Abstract
A survey of six bee viruses on a large geographic scale was undertaken by using seemingly healthy bee colonies and the PCR technique. Samples of adult bees and pupae were collected from 36 apiaries in the spring, summer, and autumn during 2002. Varroa destructor samples were collected at the end of summer following acaricide treatment. In adult bees, during the year deformed wing virus (DWV) was found at least once in 97% of the apiaries, sacbrood virus (SBV) was found in 86% of the apiaries, chronic bee paralysis virus (CBPV) was found in 28% of the apiaries, acute bee paralysis virus (ABPV) was found in 58% of the apiaries, black queen cell virus (BQCV) was found in 86% of the apiaries, and Kashmir bee virus (KBV) was found in 17% of the apiaries. For pupae, the following frequencies were obtained: DWV, 94% of the apiaries; SBV, 80% of the apiaries; CBPV, none of the apiaries; ABPV, 23% of the apiaries; BQCV, 23% of the apiaries; and KBV, 6% of the apiaries. In Varroa samples, the following four viruses were identified: DWV (100% of the apiaries), SBV (45% of the apiaries), ABPV (36% of the apiaries), and KBV (5% of the apiaries). The latter findings support the putative role of mites in transmitting these viruses. Taken together, these data indicate that bee virus infections occur persistently in bee populations despite the lack of clinical signs, suggesting that colony disease outbreaks might result from environmental factors that lead to activation of viral replication in bees.
The honey bee, Apis mellifera L., is subject to many viral infections, and a total of 15 viruses have been identified in this species and characterized (4, 8). The most commonly observed and best known honey bee viruses are 30-nm isometric particles containing a single-stranded positive RNA. These viruses include sacbrood virus (SBV) and deformed wing virus (DWV), which are assigned to the genus Iflavirus, and Kashmir bee virus (KBV), acute bee paralysis virus (ABPV), and black queen cell virus (BQCV), which are classified as members of the genus Cripavirus (family Dicistroviridae) (24). The chronic bee paralysis virus (CBPV) remains unclassified. According to epidemiological data, the distribution of these viruses in honey bee colonies appears to be worldwide (1), most likely resulting from intensive exchanges of honey bee stocks throughout the world.
Two of these viruses, SBV and CBPV, produce clinical signs that are clearly identifiable by beekeepers, whereas the majority of the viruses are believed to cause persistent, usually inapparent infections in honey bees (3, 6, 17, 20), and their associations with colony mortality remain unexplained (10, 25).
The increased number of A. mellifera L. bee colony collapses in several European countries, especially France, during the last decade has resulted in great interest in honey bee toxicology and pathology. Two principal hypotheses might explain these collapses. One of these hypotheses is that the rapid dissemination of the ectoparasitic mite Varroa destructor (5) in A. mellifera L. colonies is the main cause. This mite is now widespread in Europe, North Africa, Asia, and the Americas, and colony death occurs in 2 years if the mite infestation is not controlled by acaricide treatments. The collapse of bee colonies severely infested by V. destructor has long been attributed to viruses and has been described as bee parasitic mite syndrome (29). Generally, two hypotheses are cited that could explain the deleterious effects of varroa parasitism on honey bee colony decline. First, the mite can act as a vector and can directly inject virus particles into the insect hemolymph (11); the detection of several bee viruses in varroa mites and the high virus load strongly support this hypothesis (2, 9, 14, 31). Second, the mite can also trigger virus replication by a simple mechanical effect, cuticle piercing, or by injection of external proteins into the insect hemolymph (18). The latter hypothesis is supported by several studies that demonstrated that there was reactivation of viruses already present in the insect following experimental inoculation (3, 17). However, the association between parasitic mite syndrome and viruses is still controversial (21). The mechanisms by which viruses could be reactivated in insects are not fully understood, and predisposing factors other than mite parasitism might play a role in this phenomenon; such factors include coinfection with bacteria or protozoans (7) and the effects of chemicals released into the environment (13, 15, 16). The latter factor is suspected to play a role in triggering viral disease outbreaks in mammals (32), as well as in insects (22).
