Abstract
Breast cancer is the second leading cause of cancer throughout the world, however neuroendocrine tumors of the breast are rarely encountered. Herein we present a 75-year-old patient who was admitted to our clinic due to a mass in her breast and was operated on with a preliminary diagnosis of invasive breast carcinoma, However she was diagnosed with a neuroendocrine tumor after pathologic evaluation. The patient is the oldest one among others with a neuroendocrine tumor in the breast reported in the literature.
Keywords: Neuroendocrine tumors, breast cancer, mastectomy
Introduction
Breast cancer originates from cells within the breast. It has the second highest incidence in the world after lung cancer. Invasive ductal carcinoma, which originates from the ducts within the breast, constitutes 80% of breast cancers, whereas neuroendocrine tumors account for 0.1%. The incidence of both synchronous and metachronous cancers are higher in patients with neuroendocrine tumors when compared to the general population (1). Herein we presented a patient who was operated at our department for breast cancer, and diagnosed with neuroendocrine tumor. Further evaluations did not detect any synchronous or metachronous tumors.
Case Presentation
Seventy five-year-old female patient was referred to our clinic for evaluation of a palpable mass in her right breast that she noticed two months ago. She had four children and a history of breast-feeding each of them for at least one year. She had neither a history of using oral contraceptives or hormone replacement therapy, nor a family history for breast cancer. Her last mammography taken 3 years ago was normal. She did not comply with her routine annual follow-up appointments. On physical examination, a mass in 3×2×2 cm size was palpated at 11 o’clock position in her right breast. Both the right axilla and the left breast were normal. On mammography, a mass lesion with spicular extensions was observed on her right breast. The breast ultrasound revealed a retroareolar, hypoechoic solid mass measuring 31×21 mm and having regular borders, at 11 o’clock position on the right breast. A tru-cut biopsy was performed under ultrasound guidance, and the biopsy result was reported as invasive carcinoma.
Immunohistochemical test results showed positive nuclear staining for ER 50%, and PR 75%. C-erb b2 was negative (Score 0). Based on the findings, the patient was informed on skin sparing and modified radical mastectomy. She consented for modified radical mastectomy and was planned for surgery. Her laboratory results and chest X-ray was normal. The patient was discharged on the second postoperative day, her surgical drain and sutures were removed on the 7th postoperative day.
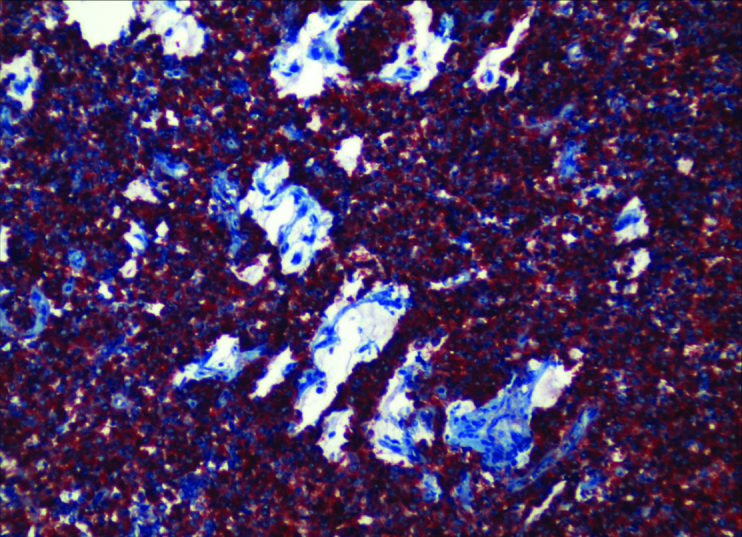
The pathological examination showed carcinoma with neuroendocrine differentiation (Figure 1, 2, 3). The pathological stage of the tumor according to WHO (World Health Organization) 2010 classification was grade 2. Mucinous differentiation was 10% and there was no intraductal component. The mitosis count was 2/10 under 10 × magnification. Ki-67 index was 5% (Figure 4). On immunohistochemical evaluation, positive nuclear staining for ER 50%, and PR 75% was detected, with negative C-erb b2 (Score 0). There was diffuse strong positive staining for chromogranin, and diffuse moderate positive staining for synaptophysin.
