Abstract
Objective
US elastography is an emerging technique that can be used during breast US examination. The increasing awareness of breast cancer led to an increase in mammography and breast US examinations. The specificity of these techniques is not high enough to prevent unnecessary biopsies. There is still a need for a more specific technique that can overcome this problem. This study aimed to evaluate the value of strain elastography in breast lesions.
Materials and Methods
In this study, 110 lesions of 96 patients were evaluated with strain elastography. Five score system was used for lesion scoring. The histopathologic results of lesions were obtained and were accepted as gold standard. The sensitivity, specificity, PPV and NPV of the technique were calculated. Histopathologic and strain elastography results were correlated.
Results
The sensitivity of US strain elastography was calculated as 83%, the specificity as 89%, the positive predictive value as 79% and the negative predictive value as 91%. There were no score 1 lesions. All score 2 lesions were benign. Score 5 had the highest true positivity rate.
Conclusion
We believe that ultrasound elastography is an effective imaging technique that can be used as an adjunct for differential diagnosis, prior to the decision to biopsy a lesion in certain cases.
Keywords: Elastography, breast elastography, breast lesions, sonoelastography
Introduction
Breast screening and diagnostic breast imaging provides early diagnosis of breast cancer. Although breast imaging modalities have high sensitivity rates, there is still need for a higher specificity in imaging to rule out malignancy in incidentally found breast lesions. Especially ultrasonography (US) examination can detect more malign masses with lower specificity, which leads to a high number of unnecessary biopsies (1, 2). US elastography, which shows the stiffness of the lesion, is being widely used in recent years. It determines the change of the size of the lesion in neutral position and under pressure. Generally, breast cancers are hard due to fibrous desmo-plastic reaction and are expected to remain stable during pressure application. There are 2 different types of techniques widely used for determination of the rigidity of the lesion with US; strain elastography and shear wave elastography techniques. In strain elastography, the observer or the patient produces the pressure either with the probe or by breathing whereas in the shear wave technique that pressure is formed by the US probe by a special sound wave, which is called shear wave (3). In this study, we aimed to evaluate the value of strain elastography technique in breast lesions by correlation of elastographic features with histopathological results.
Materials and Methods
Patients
In this study, 110 lesions of 96 patients, who were admitted for biopsy of a breast lesion, were evaluated prospectively between December 2009 and September 2010. The mean age of patients was 50 (range 19–87) years. An ethical committee approval was obtained from Medical Research Ethnic Committee of our university, and an informed consent was taken from all patients.
Conventional Breast US and US Elastography
Conventional and elastographic US studies were done with a linear matrix 5–13 MHz transducer and Siemens Antares US machine (Erlangen, Germany). All the patients underwent B mode US evaluation before elastography examination. All B mode findings were evaluated according to BI-RADS. US elastography was performed while the patient was in supine position and the transducer was placed vertical to the lesion. Compression was applied with the probe over the lesion and elastographic images were examined. The elasticity region of interest (ROI) was placed to cover the lesion and the target lesion was placed in the center.
Elastography US studies were evaluated with the color scale, which changes from blue to red according to the straining component of the examined tissue. The blue colour is seen in tissues with higher tension (soft) and red in tissues without any tension (hard). The mean tension is reflected with green color. The images of elastography were added to B-mode images. Elastography was performed by the same radiologist and the images were evaluated in real time by two radiologists. The elastography application lasted approximately 5–10 minutes and the measurement of tissue distortion was visually classified.
Elastographic images were classified according to the 5-score system of Ueno and Itoh et al (4, 5). The lesion was classified as score 1 for even strain in the entire lesion, as score 2 for strain in most of the lesion with some areas of no strain (mosaic pattern), as score 3 for strain at the periphery of the lesion with sparing of the center, as score 4 for no strain in the entire lesion and as score 5 for no strain in the entire lesion and the surrounding area (Figure 1–4). Scores 1 to 3 were considered benign while 4 and 5 were accepted as malignant.