The study of the impact of viral diseases on honey bee colonies suffers from a lack of data concerning bee virus prevalence, particularly in asymptomatic colonies. The great diversity of viruses isolated from honey bees, the lack of specific clinical signs, and the limited availability of tools for rapid and large-scale diagnoses are partially responsible for this situation. Complete or partial sequencing of several RNA viruses of the honey bee has allowed the recent development of highly sensitive methods for detection based on amplification by reverse transcription-PCR (RT-PCR) of specific viral sequences. Originally developed for diagnosis of human and animal RNA viruses, these methods have been successfully adapted to identify several RNA bee viruses, including SBV, KBV, CBPV, ABPV, BQCV, and DWV (12, 19, 28, 30, 31).
We report here the first survey, covering a 1-year period, of the prevalence and seasonal variations of six bee RNA viruses in French apiaries based on large-scale sampling of adult bees, brood, and varroa mites having different geographic origins. The samples were collected at three times (in the spring, summer, and autumn of 2002) from seemingly healthy colonies and were analyzed by using the RT-PCR technology for virus identification.
MATERIALS AND METHODS
Bee and varroa mite sampling.
A list of 1,307 volunteer beekeepers was kindly provided by French technical and professional organizations of beekeepers. A cohort of 100 beekeepers was randomly selected, and 36 beekeepers were eventually found to participate in this survey. A varroa-free apiary (apiary 429), located on Ouessant Island in the Brittany region, was included in this study. The distribution of the 36 apiaries in France is shown in Fig. 1.
FIG. 1.
Distribution of the 36 apiaries where samples were collected in France. The reference numbers of the apiaries are indicated.
Ten colonies were randomly identified in each apiary. These colonies were checked for the absence of clinical signs and were considered to be valid for honey production. Samples of adult bees (10 g or approximately 100 individuals) and brood (30 pupae) were collected at three times during 2002: in the spring (from March to 15 June), summer (from 16 June to 15 August), and autumn (from 16 August to November).
Varroa mites (100 mites per beehive) were collected at the end of August or beginning of September following colony treatment with an acaricide (Amitraz).
The samples were collected by beekeepers and immediately sent to the laboratory by express mail. The pupae were removed from their puparia by using toothpicks upon reception, and the samples were frozen at −20°C.
Sample preparation and PCR analysis.
The frozen samples were crushed in a mortar in the presence of liquid nitrogen and were homogenized in 10 mM Tris-400 mM NaCl buffer (pH 7.5). After homogenization, the samples were centrifuged for 10 min at 5,000 × g to clarify them. An aliquot of supernatant was used for extraction of total RNA with a Nucleospin RNA-II kit (Macherey-Nagel) used according to the supplier's recommendations. Total RNA was resuspended in water and quantified by spectrophotometry. An average of 2 μg of total RNA was retrotranscribed at 25°C for 10 min and at 50°C for 1 h with a Thermoscript RT-PCR kit (Invitrogen) by using random hexamers. A passive RNA reference (5 × 107 copies of tobacco mosaic virus strain INRA, which was gift from B. Alliot, ENSAM Montpellier) was introduced into each sample during RNA preparation to monitor the efficiency of RNA purification and cDNA synthesis and to reveal the presence of PCR inhibitors in the samples.
PCR assays were done as follows. Five microliters of cDNA (1/10 dilution with water) was mixed with 5 μl of 10× buffer (100 mM Tris-HCl [pH 9], 500 mM KCl, 15 mM MgCl2, 1% Triton X-100, 2 mg of bovine serum albumin per ml), 1 μl of a solution containing each deoxynucleoside triphosphate at a concentration of 10 mM, each primer at a concentration of 0.4 μM, and 1 U of Taq polymerase (Q-Biogen); the final volume of the mixture was 50 μl. The mixture was heated for 2 min at 95°C, and this was followed by 35 amplification cycles (30 s at 95°C, 30 s at 56°C, and 1 min at 72°C) and then by 7 min at 72°C to complete the polymerization. PCR products were analyzed by 1.5% agarose gel electrophoresis. The primers used in the assays are shown in Table 1. The SBV, BQCV, and ABPV primers were designed from viral genomic sequences deposited in the GenBank database under accession numbers AF092924, AF183905, and AF150629, respectively, and the corresponding PCR amplicons were sequenced to ensure their specificity.
TABLE 1.
