOx in these samples, and the data showed that these levels were also low (0.10 g/liter for OmpA and 0.17 g/liter for consensus signal peptide). Together our results indicate that the overall scFv-phOx expression levels obtained with OmpA and consensus signal peptide are low compared to those obtained with PelB. Thus, our results show that the choice of signal sequence has a strong impact on both the translocation and expression level of recombinant scFv-phOx under the conditions tested.
Acknowledgments
We are grateful to Uwe Horn for kindly providing us with plasmid pHKK. We thank Inger Løberslie, Anne Tøndervik, and Marianne Korsnes for helping with scFv-phOx analyses and certain cloning experiments, Mona Senneset for help with fermentations, and Geir Klinkenberg for help with programming the control systems needed for the high-cell-density cultivation fermentations.
The work was financed by Affitech AS and the Research Council of Norway.
REFERENCES
- 1.Bentley, W. E., N. Mirjalili, D. C. Andersen, R. H. Davis, and D. S. Kompala. 1990. Plasmid encoded protein: The principal factor in the “metabolic nurden” associated with recombinant bacteria. Biotechnol. Bioeng. 35:668-681. [DOI] [PubMed] [Google Scholar]
- 2.Betenbaugh, M. J., C. Beaty, and P. Dhurjati. 1989. Effects of plasmid amplification and recombinant gene expression on the growth kinetics of recombinant E. coli. Biotechnol. Bioeng. 33:1425-1436. [DOI] [PubMed] [Google Scholar]
- 3.Better, M., C. P. Chang, R. R. Robinson, and A. H. Horwitz. 1988. Escherichia coli secretion of an active chimeric antibody fragment. Science 242:1041-1043. [DOI] [PubMed] [Google Scholar]
- 4.Bird, R. E., K. D. Hardman, J. W. Jacobsen, S. Johnson, B. M. Kaufman, S-M. Lee, T. Lee, S. H. Pope, G. S. Riordan, and M. Whitlow. 1988. Single-chain antigen-binding proteins. Science 242:423-426. [DOI] [PubMed] [Google Scholar]
- 5.Blatny, J. B., T. Brautaset, H. C. Winther-Larsen, K. Haugan, and S. Valla. 1997. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl. Environ. Microbiol. 63:370-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blatny, J. B., T. Brautaset, H. C. Winther-Larsen, P. Karunakaran, and S. Valla. 1997. Improved broad-host-range RK2 vectors useful for high and low regulated gene expression levels in gram-negative bacteria. Plasmid 38:35-51. [DOI] [PubMed] [Google Scholar]
- 7.Brautaset, T., S. B. Petersen, and S. Valla. 2000. In vitro determined kinetic properties of mutant phosphoglucomutases and their effects on sugar catabolism in Escherichia coli. Metab. Eng. 2:104-114. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, T. L., Liao, K. W., Tzou, S. C., Cheng, C. M., Chen, B. M., and Roffler, S. R. 2004. Hapten-directed targeting to single-chain antibody receptors. Cancer Gene Ther. 11:380-388. [DOI] [PubMed] [Google Scholar]
- 9.Donini, M., V. Morea, A. Desidero, D. Pashkoulov, M. E. Villani, A. Tramontano, and E. Benvenuto. 2003. Engineering stable cytoplasmic intrabodies with designed specificity. J. Mol. Biol. 330:323-332. [DOI] [PubMed] [Google Scholar]
- 10.Durland, R. H., A. Toukdarian, F. Fang, and D. R. Helinski. 1990. Mutations in the trfA replication gene of the broad-host-range plasmid RK2 result in elevated plasmid copy number. J. Bacteriol. 172:3859-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo, J.-Q., S-Y. You, L. Li, Y-Z. Zhang, J.-N. Huang, and C-Y. Zhang. 2003. Construction and high-level expression of a single-chain Fv antibody fragment specific for acidic isoferritin in Escherichia coli. J. Biotechnol. 102:177-189. [DOI] [PubMed] [Google Scholar]
- 12.Haugan, K., P. Karunakaran, A. Tøndervik, and S. Valla. 1995. The host-range of RK2 minimal replicon copy up mutants is limited by species-specific differences in the maximum tolerable copy number. Plasmid 33:27-39. [DOI] [PubMed] [Google Scholar]