Figure 1.
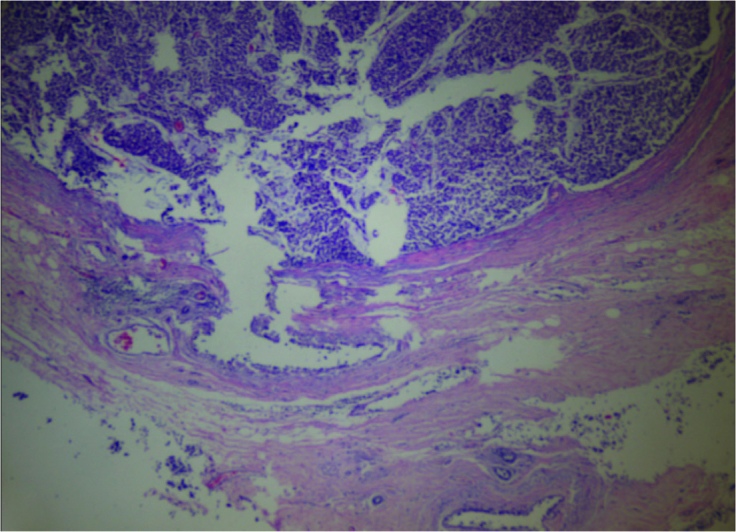
Normal breast tissue and tumor (H&E×40)
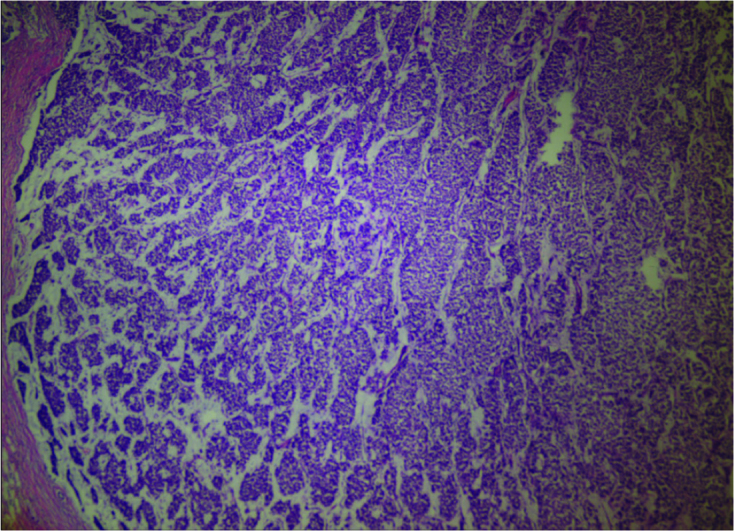
Figure 2.
Breast solid tumor and extracellular mucin (HE×100)
Figure 3.
Breast Neuroendocrin Carcinoma (Kromogranin ×200)
Figure 4.
