Abstract
The specific biofilm formation (SBF) assay, a technique based on crystal violet staining, was developed to locate plant essential oils and their components that affect biofilm formation. SBF analysis determined that cinnamon, cassia, and citronella oils differentially affected growth-normalized biofilm formation by Escherichia coli. Examination of the corresponding essential oil principal components by the SBF assay revealed that cinnamaldehyde decreased biofilm formation compared to biofilms grown in Luria-Bertani broth, eugenol did not result in a change, and citronellol increased the SBF. To evaluate these results, two microscopy-based assays were employed. First, confocal laser scanning microscopy (CLSM) was used to examine E. coli biofilms cultivated in flow cells, which were quantitatively analyzed by COMSTAT, an image analysis program. The overall trend for five parameters that characterize biofilm development corroborated the findings of the SBF assay. Second, the results of an assay measuring growth-normalized adhesion by direct microscopy concurred with the results of the SBF assay and CLSM imaging. Viability staining indicated that there was reduced toxicity of the essential oil components to cells in biofilms compared to the toxicity to planktonic cells but revealed morphological damage to E. coli after cinnamaldehyde exposure. Cinnamaldehyde also inhibited the swimming motility of E. coli. SBF analysis of three Pseudomonas species exposed to cinnamaldehyde, eugenol, or citronellol revealed diverse responses. The SBF assay could be useful as an initial step for finding plant essential oils and their components that affect biofilm formation and structure.
Selected natural products that originate in plants can influence microbial biofilm formation. For example, halogenated furanones, a class of compounds that inhibit biofilm formation by interfering with bacterial quorum sensing, were identified in a marine alga and are thought to have evolved to reduce biofouling (34). Other plant-derived compounds inhibit peptidoglycan synthesis (24), damage microbial membrane structures (10), modify bacterial membrane surface hydrophobicity (35), and modulate quorum sensing (14), all of which could influence biofilm formation. Terrestrial plants also support populations of surface-attached bacteria (3, 23) and could potentially produce phytochemicals that attenuate biofilm development through specific mechanisms. However, many plant essential oils, which are mixtures of numerous organic chemicals, contain compounds that inhibit microbial growth (2, 8, 32). Thus, a screening procedure to identify phytochemicals with specific antibiofilm activity must take into account the cytotoxicity of plant essential oils.
Crystal violet (CV) staining, a colorimetric method, has been used widely to measure biofilm formation in part because of its amenability to large screening procedures (25, 26). For many applications, particularly screening assays for surface adhesion-deficient mutants (25, 27), measuring the absolute amount of biofilm formed by CV staining is suitable. To search for antibiofilm compounds in growth-inhibitory plant essential oils, the CV assay must be modified to measure the amount of biofilm formed relative to overall growth. Also, it is generally assumed that CV binds proportionally to biomass, although there are multiple physical, chemical, and biological factors that could influence the binding of CV to biofilms. These factors include (i) structural factors that affect dye diffusion, (ii) morphological and physiological differences in individual cells that influence dye binding, and (iii) chemical interactions between plant essential oil components and CV. A direct microscopy-based evaluation of biofilm formation to assess CV-based measurements of biofilm production would help validate this approach.
In this paper we describe a CV assay that measures growth-normalized biofilm accumulation, referred to as specific biofilm formation (SBF), for locating plant essential oil components that affect biofilm formation. The SBF method is a modification of a technique employed by Pratt and Kolter (27), and like all CV staining methods, it is a method for indirect determination of biofilm formation. To evaluate the relevance of the SBF assay, two microscopic techniques were employed. First, biofilms cultivated in flow cells were examined by confocal laser scanning microscopy (CLSM) and quantitatively analyzed by using the software package COMSTAT (18). Second, adhesion was investigated by direct microscopy using the specific cell adhesion (SCA) assay, also introduced in this work. By using the SBF assay, three essential oils with different effects on biofilm formation by E. coli were compared, and the effects of their principal chemical components on biofilm structure, adhesion, cell morphology, viability, and swimming motility were measured. Finally, the SBF assay was used to investigate biofilm formation by three species of Pseudomonas following exposure to plant essential oil components.
MATERIALS AND METHODS
Strains and culture conditions.
The following strains were used in this study: Escherichia coli ATCC 33456 (36), Pseudomonas aeruginosa PAO1 (Pseudomonas Genetic Stock Center, Greenville, N.C.), Pseudomonas putida KT2440 (29), and Pseudomonas fluorescens pSMC21 (5). All strains were cultured in Luria-Bertani (LB) broth. E. coli ATCC 33456 and P. aeruginosa PAO1 were grown at 37°C; P. putida KT2440 and P. fluorescens pSMC21 were grown at 30°C.