Figure 1.
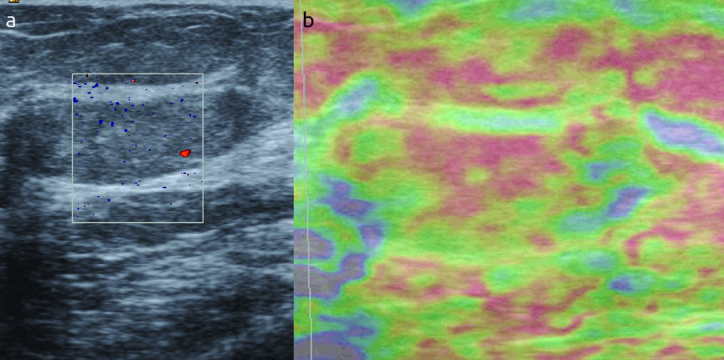
a, b. An example of score 2 in a thirty eight years old woman. (a) Mass on B-mod, (b) after compression. The histopathologic result is fibrocystic changes after tru-cut biopsy
Figure 2.
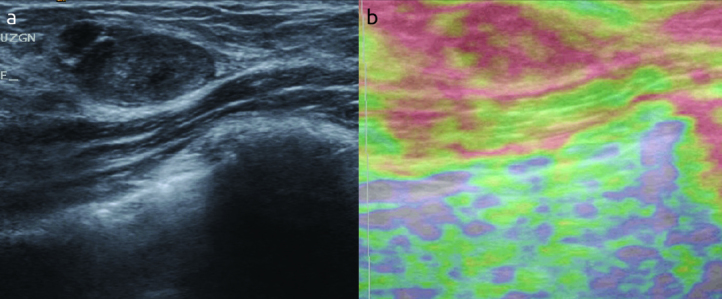
a, b. An example of score 3 in a twenty six years old woman. (a) on B-mod US, (b) after compression. The is colour changes in mass to red with some areas not showing the red color. (mosaic pattern). The histopathologic result is fibroadenoma
Figure 3.
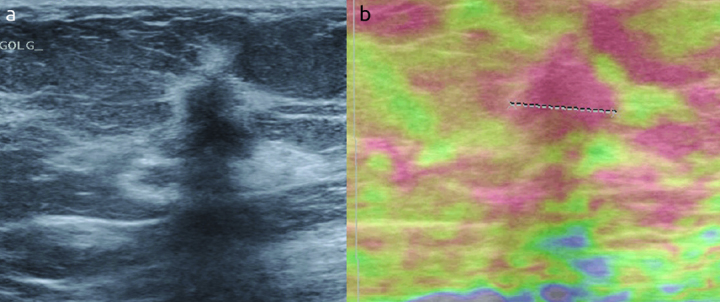
a, b. An example of score 4 in a eighty five years old woman. (a) The lesion on B-mod, (b) after compression the lesion is red but not the surrounding tissue. The histopathologic result is reported as invasive ductal carcinoma
Figure 4.
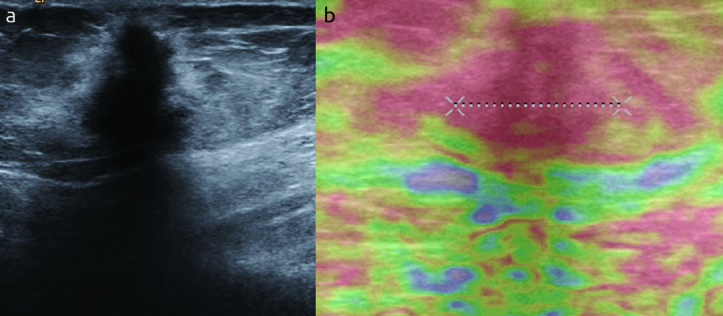
a, b. An example of score 5 in a thirty six years old woman. (a) Mass is seen on B-mod, (b) after compression the lesion and the surrounding tissue turns to red. The histopathologic result is reported as invasive ductal carcinoma
Histopathological Diagnosis
Samples were taken with 14 G core needle biopsy or excisional biopsy and evaluated by the same pathologist. The histopathological results of lesions were correlated with the elastographic findings.