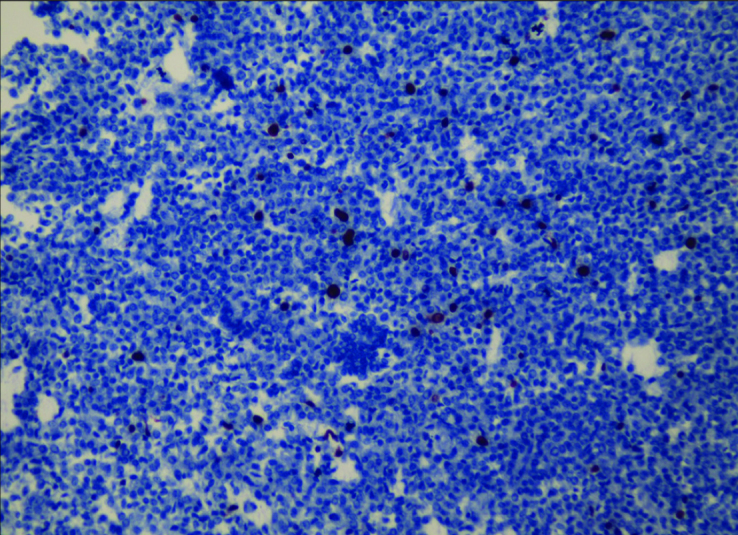
Breast Neuroendocrin Carcinoma (Ki 67×200)
The patient received adjuvant chemoradiotherapy. Abdominal and pelvic computed tomography did not reveal any synchronous tumors. Her colonoscopy and gastroscopy examinations were also normal. She is being followed-up with no additional pathologies.
Discussion and Conclusions
The incidence of breast cancer is increasing in our country, as well as the rest of the world. The Ministry of Health reported breast cancer incidence in Turkey as 37.3/100,000 in 2006, whereas this rate is currently estimated to be 50/100.000 (2). Although Pagotti and colleagues (3) mentioned that as high as 8% of breast tumors are neuroendocrine tumors, neuroendocrine variants constitute 0.1% of all breast tumors (4). Neuroendocrine tumors are derived from submucosal neuroendocrine cells, and the diagnosis can only be made by pathologic examination. Although there is no agreement on the origin of cells causing breast neuroendocrine tumors, it is believed that they arise from pluripotent stem cells (5). The presence of neuroendocrine tumors even in organs that do not contain neuroendocrine cells, such as the ovary and prostate, supports this hypothesis. Since 1983, 40 cases of neuroendocrine tumor of the breast have been reported in the literature. Their ages ranged from 40 to 70 years, the majority being over 60 years of age (6). According to Samli et al. (7) main complaints related to this disease are breast cellulitis, mastitis, breast abscess, and inflammatory events, nevertheless, our patient’s complaint was the presence of a mass in her breast. In 2003, WHO has classified neuroendocrine tumors of the breast by using immunohistochemical reagents such as chromagranin and synaptophysin, in a similar fashion to neuroendocrine tumors of the gastrointestinal system and lung (8). Synchronous tumors are almost an integral part of neuroendocrine tumors of the breast. Lopez-Bonet et al. (9) studied 1,368 neuroendocrine breast tumors, and they reported that only seven patients did not have a second tumor. In the case presented here, there was no secondary tumor and it was accepted as a primary neuroendocrine carcinoma of the breast. On physical examination, neuroendocrine tumors of the breast are found as round and irregular masses. Ultrasound and mammography are inadequate for differential diagnosis from other tumors and on MRI, homogeneous signal intensity is observed. Histological examinations of neuroendocrine tumors of the breast reveal similar results to neuroendocrine tumors located elsewhere in the body (10). Surgical treatment of breast cancer has shifted from the classic en-bloc radical mastectomy defined by Halsted to modified radical mastectomy and skin sparing breast surgery, without compromising oncologic results (11). Surgical treatment of neuroendocrine tumors of the breast is similar to other types of breast cancer. In the postoperative period, chemotherapy regimens, which include 5-fluorouracil, epirubicin, and cyclophosphamide for 6 cycles and radiotherapy are administered, and this is followed by tamoxifen therapy for 5 years (12). We used a protocol of adjuvant therapy that contained 5-fluorouracil, epirubicin and cyclophosphamide and radiotherapy, to our patient.