Chemicals.
Cinnamaldehyde and citronellol were obtained from Aldrich (Milwaukee, Wis.); eugenol was purchased from Acros Organics (Morris Plains, N.J.). All solvents were analytical grade. All plant essential oils were obtained from Aura Cacia (Weaverville, Calif.). Crystal violet solution was obtained from Becton Dickinson (Sparks, Md.).
Toxicity analyses.
Fourteen-milliliter capped polystyrene culture tubes (17 by 100 mm; Fisher Scientific, Pittsburgh, Pa.) containing 3 ml of LB medium and plant essential oils at various concentrations were inoculated with 30 μl of cells in the log phase of growth. For each concentration tested, tubes were prepared in triplicate and incubated in an orbital shaker (200 rpm). Growth was determined turbidimetrically (600 nm) at the initiation of the experiment and after 1.5 h (E. coli) or 2.5 h (Pseudomonas spp.). The essential oils that were tested were oils of Cinnamomum camphora (camphor), Cinnamomum cassia (cassia), Cinnamomum zeylanicum (cinnamon), Melaleuca alternifolia (tea tree), Cymbopogon nardus (citronella), and Zingiber officinale (ginger). The plant essential oils were diluted in methanol (20%, vol/vol) prior to use; cinnamaldehyde, eugenol, and citronellol were diluted in methanol (4%, vol/vol).
SBF assay.
Bacteria were grown in 14-ml polystyrene culture tubes containing 2 ml of LB medium and various concentrations of essential oils or individual chemicals. Compounds to be tested were dissolved in methanol as described above, and the total volume added never exceeded 1.5% of the culture volume (the amount of methanol added had no observable effect on growth). Nine identically prepared tubes were used for each concentration. Three of the nine tubes were used to measure growth in suspended culture (G tubes), three tubes were used to measure biofilm growth (B tubes), and three tubes served as controls for abiotic factors (NC tubes).
Inocula were grown to the late log phase. Subsequently, the B and G tubes received 20 μl of inoculum, and all tubes (B, G, and NC tubes) were incubated in an orbital cabinet shaker for 17 ± 1 h. Following incubation, cells in the G tubes were mixed well, and the optical densities at 600 nm (OD600) of the cultures were measured. The B and NC tubes each received 125 μl of a 0.3% solution of CV. After 15 min, the suspended culture was poured out, and the tubes were rinsed well with distilled deionized water (six rinses, approximately 4 ml per rinse). Any remaining crystal violet was dissolved in 2 ml of an ethanol-acetone (80:20) solution, and the absorbance at 570 nm of each resultant solution was measured spectrophotometrically.
Biofilm accumulation was normalized with respect to growth, which yielded the SBF. SBF was determined by using the following formula: SBF = (B − NC)/G, where B is the amount of biofilm formed, NC is the amount of CV that adhered to the polystyrene tubes due to abiotic factors, and G is the optical density of cells grown in suspended culture. At least two replicate experiments were performed for each concentration of chemical that was tested.
Flow cell determination of SCA.
Bench-scale parallel-plate flow cells (16) were used to quantify the effects of essential oil components on the initial adhesion of E. coli ATCC 33456 to a glass surface. Briefly, medium reservoirs supplemented with essential oil components were incubated at 37°C in a water bath, and filter-sterilized air was pumped into the growth medium at a flow rate of 8.0 ml min−1. The aerated media were inoculated with E. coli ATCC 33456 (OD600, 0.03) and recirculated through the flow cells at a flow rate of 0.84 ml min−1. After 2 h, the optical density of the suspended cells in each medium reservoir was measured, the flow cell apparatus was reconfigured to obtain a continuous-flow arrangement, and the channels were rinsed for 20 min with 50 mM phosphate buffer. After rinsing, the flow cells were examined with a Nikon Eclipse E600 microscope equipped with differential interference contrast optics, and four fields were obtained from randomly selected positions along the length of the channel by using a ×40 Plan Fluor objective. To determine the SCA, the number of cells per field was determined, multiplied to obtain the number of cells attached per square millimeter, and divided by the optical density of the suspended cells that grew concurrently in the medium reservoir. At least eight fields per concentration tested were obtained to estimate the SCA.
Cultivation of biofilms for viability staining and structure analysis.