Statistical Analysis
Diagnostic sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of elastography technique were evaluated by comparing with histopathologic results. The correlation between elastographic results and histopathologic findings was tested with Fisher test. SPSS 12.0 software was used for analysis and p<0,05 was accepted as significant.
Results
In this study, 110 lesions of 96 patients were examined with US and classified according to BI-RADS for B mode findings. In 96 patients the mean age was 50 (range 19–87). The mean diameter of the lesions was 1.9 cm (range 0.5–9 cm). Core needle biopsy was performed in 102 lesions and 8 patients with single lesion underwent excisional biopsy. In US elastography, 110 lesions were scored. There were no score 1 lesions. Fifty lesions were classified as score 2, 21 lesions as score 3, 17 lesions as score 4 and 22 lesions as score 5.
The histopathological evaluation of 110 lesions showed 37 malignant lesions (33.7%) while the rest was benign. In the comparison of histopathologic findings with US elastography; all 50 lesions of score 2 were benign; 15 of 21 score 3 lesions were benign while 6 were malignant; 6 of 17 score 4 lesions were benign and 11 were malignant; 20 of 22 score 5 lesions were malignant while 2 were benign.
The sensitivity of US strain elastography was calculated as 83%, the specificity as 89%, the positive predictive value as 79% and the negative predictive value as 91% (Table 1). In the individual analysis for different scores; NPV was found as 100% for score 2 and 72% for score 3, and PPV was identified as 65% for score 4 and 91% for score 5.
Table 1.
True positive (TP), true negative (TN), false positive (FP) and false negative (FN) values for elastography scores
| Score 2 | Score 3 | Score 4 | Score 5 | Sum | |
|---|---|---|---|---|---|
| TP | 0 | 0 | 11 | 20 | 31 |
| TN | 50 | 15 | 0 | 0 | 65 |
| FP | 0 | 0 | 6 | 2 | 8 |
| FN | 0 | 6 | 0 | 0 | 6 |
| SUM | 50 | 21 | 17 | 22 | 110 |
Sensitivity: 83%, Specificity: 89%, PPV: 79%, NPV: 91%, p<0.001 TP: True positive; TN: true negative; FP: false positive; FN: false negative
The distribution of 37 malignant lesions were as follows; 25 (68%) invasive ductal carcinoma, 5 (13%) invasive lobular carcinoma, 4 (10%) mixed invasive ductal and lobular carcinoma, 1 (3%) solid type mucinous carcinoma, 1 (3%) invasive-atypical medullary carcinoma, and 1 (3%) intracystic papillary carcinoma.
There were 8 false positive and 6 false negative results. Among the 8 false positive lesions; 2 patients with phylloides tumor (40 mm and 50 mm in size, respectively) were interpreted as score 5, three complex fibro adenomas (10 mm, 10 mm and 20 mm in size, respectively), two intraductal papilloma (30 mm and 26 mm in size, respectively) and one lacteal adenoma (43 mm in size) were classified as score 4.
Among the 6 (16%) false negative results, one solid type mucinous carcinoma (17 mm in size), one invasive lobular carcinoma (37 mm in size), one invasive ductal carcinoma (90 mm in size), one micro invasive ductal carcinoma in situ (20 mm in size), one invasive atypical medullary carcinoma (35 mm in size) and one mixed invasive ductal and lobular carcinoma (7 mm in size) were classified as score 3. None of the malignant lesions were classified as score 2 or 1.