Primers used for PCR diagnosis
| Virus | Forward primer | Reverse primer | Length (bp)a | Reference |
|---|---|---|---|---|
| DWV | TTTGCAAGATGCTGTATGTGG | GTCGTGCAGCTCGATAGGAT | 395 | 30 |
| SBV | GGATGAAAGGAAATTACCAG | CCACTAGGTGATCCACACT | 426 | |
| CBPV | AGTTGTCATGGTTAACAGGATACGAG | TCTAATCTTAGCACGAAAGCCGAG | 455 | 27 |
| ABPV | TGAGAACACCTGTAATGTGG | ACCAGAGGGTTGACTGTGTG | 452 | |
| BQCV | GGACGAAAGGAAGCCTAAAC | ACTAGGAAGAGACTTGCACC | 424 | |
| KBV | GATGAACGTCGACCTATTGA | TGTGGGTTGGCTATGAGTCA | 393 | 29 |
| TMVb | AAAAACAGTCCCCAACTTCC | AAGGAGGATTCTCTCGCTGT | 600 |
Length of DNA fragment amplified.
TMV, tobacco mosaic virus.
Statistical analysis.
The results were expressed as the presence or absence of a virus in an apiary or in a colony. The data include data for adult bees, bee pupae, and varroa samples collected in the three seasons. The independence of the variables was estimated with a chi-square test, including Yates correction, by using the Statbox software (Grimmersoft).
Nucleotide sequence accession numbers.
The sequences of some isolates were deposited in the GenBank database under accession numbers AY669845, AY669846, AY669847, AY669848, AY669849, AY669850, AY669851, AY669852, and AY669853.
RESULTS
Virus frequencies in apiaries.
The data for PCR detection of six bee viruses in the 36 apiaries are shown in Table 2. Except for apiary 429 (Ouessant Island), where no virus and no varroa were ever detected, viruses were found in all of the apiaries analyzed. No significant differences between geographical regions were observed. A large majority of the apiaries were found to be infected by several viruses, as 92% of the apiaries were found to be positive for at least three different viruses(31% of the apiaries contained three viruses, 36% contained four viruses, and 25% contained five viruses). As a whole, higher virus frequencies were detected in adult populations than in brood populations. In adults during the year DWV was found at least once in 97% of the apiaries, SBV was found in 86% of the apiaries, CBPV was found in 28% of the apiaries, ABPV was found in 58% of the apiaries, BQCV was found in 86% of the apiaries, and KBV was found in 17% of the apiaries. For pupae, the following frequencies were obtained: DWV, 94% of the apiaries; SBV, 80% of the apiaries; CBPV, none of the apiaries; ABPV, 23% of the apiaries; BQCV, 23% of the apiaries; and KBV, 6% of the apiaries (Table 3). CBPV was never detected in pupae. To avoid misinterpretation due to sequence homologies between ABPV and KBV, we sequenced the KBV amplicons. They were all 98% identical to the KBV sequence deposited in the GenBank database (accession no. AF197905).
TABLE 2.
Seasonal variation of DWV, SBV, ABPV, BQCV, CBPV, and KBV in bee and Varroa samples from 36 apiaries
| Apiary | Regiona | DWV
|
SBV
|
CBPV
|
ABPV
|
BQCV
|
KBV
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults, spring | Adults, summer | Adults, autumn | Pupae, spring | Pupae, summer | Pupae, autumn | Varroac | Adults, spring | Adults, summer | Adults, autumn | Pupae, spring | Pupae, summer | Pupae, autumn | Varroac | Adults, spring | Adults, summer | Adults, autumn | Pupae, spring | Pupae, summer | Pupae, autumn | Varroac | Adults, spring | Adults, summer | Adults, autumn | Pupae, spring | Pupae, summer | Pupae, autumn | Varroac | Adults, spring | Adults, summer | Adults, autumn | Pupae, spring | Pupae, summer | Pupae, autumn | Varroac | Adults, spring | Adults, summer | Adults, autumn | Pupae, spring | Pupae, summer | Pupae, autumn | Varroac | ||