Biofilms of E. coli ATCC 33456 were cultivated by using the flow cell technique described above, with the following changes: (i) after 2 h, the medium bottles were replaced, and the flow cell apparatus was reconfigured to obtain a continuous-flow arrangement with a flow rate of 0.35 ml min−1 for 18 h; and (ii) after 18 h, the biofilms were rinsed for 20 min with a sterile 50 mM KCl-NaCl solution (pH 7.0). After rinsing, 1 ml of a 1:1,000-diluted Live/Dead dye solution (Molecular Probes, Eugene, Oreg.) was pumped into the flow cell apparatus. After 15 min, the flow cells were rinsed for another 5 min and subsequently examined by CLSM. Experiments were conducted in triplicate, and at least 10 fields per condition tested were collected.
Quantitative analysis of biofilm structure.
Image stacks collected by CLSM were evaluated by using the digital image analysis program COMSTAT (18), which was designed for quantifying features of biofilm structure. The parameters analyzed by COMSTAT included maximum thickness, total biomass, substratum coverage, biomass, average diffusion distance, roughness coefficient, and surface-to-volume ratio. The COMSTAT analysis was performed with data collected in the green channel (SYTO 9-stained cells).
Statistical analysis.
Differences in biofilm structure measured with COMSTAT were tested for significance by using Student's t test. The error bars in all of the graphs below indicate the standard error of the mean.
Swimming motility assay.
The swimming motility of E. coli ATCC 33456 was tested by using a modification of a previously described technique (6). Petri dishes (100 by 15 mm; Fisher Scientific) containing 20 ml of LB broth and 0.3% Bacto Agar (Difco, Detroit, Mich.) were used. Cinnamaldehyde, eugenol, and citronellol were diluted in methanol (4%, vol/vol) and added at concentrations that resulted in 60% inhibition of planktonic cell growth in the toxicity analyses. The plates were inoculated by using sterilized toothpicks and were incubated for 17 ± 1 h in a 30°C incubator, and the diameter of each motility halo was measured. Six replicates were used for each treatment.
Gas chromatography.
Analysis of the components of C. cassia, C. zeylanicum, and C. nardus oils was conducted by gas chromatography with flame ionization detection. Analyses were performed with a Autosystem XL gas chromatograph (Perkin-Elmer, Wellesley, Mass.) by using a Supelco SPB-20 column (length, 30 m; inside diameter, 0.32 mm; Supelco, Bellefonte, Pa.). The injector temperature was 250°C, and the detector temperature was 300°C. The carrier gas was helium (1.3 ml min−1), and the detector gases were hydrogen (45 ml min−1) and air (450 ml min−1). Oils of C. cassia and C. zeylanicum were analyzed with the following temperature program: the initial column temperature was 150°C, the oven temperature was increased at a rate of 10°C min−1 until it was 260°C, and the temperature was kept at 260°C for 7 min. C. nardus oil was analyzed with the following temperature program: the initial column temperature was 210°C and was kept at this level for 2 min, and then the oven temperature was increased at a rate of 5°C min−1 until it was 260°C.
RESULTS
Effects of plant essential oils on growth and biofilm formation.
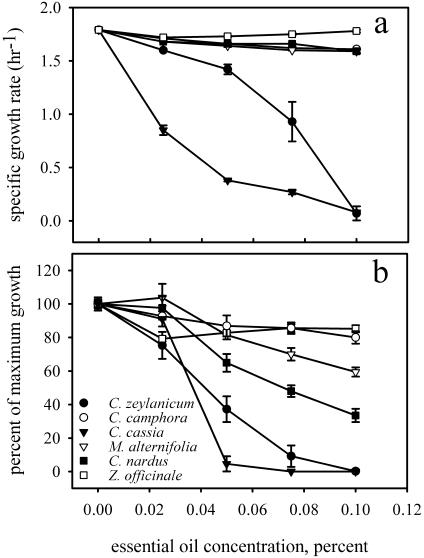
Six plant essential oils were screened for the ability to inhibit the growth of E. coli ATCC 33456 (Fig. 1a). Z. officinale essential oil had no effect on growth, and essential oils derived from C. camphora and M. alternifolia slightly inhibited the growth of E. coli ATCC 33456. C. zeylanicum and C. cassia essential oils substantially reduced the growth rate of E. coli ATCC 33456. C. nardus had a minimal effect on growth after 2 h, but growth inhibition was evident after 15 h (Fig. 1b). C. zeylanicum, C. cassia, and C. nardus were selected for further study.
FIG. 1.
Plant essential oil toxicity for E. coli ATCC 33456. (a) Toxicity measured by using the specific growth rate. (b) Toxicity expressed as a percentage of growth compared to the growth in LB medium after 15 h.