- 13.Horn, U., W. Strittmatter, A. Krebber, U. Knüpfer, M. Kujau, R. Wenderoth, K. Müller, S. Matzku, A. Plückthun, and D. Riesenberg. 1996. High volumetric yields of functional dimeric miniantibodies in Escherichia coli, using an optimized expression vector and high-cell-density fermentation under non-limited growth conditions. Appl. Microbiol. Biotechnol. 46:524-532. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys, D. P. 2003. Production of antibodies and antibody fragments in Escherichia coli and a comparison of their functions, uses and modification. Curr. Opin. Drug Discov. Dev. 6:188-196. [PubMed] [Google Scholar]
- 15.Kipriyanov, S. M., G. Moldenhauer, and M. Little. 1997. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. J. Immunol. Methods 200:69-77. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, P. K. R., H-E. Maschke, K. Friehs, and K. Schügerl. 1991. Strategies for improving plasmid stability in genetically modified bacteria in bioreactors. Trends Biotechnol. 9:279-284. [DOI] [PubMed] [Google Scholar]
- 17.Marks, J. D., A. D. Griffiths, D. Andrew, M. Malmquist, T. Clackson, J. Bye, and G. Winter. 1992. By-passing immunization: building high affinity human antibodies by chain shuffling. Bio/Technology 10:779-783. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 19.Oelschlaeger, P., S. Lange, J. Schmitt, M. Siemann, M. Reuss, and R. D. Schmid. 2003. Identification of factors impeding the production of a single-chain antibody fragment in Escherichia coli by comparing in vivo and in vitro expression. Appl. Microbiol. Biotechnol. 61:123-132. [DOI] [PubMed] [Google Scholar]
- 20.Pecota, D. C., C. S. Kim, K. Wu, K. Gerdes, and T. K. Wood. 1997. Combining the hok/sok, parDE, and pnd postsegregational killer loci to enhance plasmid stability. Appl. Environ. Microbiol. 63:1917-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce, J. J., C. Turner, E. Keshacarz-Moore, and P. Dunnhill. 1997. Factors determining more efficient large-scale release of a periplasmic enzyme from E. coli using lysozyme. J. Biotechnol. 58:1-11. [DOI] [PubMed] [Google Scholar]
- 22.Pines, O., and M. Inouye. 1999. Expression and secretion of proteins in E. coli. Mol. Biotechnol. 12:25-34. [DOI] [PubMed] [Google Scholar]
- 23.Pini, A., and L. Bracci. 2000. Phage display of antibody fragments. Curr. Protein Peptide Sci. 1:155-169. [DOI] [PubMed] [Google Scholar]
- 24.Ramos, J. L., C. Michan, F. Rojo, D. Dwyer, and T. Timmis. 1990. Signal-regulator interactions: Genetic analysis of the effector binding site of XylS, the benzoate-activated positive regulator of Pseudomonas TOL plasmid meta-cleavage pathway operon. J. Mol. Biol. 211:373-382. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Skerra, A., and A. Plückthun. 1988. Assembly of functional immunoglobulin Fv fragment in Escherichia coli. Science 240:1038-1041. [DOI] [PubMed] [Google Scholar]
- 27.Stenström, C. M., E. Holmgren, and L. A. Isakson. 2001. Cooperative effects by the initiation codon and its flanking regions on translation initiation. Gene 273:259-265. [DOI] [PubMed] [Google Scholar]
- 28.Summers, D. K. 1999. The kinetics of plasmid loss. Trends Biotechnol. 9:273-278. [DOI] [PubMed] [Google Scholar]
- 29.Winther-Larsen, H. C., K. D. Josefsen, T. Brautaset, and S. Valla. 2000. Parameters affecting gene expression from the Pm promoter in gram-negative bacteria. Metab. Eng. 2:79-91. [DOI] [PubMed] [Google Scholar]
- 30.Winther-Larsen, H. C., J. B. Blatny, B. Valand, T. Brautaset, and S. Valla. 2000. Pm promoter expression mutants and their use in broad-host-range RK2 plasmid vectors. Metab. Eng. 2:92-103. [DOI] [PubMed] [Google Scholar]