| 910 | AL | −b | − | + | − | − | − | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 333 | AQ | + | + | + | − | + | + | NA | − | + | − | − | + | − | NA | − | − | − | − | − | − | NA | − | − | + | − | − | − | NA | − | + | + | − | + | − | NA | − | − | − | − | − | − | NA |
| 497 | AQ | + | + | + | + | + | + | + | + | + | + | + | + | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 878 | AQ | + | + | + | + | + | + | + | + | + | + | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| 855 | AU | + | + | + | + | − | − | + | − | + | + | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 649 | AU | + | + | + | − | + | + | + | − | + | + | − | − | + | − | + | − | − | − | − | − | − | − | + | − | + | + | − | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 208 | BN | + | + | + | + | + | + | + | − | + | + | − | + | − | + | − | + | − | − | − | − | − | − | + | + | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 426 | BR | + | + | + | + | + | − | + | − | − | − | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 429 | BR | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA |
| 150 | CA | + | + | + | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 746 | CA | + | + | + | + | + | + | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + | + | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 751 | CA | + | + | + | + | + | + | + | − | + | + | − | + | − | − | + | + | − | − | − | − | − | − | + | − | − | − | − | − | + | + | + | − | + | − | − | − | − | − | − | − | − | − |
| 624 | CE | + | + | + | + | + | + | NA | + | + | − | + | − | − | NA | − | − | + | − | − | − | NA | − | + | − | − | + | − | NA | + | + | + | − | − | − | NA | − | − | − | − | − | − | NA |
| 413 | CE | + | + | + | + | + | + | + | + | + | + | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 1005 | HN | + | + | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 802 | LO | + | + | + | − | − | + | + | + | + | − | + | + | − | + | − | − | − | − | − | − | − | − | − | + | − | + | − | + | + | + | + | − | − | − | − | − | − | − | − | − | − | |
| 702 | LR | − | + | + | − | + | + | NA | − | − | − | − | − | − | NA | − | − | + | − | − | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA |
| 900 | LR | + | + | + | − | + | + | + | + | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | + | + | − | − | + | − | − |
| 125 | MP | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 1051 | MP | + | + | + | − | − | + | + | + | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | + | − | − | − | − | − | − | − | − | − |
| 1049 | MP | + | + | + | − | − | − | NA | + | + | − | + | + | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA | + | + | + | − | − | − | NA | − | − | − | − | − | − | NA |
| 137 | MP | + | + | + | + | + | + | NA | + | + | − | + | − | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA | + | − | − | − | − | − | NA | − | − | − | − | − | − | NA |
| 814 | NO | + | + | + | + | + | NA | NA | − | − | − | − | − | NA | NA | − | + | + | − | − | NA | NA | − | + | + | − | − | NA | NA | − | − | − | − | − | NA | NA | − | − | − | − | − | NA | NA |
| 1182 | PA | + | − | + | − | + | + | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − | + | + | − | + | + | + | + | + | + | − | + | − | − | + | + | + | − | + | + | + |
| 1196 | PA | + | + | + | − | − | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 242 | PC | + | + | + | + | + | + | + | + | + | + | − | + | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 28 | PI | + | + | + | + | + | + | + | + | + | + | + | + | − | + | − | − | − | − | − | − | − | + | + | + | − | − | + | + | + | + | + | − | + | − | − | − | − | − | − | − | − | − |
| 727 | PL | + | + | + | − | + | + | + | − | + | + | − | − | + | + | − | − | + | − | − | − | − | + | + | + | − | − | − | + | + | + | − | − | + | − | − | − | − | − | − | − | − | − |