The effects of C. zeylanicum, C. cassia, and C. nardus essential oils on biofilm formation by E. coli ATCC 33456 were evaluated by the SBF assay. After 17 h, C. cassia reduced the extent of biofilm formation as a function of increasing essential oil concentration, whereas the SBF increased in response to C. zeylanicum and C. nardus essential oils. Representative changes in SBF values compared to controls were −100% ± 22% for C. cassia, 241% ± 38% for C. zeylanicum, and 586% ± 54% for C. nardus following exposure to essential oil concentrations that resulted in 73% ± 4% inhibition of planktonic growth. The corresponding OD600 values for suspended cultures ranged from 0.72 ± 0.07 for cells exposed to C. nardus to 0.92 ± 0.20 for cells exposed to C. cassia.
Effects of essential oil principal components on growth.
The principal chemical components of the three essential oils were quantified by gas chromatography with flame ionization detection. The major components of C. zeylanicum and C. cassia were cinnamaldehyde and eugenol, which comprised 2.1 and 53.4% of C. zeylanicum extract and 55.3 and 0.4% of C. cassia extract, respectively. The major component of C. nardus was citronellol, which comprised 48.8% of the total compounds present. The identities of the principal essential oil components were confirmed by comparison with the retention times of cinnamaldehyde, eugenol, and citronellol standards.
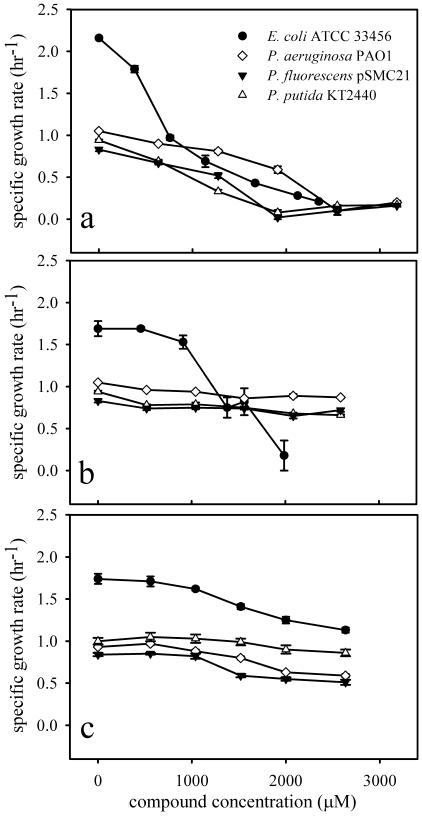
The effects of cinnamaldehyde, eugenol, and citronellol on the growth of E. coli ATCC 33456, P. aeruginosa PAO1, P. putida KT2440, and P. fluorescens pSMC21 were evaluated (Fig. 2). Cinnamaldehyde significantly inhibited the growth of all four species that were tested. Although eugenol strongly inhibited the growth of E. coli ATCC 33456, the growth of the three Pseudomonas spp. was reduced by only 20% ± 5% at a eugenol concentration of 2,600 μM (425 ppm), the maximum concentration tested. The growth of all four species was moderately inhibited by citronellol after 2 h. Like the results obtained with C. nardus essential oil, a delay in toxicity was observed when E. coli ATCC 33456 was treated with citronellol. By 18 h, 1,850 μM (290 ppm) citronellol inhibited E. coli growth by 60% compared to controls, and 2,650 μM (415 ppm) inhibited growth by 81%.
FIG. 2.
Effects of cinnamaldehyde (a), eugenol (b), and citronellol (c) on the specific growth rate of E. coli ATCC 33456 and selected Pseudomonas strains.
Effects of cinnamaldehyde, eugenol, and citronellol on E. coli ATCC 33456 biofilm formation and structure, viability, and swimming motility.