Discussion and Conclusions
Breast biopsy generally increases the cost per individual if used as a gold standard for differentiation of malignant and benign lesions of the breast. Especially in breast cancer screening programs, approximately 75% of breast biopsies are performed for benign lesions (1). B mode US features of breast lesions were well defined by Stavros et al. (2) which guided in the differentiation of benign and malignant breast masses, and decreased the need for biopsies. Although the sensitivity of B mode in conventional US is high (98%) for these lesions, the specificity is still low (68%) (2). US elastography is a recent technique that may improve classification of these lesions and may increase the specificity of US. Recently US elastography became an important tool in the evaluation of soft tissues.
Ueno and Itoh (4, 5) offered the first elasticity grading system. In this 5 score classification system color spectrum changes are taken in a dynamic form, and it is suggested to have a higher specificity in differential diagnosis. This scoring system has been used in several studies and the effectiveness of elastography as an adjunct to B mode ultrasonography was investigated (6–15). Modified versions of Ueno’s classification by Scaperrotta et al (16) and Regini et al (17) also confirmed higher specificity for US elastography. Scaperrotta et al. (16) modified the classification system that was used by Itoh et al., and suggested a grading system with 3 scores. They reported the sensitivity and specificity of this system as 80% and 80.9%, respectively. In this study, the authors have concluded that elastography can increase the specificity of conventional ultrasonography up to 88.7%, especially in non-palpable lesions. Although some studies did not detect an improvement in specificity with the addition of US elastography (14, 17), a number of other studies showed an increase in specificity (6–13). Cho et al. (10) studied 100 non-palpable breast lesions and found the sensitivity as 82% and the specificity as 84%, and concluded that there were no significant differences between conventional US and elastography in the evaluation of solid breast masses. They reported that biopsy was not required in BI-RADS category 4a lesions with an elastography score of 1 (11). Burnside et al. (18) identified an increase in the mean specificity (0.257 vice versa 0.132, P<0.001) and sensitivity (0.993 vice versa 0.997, P>0.99) when US tension imaging is compared along with B-mode US. They concluded that the inter and intra-observer variability in the evaluation of US tension imaging affected the diagnostic performance and imaging quality, and that elastography mode helped less experienced performers more than the more experienced ones in terms of detection of malignant lesions. Two recent meta-analysis on strain elastography reported sensitivity rates of 88% and 83% and specificity rates of 83% and 84% (19, 20). Our findings are comparable with the recent literature with a sensitivity of 83% and specificity of 89%. The positive predictive value was 79% and negative predictive value was 91%.
In our study, four of six malignant lesions in the false negative group and six of eight benign lesions in the false positive group were larger than 2 cm in size. The false negative and positive results may have been due to the large size of these lesions, as reported in some previous studies (6).
There were five benign lesions with elastography scores of 2, with at least three malignant features and were classified as BI-RADS category 4b according to B mode US. The rest 45 lesions were either BI-RADS 3 or 4a. All 50 lesions with an elasticity score of 2 were proved to be benign histopathologically, and the NPV for score 2 was calculated as 100%. We believe that lesions evaluated as score 2 can be accepted as benign, unless they present suspicious malignant features on B Mode US. If this property had been taken into consideration, the biopsy of 45 of these 50 lesions (90%) could have been prevented. Our findings are comparable with the recent literature that biopsies of BI-RADS 3 and 4 a lesions can be prevented with the aid of strain elastography (10, 21, 22).
When histopathological results of lesions with an elasticity score of 3 are compared, 15 of 21 lesions were benign, with 6 false negative malignancies. Among these, three lesions had high risk features according to B Mode US findings on the gray scale ultrasonography and was classified as BI-RADS 4c category. One of them was identified as mixed invasive ductal and lobular carcinoma 7 mm in size, the other one as an invasive lobular carcinoma with 37 mm in size, and the last one as invasive ductal carcinoma 90 mm in size. The other 3 lesions were either BI-RADS 4a or b lesions. The histopathological findings were medullary carcinoma, mucinous carcinoma and micro invasive ductal carcinoma in situ. Although in general breast cancer is stiff, ( 4, 8 internet) some cancer types may have benign features depending on their histopathologic subtypes and lead to misdiagnosis (5, 6, 16). Ductal carcinoma in situ, invasive lobular, papillary, mucinous, and medullary carcinomas, and some tumors with central necrosis may be softer than invasive ductal carcinoma with a fibrous desmo-plastic reaction (5, 7, 9). We identified similar findings in our results with a high false negative ratio (28%) in lesions with an elasticity score of 3. The evaluation of these score 3 lesions should be done with care, and they should be compared with other imaging findings.