| 355 | RA | + | + | + | − | + | − | NA | + | + | + | + | + | − | NA | − | − | − | − | − | − | NA | − | + | + | − | − | − | NA | + | + | + | − | − | − | NA | + | + | + | − | − | − | NA |
| 626 | RA | + | + | + | − | + | + | NA | + | + | + | + | + | + | NA | − | − | − | − | − | − | NA | + | − | − | − | − | − | NA | + | + | + | − | − | − | NA | − | − | − | − | − | − | NA |
| 380 | RA | + | + | + | + | + | + | NA | + | + | + | + | + | − | NA | − | − | − | − | − | − | NA | + | + | − | − | − | − | NA | + | + | + | − | − | − | NA | − | − | − | − | − | − | NA |
| 992 | RA | + | + | + | + | + | + | NA | + | + | − | + | − | − | NA | − | − | − | − | − | − | NA | − | + | + | − | + | − | NA | + | + | + | + | − | − | NA | − | − | − | − | − | − | NA |
| 591 | RA | + | + | + | − | + | + | NA | + | + | + | + | + | + | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | NA | + | + | + | − | + | − | NA | + | + | + | − | − | − | NA |
| 585 | RA | + | + | + | + | + | + | NA | + | + | + | − | + | − | NA | − | − | − | − | − | − | NA | − | − | + | − | − | − | NA | + | + | + | − | − | − | NA | + | + | + | − | − | − | NA |
| 106 | RA | + | + | + | + | + | + | + | + | + | − | + | + | − | − | − | − | − | − | − | − | − | + | + | + | − | − | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| 934 | RA | + | + | + | + | + | + | NA | + | + | + | + | + | − | NA | − | − | − | − | − | − | NA | − | − | + | − | − | − | NA | + | + | + | − | − | − | NA | + | + | + | − | − | − | NA |
| No. (%) of positive apiaries | 33 (92) | 33 (92) | 35 (97) | 20 (56) | 29 (81) | 29 (83) | 22 (100) | 24 (67) | 31 (86) | 24 (67) | 17 (47) | 23 (64) | 7 (20) | 10 (45) | 4 (11) | 3 (8) | 7 (19) | 0 | 0 | 0 | 0 | 5 (14) | 15 (42) | 14 (39) | 1 (3) | 6 (17) | 4 (11) | 8 (36) | 28 (78) | 29 (81) | 27 (75) | 1 (3) | 7 (19) | 0 | 0 | 6 (17) | 6 (17) | 5 (14) | 0 | 2 (6) | 1 (3) | 1 (5) | |
PA, Provence Alpes Côte d'Azur; LR, Languedoc Roussillon; RA, Rhône Alpes; NO, Nord; MP, Midi Pyrénées; CA, Champagne Ardennes; LO, Lorraine; AU, Auvergne Picardie; BR, Bretagne; PL, Pays de Loire; BN, Basse Normandie; CE, Centre; AL, Alsace; AQ, Aquitaine; HN, Haute Normandie; PC, Poitou Charentes.
−, negative; +, positive; NA, sample not available.
The Varroa samples were collected in August or September.
TABLE 3.
Frequencies of infected apiaries during 2002
| Virus | Frequency (%)a
|
||
|---|---|---|---|
| Adults | Pupae | Mites | |
| DWV | 97 | 94 | 100 |
| SBV | 86 | 80 | 45 |
| CBPV | 28 | 0 | 0 |
| ABPV | 58 | 23 | 36 |
| BQCV | 86 | 23 | 0 |
| KBV | 17 | 6 | 5 |
An apiary was considered positive for DWV, SBV, CBPV, ABPV, BQCV, or KBV if at least 1 of 10 colonies was positive in the spring, summer, or autumn.
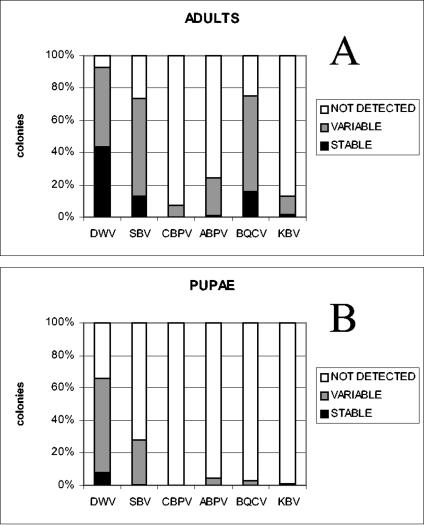
The analysis performed with samples collected in the spring, summer, and autumn showed that the majority of the colonies were infected only once or twice during the year (designated variable), as shown in Fig. 2. These results were due to the appearance of newly infected colonies during the year or to positive colonies that were reported to be negative later in the year.
FIG. 2.
Frequencies of bee virus infections in samples of adult bees (A) and pupae (B) collected from 360 colonies. The data are expressed as percentages. The detection of DWV, SBV, CBPV, ABPV, BQCV, or KBV in the spring, summer, and autumn in the same colony is referred to as stable. Viral infections that were detected once or twice during the year in the same colony are referred to as variable. Not detected refers to colonies in which virus was not detected at any time of the year.