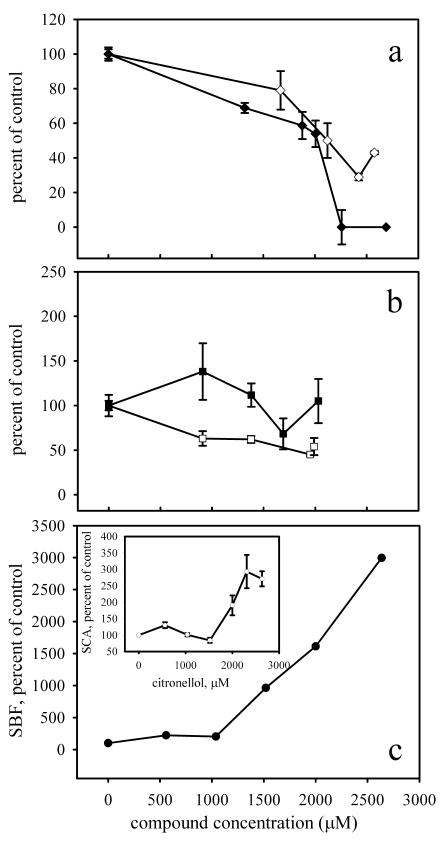
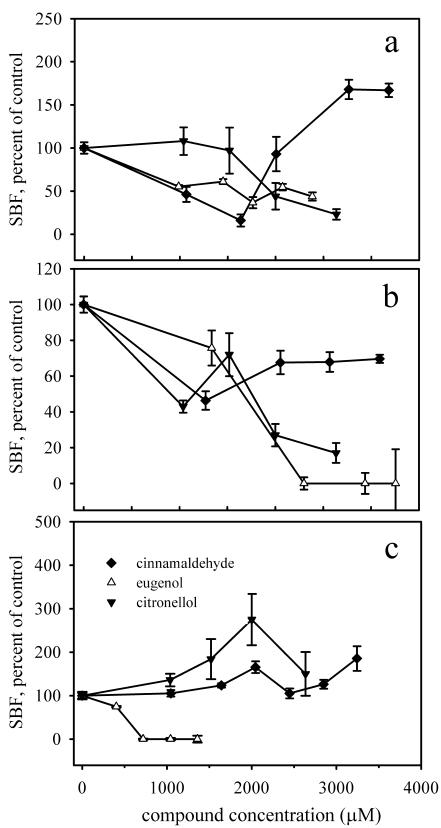
The SBF and SCA assays revealed the same general trends for cinnamaldehyde, eugenol, and citronellol in response to increasing concentrations (Fig. 3). For example, the SBF after exposure to 2,010 μM (265 ppm) cinnamaldehyde was reduced by 46% ± 8% compared to the SBF measured for the control treatment. Similarly, representative SCA values were 100,239 ± 18,809 cells mm−2 OD600 unit−1 for cells grown in LB medium only and 50,600 ± 19,448 cells mm−2 OD600 unit−1 for cells grown in the presence of 2,120 μM (280 ppm) cinnamaldehyde, a reduction of 50% ± 10% (P < 0.001).
FIG. 3.
Comparison of SBF and SCA assays as a function of increasing plant essential oil component concentration. The activities are the activities relative to the activity of E. coli ATCC 33456 in LB medium. (a) Cinnamaldehyde; (b) eugenol; (c) citronellol. (Inset) SCA assay with citronellol. Solid symbols, SBF assay; open symbols, SCA assay.
To measure the effects of cinnamaldehyde, eugenol, and citronellol on E. coli ATCC 33456 biofilm structure and viability, flow cell-grown biofilms were investigated by CLSM. Biofilms were grown for 18 h, which was similar to the growth period for the SBF assay. Essential oil components were added at concentrations that caused 60% inhibition of the planktonic cell growth (cinnamaldehyde, 2,170 μM [285 ppm]; eugenol, 1,690 μM [275 ppm]; citronellol, 1,850 μM [290 ppm]). Quantitative analysis of biofilm structure by using COMSTAT indicated several significant differences that resulted from exposure to plant essential oil components (Table 1).
TABLE 1.
COMSTAT analysis of E. coli ATCC 33456 biofilm structurea
| Medium or compound | No. of samples | Total biomass (μm3/μm2) | Maximum thickness (μm) | Substratum coverage (%) | Roughness coefficient | Avg diffusion distance (μm) | Surface-to-biovolume ratio (μm2/μm3) |
|---|---|---|---|---|---|---|---|
| LB medium | 10 | 1.5 ± 0.9b | 46.2 ± 5.7 | 34 ± 19 | 1.7 ± 0.2 | 0.02 ± 0.02 | 4.1 ± 0.7 |
| Cinnamaldehyde | 20 | 1.6 ± 0.7f | 18.3 ± 1.8d,f | 16 ± 5d,f | 1.5 ± 0.2d,e | 0.05 ± 0.03c,f | 2.0 ± 0.2d,f |
| Eugenol | 19 | 4.1 ± 2.8c | 22.2 ± 6.1d | 57 ± 19c | 1.1 ± 0.5d | 0.18 ± 0.18c | 2.8 ± 1.0d |
| Citronellol | 10 | 4.8 ± 2.0c | 28.5 ± 10.0d | 52 ± 18c | 0.9 ± 0.3d | 0.12 ± 0.09c | 3.5 ± 0.5 |
Biofilms were grown for 18 h in flow cells. Essential oil components were added at concentrations that resulted in 60% inhibition of the planktonic growth rate.
All values are means ± standard deviations.
Significantly greater (P < 0.05) than the corresponding value for the LB medium control.