In this study, we accepted elasticity score 4 as malignant. When compared with the histopathological results; 6 of the 17 lesions were benign with a 91% false positivity rate. Three of these 6 lesions had BI-RADS 4b category and the remaining three were 4a. Especially different subtypes of fibroadenomas can show variable features sometimes depending on their development or involution phase (5, 6, 16).
Twenty of the 22 lesions classified as score 5 by elastography were malignant. Score 5 had a high rate of PPV (91%). Both of the false positive lesions were phylloides tumors. These patients should be managed and followed-up diligently if they are found to be benign on histopathologic evaluation.
Our study limitations were as follows:
First; Most of our lesions were large in size with a diameter higher than 20 mm. There were only a few lesions smaller than 5 mm. This may be considered as a limitation as there are studies implying higher sensitivity in smaller lesions. Giuseppetti et al. (6) reported a better diagnostic performance in lesions smaller than 2 cm. On the other hand, Scaperrotta et al (16) did not find statistically significant differences in the diagnostic performance between small and large lesions. A recent meta-analysis of Sadigj G et al. (23) showed that regardless of the lesion size, US elastography had a higher specificity and lower sensitivity as compared to B mode US in characterizing breast masses.
Second; the effects of breast density and composure on elastography results were not evaluated in our study. The studies imply that extreme differences in the density of the breast parenchyma may cause false negative or positive findings as elasticity depends on the surrounding tissue (17).
Third; there are limitations related to the technique itself. When compared to shear wave elastography, strain elastography is clearly operator dependent and depends on subjective analysis without quantitative measurements. It is crucial to keep steady compression with the probe, avoiding lateral or angulated movements, to obtain optimal images. The operator should place enough normal surrounding tissue inside the ROI for a precise assessment. Besides, intraobserver or interobserver variability was not taken into account.
US strain elastography has a high accuracy rate in the differential diagnosis of breast lesions. In this study, elastography scores 4 and 5 lesions were accepted as malignant and the sensitivity was calculated as 83%, specificity as 89%, positive predictive value as 79% and the negative predictive value as 91%. These findings demonstrate the efficacy of US elastography in routine clinical practice. Lesions with elastography score of 2 can be assessed as benign, unless they have BI-RADS scores higher than 4a, and thus unnecessary biopsies can be prevented. Lesions with elastographic score of 5 have a high risk for malignancy, so the histopathologic and radiologic correlation of these lesions must be done carefully. Follow-up for malignancy should be recommended in these patients, if biopsy findings do not reveal malignancy.
We believe that ultrasound elastography is an effective imaging technique that can be used as an adjunct for differential diagnosis of breast lesions, prior to the decision to biopsy a lesion in certain cases.
Footnotes
Ethics Committee Approval: Ethics committee approval was received from Marmara University for this study.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.A.; Design - E.A., A.O.A.; Supervision - E.A., A.O.A., H.K.; Funding - E.A., A.O.A., H.K.; Materials - E.A., A.O.A., H.K.; Data Collection and/or Processing - A.O.A; Analysis and/or Interpretation - E.A., A.O.A.; Literature Review - A.O.A., R.E.; Writer - A.O.A., R.E.; Critical Review - E.A.
Financial Disclosure: This study was conducted by a Grant received form BAP Department of the University.
Conflict of Interest: No conflict of interest was declared by the authors.