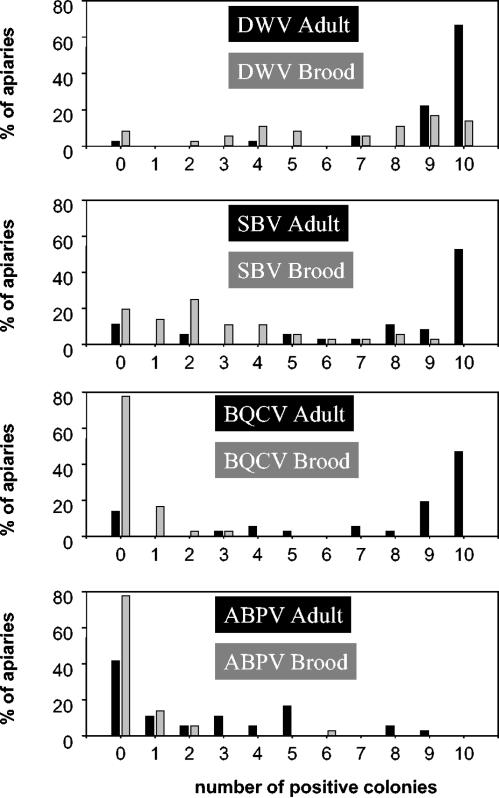
Viral infections were distributed differently among colonies of the same apiary. Figure 3 shows the percentages of apiaries in which 0 to 10 of 10 colonies were found to be positive for DWV, SBV, BQCV, or ABPV when adults or pupae were examined. The data for CBPV and KBV are not shown due to the few cases reported for these viruses. In adult bees, DWV, SBV, and BQCV infections were prevalent in a large majority of the colonies in each apiary, whereas ABPV infections were often found in few colonies in the same apiary.
FIG. 3.
Percentages of apiaries in which 0 to 10 colonies were infected by DWV, SBV, BQCV, or ABPV at the adult or larval stage.
Seasonal variation of viral infections.
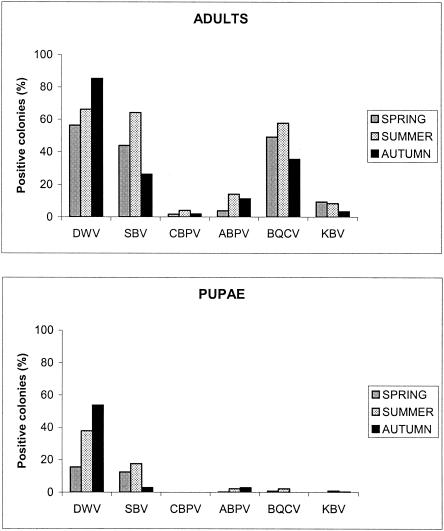
A total of 360 colonies were analyzed in the spring, summer, and autumn of 2002. Although virus infections were detected during the year, some seasonal variations in virus frequencies were observed with both adults and pupae (Fig. 4). SBV was prominently found in the spring and summer in both adults and pupae (for spring adults versus summer adults, df = 1, χ2 = 31.03, and P < 0.001; for summer adults versus autumn adults, df = 1, χ2 = 9.91, and P < 0.001; for spring pupae versus summer pupae, df = 1, χ2 = 8.94, and P < 0.001; for summer pupae versus autumn pupae, df = 1, χ2 = 3.17, and P < 0.07). In adults, the SBV frequencies in colonies were 44, 64, and 26% in the spring, summer, and autumn, respectively. In pupae, the values were 13, 18, and 3%, respectively. The same distribution was observed for BQCV infections in adults, as the frequencies were 49, 58, and 36% in the spring, summer, and autumn, respectively (for spring adults versus summer adults, df = 1, χ2 = 21.56, and P < 0.001; for summer adults versus autumn adults, df = 1, χ2 = 21.48, and P < 0.001). In contrast, the DWV infections increased during the year both in adults and in pupae. The recorded DWV frequencies in the spring, summer, and autumn were 56, 66, and 85%, respectively, for adults (for spring adults versus summer adults, df = 1, χ2 = 34.88, and P < 0.001; for summer adults versus autumn adults, df = 1, χ2 = 20.55, and P < 0.001) and 16, 38, and 54%, respectively, for pupae (for spring pupae versus summer pupae, df = 1, χ2 = 16.01, and P < 0.001; for summer pupae versus autumn pupae, df = 1, χ2 = 26.83, and P < 0.001). Similarly, ABPV infections were prominently found during the summer in adult bees (for summer adults versus autumn adults, df = 1, χ2 = 11.42, and P < 0.001). Conversely, KBV infections tended to regress in autumn, while CBPV infections were detected throughout the year but at low frequencies.