Significantly less (P < 0.05) than the corresponding value for the LB medium control.
Significantly greater (P < 0.05) than the corresponding value for citronellol.
Significantlly less (P < 0.05) than the corresponding value for citronellol.
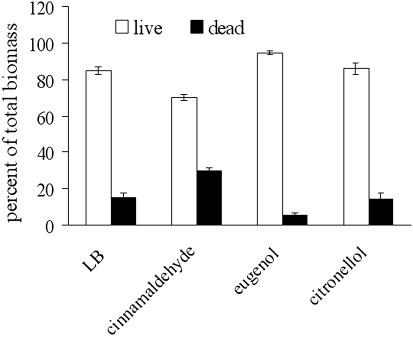
Viability staining indicated that both live and dead cells were present in all the biofilms tested (Fig. 4). No more than 30% of the total population was killed by the chemical treatments, although the concentrations of essential oil components used reduced planktonic growth by approximately 60%. Cinnamaldehyde-treated biofilms had a significantly higher (P < 0.001) percentage of dead cells than the biofilms that received the other treatments, and many cells were abnormally long and appeared to be stressed. Cinnamaldehyde reduced the swimming motility of E. coli ATCC 33456 by 60% ± 8% compared to controls (P < 0.001). In contrast, eugenol (P < 0.29) and citronellol (P < 0.26) had no significant effect.
FIG. 4.
Cell viability in E. coli ATCC 33456 biofilms after 18 h of growth in the presence of plant essential oil components. Open bars, live cells; solid bars, dead cells.
Effects of cinnamaldehyde, eugenol, and citronellol on biofilm formation by Pseudomonas spp.
To determine whether the SBF assay could identify differences in biofilm formation by other bacteria, the effects of cinnamaldehyde, eugenol, and citronellol on biofilm formation by three Pseudomonas species were investigated (Fig. 5). For P. aeruginosa PAO1, biofilm development was significantly inhibited by both eugenol and citronellol. In contrast, the specific biofilm formation by P. aeruginosa PAO1 decreased initially but subsequently increased at cinnamaldehyde concentrations greater than 2,000 μM (265 ppm). Biofilm development by P. putida KT2440 was reduced by increasing concentrations of all three chemicals. The SBF of P. fluorescens pSMC21 was not substantially inhibited at any concentration of cinnamaldehyde or citronellol. On the other hand, biofilm development was significantly inhibited by eugenol (no biofilm formed at any concentration higher than 700 μM [115 ppm]).
FIG. 5.
Effects of cinnamaldehyde, eugenol, and citronellol on SBF by Pseudomonas species. (a) P. aeruginosa PAO1; (b) P. putida KT2440; (c) P. fluorescens pSMC21.
DISCUSSION
CV staining has been widely adopted by microbiologists to investigate mutants with respect to adhesion or biofilm formation (13, 15, 19, 25, 27) and attachment to diverse surfaces (4, 22) and to compare biofilm development in different pathogens (20, 21). Its greatest features are that it is inexpensive, relatively quick, and adaptable for use in high-throughput screening with microtiter plates (11, 25, 26). Adhesion assays have also been developed based on staining with safranin (7, 31) and trypan blue (7). The SBF assay was conceived as a simple CV-based approach to find plant essential oils with antibiofilm properties. This assay is significant because it incorporates growth and biofilm accumulation in a single parameter, illustrating the relative tendency of cells in a population to attach to surfaces in response to changing essential oil concentrations. The SBF assay differs in this regard from other CV-based studies of biofilms that have measured the corresponding growth of cells (11) or cellular activity (26), and it could be used in investigations of the influence of chemicals on biofilm development.
The divergent responses of E. coli ATCC 33456 to cinnamaldehyde and citronellol measured by the SBF assay were unexpected. To establish whether the observed trends in SBF concurred with the microscopy-based direct assays of biofilm development, three data analyses were considered. First, the effects of cinnamaldehyde, eugenol, and citronellol on biofilm formation by E. coli ATCC 33456 were compared (Table 1). Five parameters measuring biomass calculated by COMSTAT (total biomass, maximum biofilm thickness, substratum coverage, surface-to-biovolume ratio, and average diffusion distance) were significantly greater for biofilms exposed to citronellol than for biofilms exposed to cinnamaldehyde. This result strongly agreed with the trend determined by the SBF assay. In contrast, the COMSTAT parameters for eugenol were not significantly different than those for citronellol, and they were significantly different from those for cinnamaldehyde for only one parameter (substratum coverage), a finding that was analogous to the intermediate response of eugenol seen in the SBF assay. Second, the changes in COMSTAT parameters for treated biofilms and untreated controls were compared. For cinnamaldehyde, decreases in three of five parameters characterizing biomass compared to biofilms grown on LB medium alone were observed, and there was an increase in one parameter. For eugenol, there were increases in three parameters and decreases in two. For citronellol, there were increases in three parameters and a decrease in one. In general, these results followed the trend that was observed in the SBF assay. Third, the SCA assay, measuring growth-normalized adhesion by direct microscopy, revealed tendencies similar to those observed with the SBF assay in response to increasing concentrations of each of the plant essential oil components tested (Fig. 3). On the whole, the results of the microscopy-based analyses corroborated the overall trend in biofilm accumulation determined by the SBF assay.