References
- 1.Poplack SP, Carney PA, Weiss JE, Titus-Ernstoff L, Goodrich ME, Tosteson AN. Screening mammography: costs and use of screening-related services. Radiology. 2005;234:79–85. doi: 10.1148/radiol.2341040125. http://dx.doi.org/10.1148/radiol.2341040125. [DOI] [PubMed] [Google Scholar]
- 2.Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123–134. doi: 10.1148/radiology.196.1.7784555. http://dx.doi.org/10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- 3.Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: Principles and technique. Diagnostic and Interventional Imaging. 2013;94:487–495. doi: 10.1016/j.diii.2013.01.022. http://dx.doi.org/10.1016/j.diii.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Ueno E, Iboraki P. Clinical application of US elastography in the diagnosis of breast disease. Paper presented at: European Congress of Radiology; March 5–9, 2004; Vienna, Austria. [Google Scholar]
- 5.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. http://dx.doi.org/10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 6.Giuseppetti GM, Martegani A, Di Cioccio B, Baldassarre S. Elastosonography in the diagnosis of the nodular breast lesions: preliminary report. Radiol Med. 2005;110:69–76. [PubMed] [Google Scholar]
- 7.Thomas A, Fischer T, Frey H, Ohlinger R, Grunwald S, Blohmer JU, Winzer KJ, Weber S, Kristiansen G, Ebert B, Kümmel S. Real-time Elastography: an advanced method of ultrasound-first results in 108 patients with breast lesions. Ultrasound Obstet Gynecol. 2006;28:335–340. doi: 10.1002/uog.2823. http://dx.doi.org/10.1002/uog.2823. [DOI] [PubMed] [Google Scholar]
- 8.Tardivon A, El Khoury C, Thibault F, Wyler A, Barreau B, Neuen-schwander S. Elastography of the breast: a prospective study of 122 lesions [in French] J Radiol. 2007;88:657–662. doi: 10.1016/s0221-0363(07)89872-6. http://dx.doi.org/10.1016/S0221-0363(07)89872-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhi H, Ou B, Luo BM, Feng X, Wen YL, Yang HY. Comparison of ultrasound elastography, mammography, and sonography in the diagnosis of solid breast lesions. J Ultrasound Med. 2007;26:807–815. doi: 10.7863/jum.2007.26.6.807. [DOI] [PubMed] [Google Scholar]
- 10.Cho N, Moon WK, Park JS, Cha JH, Jang M, Seong MH. Nonpalpable breast masses: evaluation by US elastography. Korean J Radiol. 2008;9:111–118. doi: 10.3348/kjr.2008.9.2.111. http://dx.doi.org/10.3348/kjr.2008.9.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan SM, Teh HS, Mancer JF, Poh WT. Improving B-mode ultrasound evaluation of breast lesions with real-time ultrasound Elastography: a clinical approach. Breast. 2008;17:252–257. doi: 10.1016/j.breast.2007.10.015. http://dx.doi.org/10.1016/j.breast.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhu QL, Jiang YX, Liu JB, Liu H, Sun Q, Dai Q, Chen X. Real-time ultrasound elastography: its potential role in assessment of breast lesions. Ultrasound Med Biol. 2008;34:1232–1238. doi: 10.1016/j.ultrasmedbio.2008.01.004. http://dx.doi.org/10.1016/j.ultrasmedbio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Vanhoutte A, Fellah L, Galant C, d’Hoore W, Berlière M, Leconte I. Contribution of sonoelastography to the characterization of breast lesions. JBR-BTR. 2008;91:187–194. [PubMed] [Google Scholar]