FIG. 4.
Seasonal variation of viral infection rates in honey bee colonies.
Bee viruses in varroa.
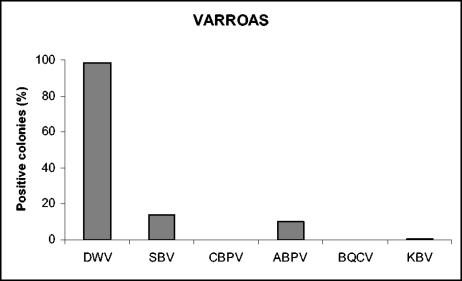
We received varroa samples from only 22 of the 36 apiaries. Four different viruses were detected in mites; DWV was present in 100% of the apiaries, SBV was present in 45% of the apiaries, ABPV was present in 36% of the apiaries, and KBV was present in 5% of the apiaries (Table 2). No BQCV or CBPV was detected in mites. The frequencies calculated for the colonies from which mite samples were collected were slightly different, as follows: 98% for DWV, 14% for SBV, 10% for ABPV, and 1% for KBV (Fig. 5). Four distinct viruses were detected in the mites collected from apiary 1182, and three distinct viruses were detected in the mites collected from apiaries 802, 28, and 727 (Table 2).
FIG. 5.
Prevalence of viral infections in V. destructor samples.
DISCUSSION
Several reports on the presence of honey bee viruses in A. mellifera L. populations in different countries have been published (6, 10, 20, 23, 25, 27), some of them before the spread of V. destructor in Europe. Most of these reports were based on symptomatic or dead bees collected at the hive entrance, sometimes after colony collapse; i.e., most of the time the data were obtained from a few samples that were not representative of the natural occurrence of virus infections in bee colonies. Moreover, the techniques used for virus detection, which were essentially based on serology, did not permit the workers to detect the low virus levels typical of persistent inapparent infections.
Here, we describe the first survey of the prevalence of six honey bee viruses in seemingly healthy bee colonies randomly chosen from 36 apiaries, in which the RT-PCR technique was used. The samples came from different geographic areas in France, including plains with intensive industrial crops, valleys, and mountains.
DWV.
Of the six viruses identified by PCR in this study, DWV was by far the most frequently detected in colonies, both in bee samples (adults and brood) and in varroa mites. For adults and pupae, the frequency of DWV-infected colonies increased from spring to autumn. The seasonal variations in DWV incidence were much more pronounced for pupae than for adult bees. In mites, DWV was found in 98% of the samples analyzed. These data confirm the putative role of varroa in the transmission of this virus (14, 26, 31). Interestingly, a large number of DWV-positive colonies were detected both for adult bees and for pupae or larvae throughout the year. According to Bailey and Ball (8), DWV is thought to be responsible for wing deformities when infection occurs during the white-eyed pupation stage of bee development. However, the mechanisms that lead to these symptoms are not clearly understood, and usually only a few bees in a colony severely infested by V. destructor display such deformities. DWV has been cited as potentially responsible for bee colony collapse (25), but other studies have shown that this virus might be considered poorly pathogenic (14, 31).
SBV.
The sacbrood virus was also found in the majority of bee colonies and was found more frequently in adults than in pupae, but unlike DWV, the SBV frequencies were much higher during the spring and summer. Similar SBV frequencies were previously found in healthy workers and pupae in Australia by an indirect SBV detection method (3). Likewise, Bailey and coworkers found that 33% of apparently healthy colonies examined in Great Britain were infected, with few larvae exhibiting sacbrood clinical signs (6). This latter work also showed that the occurrence of sacbrood was greater in the spring and in the summer. The seasonal variation in sacbrood virus frequencies observed both in adult bees and in pupae might reflect a difference in bee susceptibility to the virus or changes in the environment, such as the quality of the pollen consumed by larvae. Bees could also develop a kind of molecular defense mechanism to reduce virus multiplication. However, we cannot exclude the putative role of V. destructor in SBV propagation as the chi-square test showed that there was a positive correlation between the prevalence of SBV in varroa samples and the presence of SBV in adults (df = 1, χ2 = 0.26, P = 0.61) and pupae (df = 1, χ2 = 1.57, P = 0.21) in samples collected in the summer.