Swimming motility has been linked to biofilm formation in several kinds of bacteria (33), is mediated by flagella (17), and initiates cell-to-surface contact. In some cases, flagellar motility has been found to be essential for normal biofilm formation (27). However, flagellar motility was not required for initial adhesion and biofilm formation by curli-producing strains of E. coli (28) or for biofilm formation by E. coli strains carrying conjugative plasmids (30). In this work, cinnamaldehyde significantly reduced the swimming motility of E. coli ATCC 33456. We hypothesize that cinnamaldehyde may have reduced biofilm formation by E. coli ATCC 33456 in part by interfering with its ability to reach the substratum, a finding consistent with the results of the SCA assay.
Several patterns of SBF were observed in response to increasing concentrations of the three essential oil components tested in this study, suggesting that the compounds may interact with microorganisms through different mechanisms. For example, the SBF for E. coli in response to cinnamaldehyde decreased gradually, but it declined rapidly at concentrations greater than 1,750 μM, possibly due to accumulated cell membrane damage, which was detected during viability staining. In contrast, over a similar concentration range, the SBF of P. aeruginosa first decreased and subsequently increased to a level higher than that in the absence of cinnamaldehyde. This behavior suggested that there was activation of a stress-induced response that led to increased CV binding, possibly by increased exopolysaccharide production, as observed elsewhere in response to toxicity (1, 12). Similarly, citronellol substantially increased the SBF of E. coli ATCC 33456, possibly by acting as a poly-l-lysine-like adhesive (9), but it decreased the SBF of P. aeruginosa and P. putida at equivalent concentration ranges, indicating that there was a different interaction with these microorganisms. By characterizing SBF patterns for chemicals with well-understood mechanisms of activity, the SBF assay could provide insight into the mode of action of new compounds. However, direct microscopy would ultimately be required to evaluate the effects on biofilm formation and structure.
Acknowledgments
We thank Arne Heydorn and Sorin Molin, Technical University of Denmark, for sharing COMSTAT, Robert Simmons for his assistance with CLSM, and Don Ahearn for his helpful review of the manuscript. We are grateful to H. O'Connell, B. Cierny, A. Esfandiarinia, F. Mokuolu, C. Morris, and B. Sapkota for their assistance with lab work.
This research was supported by a Georgia State University Research Program enhancement grant.
REFERENCES
- 1.Aquino, S. F., and D. C. Stuckey. 2004. Soluble microbial products formation in anaerobic chemostats in the presence of toxic compounds. Water Res. 38:255-266. [DOI] [PubMed] [Google Scholar]
- 2.Baratta, M. T., H. J. D. Dorman, S. G. Deans, A. C. Figueiredo, J. G. Barroso, and G. Ruberto. 1998. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr. J. 13:235-244. [Google Scholar]
- 3.Beattie, G. A., and S. E. Lindow. 1999. Bacterial colonization of leaves: a spectrum of strategies. Phytopathology 89:353-359. [DOI] [PubMed] [Google Scholar]
- 4.Blackman, I. C., and J. F. Frank. 1996. Growth of Listeria monocytogenes as a biofilm on various food-processing surfaces. J. Food Prot. 59:827-831. [DOI] [PubMed] [Google Scholar]
- 5.Bloemberg, G. V., G. A. O'Toole, B. J. Lugtenberg, and R. Kolter. 1997. Green fluorescent protein as a marker for Pseudomonas spp. Appl. Environ. Microbiol. 63:4543-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkart, M., A. Toguchi, and R. M. Harshey. 1998. The chemotaxis system, but not chemotaxis, is essential for swarming motility in Escherichia coli. Proc. Natl. Acad. Sci. USA 95:2568-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen, G. D., W. A. Simpson, A. L. Bisno, and E. H. Beachey. 1982. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowan, S. E., D. Liepmann, and J. D. Keasling. 2001. Development of engineered biofilms on poly-l-lysine patterned surfaces. Biotechnol. Lett. 23:1235-1241. [Google Scholar]
- 10.Cox, S. D., C. M. Mann, J. L. Markham, H. C. Bell, J. E. Gustafson, J. R. Warmington, and S. G. Wyllie. 2000. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 88:170-175. [DOI] [PubMed] [Google Scholar]