- 14.Sohn YM, Kim MJ, Kim EK, Kwak JY, Moon HJ, Kim SJ. Sonographic elastography combined with conventional sonography: how much is it helpful for diagnostic performance? J Ultrasound Med. 2009;28:413–420. doi: 10.7863/jum.2009.28.4.413. [DOI] [PubMed] [Google Scholar]
- 15.Raza S, Odulate A, Ong EM, Chikarmane S, Harston CW. Using real-time tissue elastography for breast lesion evaluation: our initial experience. J Ultrasound Med. 2010;29:551–563. doi: 10.7863/jum.2010.29.4.551. [DOI] [PubMed] [Google Scholar]
- 16.Scaperrotta G, Ferranti C, Costa C, Mariani L, Marchesini M, Suman L, Folini C, Bergonzi S. Role of sonoelastography in non-palpable breast lesions. Eur Radiol. 2008;18:2381–2389. doi: 10.1007/s00330-008-1032-8. http://dx.doi.org/10.1007/s00330-008-1032-8. [DOI] [PubMed] [Google Scholar]
- 17.Regini E, Bagnera S, Tota D, Campanino P, Luparia A, Barisone F, Durando M, Mariscotti G, Gandini G. Role of sonoelastography in characterising breast nodules: preliminary experience with 120 lesions. Radiol Med. 2010;115:551–562. doi: 10.1007/s11547-010-0518-z. http://dx.doi.org/10.1007/s11547-010-0518-z. [DOI] [PubMed] [Google Scholar]
- 18.Burnside ES, Hall TJ, Sommer AM, Hesley GK, Sisney GA, Svensson WE, Fine JP, Jiang J, Hangiandreou NJ. Differentiating benign from malignant solid breast masses with US strain imaging. Radiology. 2007;245:401–410. doi: 10.1148/radiol.2452061805. http://dx.doi.org/10.1148/radiol.2452061805. [DOI] [PubMed] [Google Scholar]
- 19.Sadigh G, Carlos RC, Neal CH, Dwamena BA. Accuracy of quantitative ultrasound elastography for differentiation of malignant and benign breast abnormalities: a meta-analysis. Breast Cancer Res Treat. 2012;134:923–931. doi: 10.1007/s10549-012-2020-x. http://dx.doi.org/10.1007/s10549-012-2020-x. [DOI] [PubMed] [Google Scholar]
- 20.Gong X, Xu Q, Xu Z, Xiong P, Yan W, Chen Y. Real-time elastography for the differentiation of benign and malignant breast lesions: a meta-analysis. Breast Cancer Res Treat. 2011;130:11–18. doi: 10.1007/s10549-011-1745-2. http://dx.doi.org/10.1007/s10549-011-1745-2. [DOI] [PubMed] [Google Scholar]
- 21.Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, Ohlinger R, Mendelson EB, Balu-Maestro C, Locatelli M, Tourasse C, Cavanaugh BC, Juhan V, Stavros AT, Tardivon A, Gay J, Henry JP, Cohen-Bacrie C BE1 Investigators. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012;262:435–449. doi: 10.1148/radiol.11110640. http://dx.doi.org/10.1148/radiol.11110640. [DOI] [PubMed] [Google Scholar]
- 22.Schäfer FK, Hooley RJ, Ohlinger R, Hahne U, Madjar H, Svensson WE, Balu-Maestro C, Juhan V, Athanasiou A, Mundinger A, Order B, Locatelli M, Cosgrove D, Wolf OJ, Henry JP, Moutfi M, Gay JM, Cohen-Bacrie C. ShearWave™ Elastography BE1 multinational breast study: additional SWE™ features support potential to downgrade BI-RADS®-3 lesions. Ultraschall Med. 2013;34:254–259. doi: 10.1055/s-0033-1335523. http://dx.doi.org/10.1055/s-0033-1335523. [DOI] [PubMed] [Google Scholar]
- 23.Sadigh G, Carlos RC, Neal CH, Wojcinski S, Dwamena BA. Impact of breast mass size on accuracy of ultrasound elastography vs. conventional B-mode ultrasound: a meta-analysis of individual participants. Eur Radiol. 2013;23:1006–1014. doi: 10.1007/s00330-012-2682-0. http://dx.doi.org/10.1007/s00330-012-2682-0. [DOI] [PubMed] [Google Scholar]