In conclusion, primary neuroendocrine tumor of the breast is a rare entity that may be detected at any age. Its treatment includes surgery and chemoradiotherapy protocols similar to other types of breast cancer, and the patient should be evaluated for the presence of synchronous tumors.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.A.B., A.K.; Design - M.A.B.; Supervision - M.U.K., H.A.; Funding - A.K., M.A.B., Y.Ö.; Materials - A.K., Y.Ö.; Data Collection and/or Processing - M.A.B.; Analysis and/or Interpretation - M.A.B., M.U.K.; Literature Review - M.A.B., H.A.; Writer - MAB.; Critical Review - A.K., H.A.
Financial Disclosure: The authors declared that this study has received no financial support.
Conflict of Interest: No conflict of interest was declared by the authors.
References
- 1.Prommegger R, Ensinger C, Steiner P, Sauper T, Profanter C, Margreiter R. Neuroendocrine tumors and second primary malignancy, a relationship with clinical impact? Anticancer Res. 2004;24:1049–1051. [PubMed] [Google Scholar]
- 2.Polat AK, Soran A. Metastatik meme kanseri tanısı alanlarda primer tümör cerrahisinin yeri. Ulusal Cerrahi Dergisi. 2010;26:185–191. [Google Scholar]
- 3.Pagotti M, Marci L, Finzi G, Capella C, Eusebi V, Bussolati G. Neuroendocrine differentiation in carcinoma of the breast: A study of 51 cases. Semin Diagn Pathol. 1989;6:174–188. [PubMed] [Google Scholar]
- 4.Günhan BI, Zekioglu O, Ustün EE, Memis A, Erhan Y. Neuroendocrine differentiated breast carcinoma: imaging features correlated with clinical and histophatological findings. Eur Radiol. 2003;13:788–793. doi: 10.1007/s00330-002-1567-z. [DOI] [PubMed] [Google Scholar]
- 5.Adegbola T, Connolly CE, Mortimer G. Small cell neuroendocrine carcinoma of the breast: a report of three cases and review of the literature. J Clin Pathol. 2005;58:775–778. doi: 10.1136/jcp.2004.020792. http://dx.doi.org/10.1136/jcp.2004.020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nawawi O, Goh KY, Rahmat K. A Rare Case of Primary Infiltrating Neuroendocrine Carcinoma of the Breast. Iran J Radiol. 2012 Nov;9:212–216. doi: 10.5812/iranjradiol.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samli B, Celik S, Evrensel T, Orhan B, Tasdelen I. Primary neuroendocrine small cell carcinoma of the breast. Arch Pathol Lab Med. 2000;124:296–298. doi: 10.5858/2000-124-0296-PNSCCT. [DOI] [PubMed] [Google Scholar]
- 8.Böcker W. WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics. Verh Dtsch Ges Pathol. 2002;86:116–119. [PubMed] [Google Scholar]
- 9.López-Bonet E, Alonso-Ruano M, Barraza G, Vazquez-Martin A, Bernadó L, Menendez JA. Solid neuroendocrine breast carcinomas: Incidence, clinico-pathological features and immunohistochemical profiling. Oncol Rep. 2008;20:1369–1374. [PubMed] [Google Scholar]
- 10.Alonso-Ruano M, López-Bonet E, Huerta-Anaya MV, Vila-Camps E, Bernadó L, Tuca-Rodríguez F, Suarez-Pumariega P, Menendez JA. Synchronous solid neuroendocrine breast carcinoma and abdominal lymphoma: a case report and review of the literature. oncol lett. 2013;5:459–462. doi: 10.3892/ol.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Çakabay B, Aksel B, Ünal AE, Bayar S, Kocaoğlu H, Demirci S, Akgül H. Primer meme kanserinin lokal kontrolünde radyofrekans ablasyon bir seçenek olabilir mi? Ulusal Cerrahi Dergisi. 2011;27:55–59. [Google Scholar]
- 12.Watrowski R, Jäger C, Mattern D, Horst C. Neuroendocrine carcinoma of the breast--diagnostic and clinical implications. Anticancer Res. 2012;32:5079–5082. [PubMed] [Google Scholar]