- 11.Djordjevic, D., M. Wiedmann, and L. A. McLandsborough. 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 68:2950-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang, H. H., L. C. Xu, and K. Y. Chan. 2002. Effects of toxic metals and chemicals on biofilm and biocorrosion. Water Res. 36:4709-4716. [DOI] [PubMed] [Google Scholar]
- 13.Favre-Bonte, S., T. Kohler, and C. Van Delden. 2003. Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J. Antimicrob. Chemother. 52:598-604. [DOI] [PubMed] [Google Scholar]
- 14.Gao, M., M. Teplitski, J. B. Robinson, and W. D. Bauer. 2003. Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol. Plant-Microbe Interact. 16:827-834. [DOI] [PubMed] [Google Scholar]
- 15.Genevaux, P., S. Muller, and P. Bauda. 1996. A rapid screening procedure to identify mini-Tn10 insertion mutants of Escherichia coli K-12 with altered adhesion properties. FEMS Microbiol. Lett. 142:27-30. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert, E., and J. Keasling. 2004. Bench scale flow cell for nondestructive imaging of biofilms, p. 109-118. In J. Spencer and A. Ragout de Spencer (ed.), Environmental microbiology methods and protocols. Humana Press, Totowa, N.J.
- 17.Harshey, R. M. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57:249-273. [DOI] [PubMed] [Google Scholar]
- 18.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersbøll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 19.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, Y., H. K. Yip, Y. H. Samaranayake, J. Y. Yau, and L. P. Samaranayake. 2003. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J. Clin. Microbiol. 41:2961-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, X., Z. Yan, and J. Xu. 2003. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 149:353-362. [DOI] [PubMed] [Google Scholar]
- 22.Merritt, K., V. M. Hitchins, and S. A. Brown. 2000. Safety and cleaning of medical materials and devices. J. Biomed. Mater. Res. 53:131-136. [DOI] [PubMed] [Google Scholar]
- 23.Morris, C. E., and J.-M. Monier. 2003. The ecological significance of biofilm formation by plant-associated bacteria. Annu. Rev. Phytopathol. 41:429-453. [DOI] [PubMed] [Google Scholar]
- 24.Ogunlana, E. O., S. Hoeglund, G. Onawunmi, and O. Skoeld. 1987. Effects of lemongrass oil on the morphological characteristics and peptidoglycan synthesis of Escherichia coli cells. Microbios 50:43-59. [PubMed] [Google Scholar]
- 25.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 26.Pitts, B., M. A. Hamilton, N. Zelver, and P. S. Stewart. 2003. A microtiter-plate screening method for biofilm disinfection and removal. J. Microbiol. Methods 54:269-276. [DOI] [PubMed] [Google Scholar]
- 27.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 28.Prigent-Combaret, C., G. Prensier, T. T. Le Thi, O. Vidal, P. Lejeune, and C. Dorel. 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environ. Microbiol. 2:450-464. [DOI] [PubMed] [Google Scholar]
- 29.Ramos-Diaz, M. A., and J. I. Ramos. 1998. Combined physical and genetic map of the Pseudomonas putida KT2440 chromosome. J. Bacteriol. 180:6352-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reisner, A., A. J. Janus, M. A. Haagensen, E. L. Z. Schembri, and S. Molin. 2003. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48:933-946. [DOI] [PubMed] [Google Scholar]
- 31.Romeo, T., M. Gong, M. Y. Liu, and A. M. Brun-Zinkernagel. 1993. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J. Bacteriol. 175:4744-4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shelef, L. A. 1983. Antimicrobial effects of spices. J. Food Saf. 6:29-44. [Google Scholar]
- 33.Soutourina, O. A., and P. N. Bertin. 2003. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol. Rev. 27:505-523. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg, P. D., R. Schneider, and S. Kjelleberg. 1997. Chemical defenses of seaweeds against microbial colonization. Biodegradation 8:211-220. [Google Scholar]
- 35.Turi, M., E. Turi, S. Koljalg, and M. Mikelsaar. 1997. Influence of aqueous extracts of medicinal plants on surface hydrophobicity of Escherichia coli strains of different origin. APMIS 105:956-962. [PubMed] [Google Scholar]
- 36.Wang, Y.-T., and H. Shen. 1995. Bacterial reduction of hexavalent chromium. J. Ind. Microbiol. 14:159-163. [DOI] [PubMed] [Google Scholar]