BQCV.
BQCV was very prevalent in colonies, especially in the adult bee population, in which the frequency reached 58% in the summer. In pupae, however, BQCV infections were scarcely detected; the maximum frequency was 2% in the summer. As shown in Table 2, adults in 24 apiaries were found to be positive for this virus throughout the year, which is an indication that BQCV infections (probably inapparent infections) can be maintained throughout the year. It was reported previously that this virus is found in close association with the protozoan Nosema apis, a parasite of the honey bee, and that in laboratory conditions per os infection of adult bees with BQCV is totally dependent on the presence of the parasite (7). The prevalence of N. apis infections in the samples which we analyzed (data not shown) may confirm these observations. Dissemination of BQCV by varroa mites appears to be improbable as this virus was never detected in any of the mite samples that we examined.
CBPV.
Likewise, all the varroa samples that we analyzed were negative for CBPV. These results suggest that the contribution of mites to the dissemination of this virus in the colony, if any, is small. Our data showed that CBPV was found only in adult bees, and the maximum frequency in colonies was only 4%, while CBPV infections recorded throughout the year occurred in 28% of the apiaries involved in our study (Table 2). The sporadic detection of CBPV during the year suggests that this virus might persist at undetectable levels in healthy colonies. Even if CBPV was shown to replicate in larvae after experimental inoculation (8), no CBPV could be detected in larvae or pupae in the samples that we examined. Taken together, our findings for CBPV prevalence are in agreement with previous observations showing that outbreaks of severe disease are erratic and exhibit no seasonal pattern (6, 28) and that CBPV transmission might result preferentially from contact between adults of the same colony (8).
ABPV.
ABPV is known to persist as inapparent infections in seemingly healthy bee colonies (6), but its presence was found to be correlated with the mortality of colonies infested with V. destructor (1). Twenty of the 195 varroa samples that we examined were positive for ABPV. The incidence of the virus in colonies was higher in the summer and autumn. Compared to the population dynamics of V. destructor, this suggests that the mite has a role in spreading this virus. However, the virus was also present in bee samples from apiaries where no ABPV-positive varroa mites were detected, suggesting that ABPV transmission may occur by contact between individual bees. The presence throughout the year of ABPV-positive adult bees in 21 of the 36 apiaries (Table 2) indicates that persistent ABPV infections can occur in honey bee colonies.
KBV.
KBV has been found frequently in Australia and in the United States but had never been identified in France previously. Here, we report the presence of KBV in 6 of 36 apiaries, and despite its lower prevalence, the distribution pattern of KBV appears to be slightly different than that of ABPV as the viral infections were shown to regress in autumn.
The data presented here clearly show that virus infections in apiaries are quite common. Furthermore, multiple infections were the rule in all the apiaries, and no significant geographical localization could be established for a given virus. These results provide clear evidence that bee virus infections occur persistently in bee colonies, despite the lack of clinical signs. One of the consequences of this is that outbreaks of virus disease leading to colony collapse probably result from external factors that provide favorable conditions (i) for the dissemination of the virus between individuals of the same colony and between different colonies and (ii) for the spread and replication of a virus from primary infection sites (e.g., gut epithelial cells) to critical targets (e.g., the nervous system) in bees. Although the lack of virus detection for apiary 429 (Ouessant Island) can be related to the geographical isolation of this apiary, it is worth noting that this finding correlates very well with the lack of varroa mites on the island. The high frequencies of viruses in mites suggest that V. destructor probably contributes efficiently to the outbreak of bee virus diseases, acting both as a vector and as an activator of virus replication. The latter phenomenon is a very challenging field for understanding the relationships among viruses, honey bee colonies, and V. destructor better.
Acknowledgments
We thank French beekeepers who participated in this survey and French beekeeper organizations (ANERCEA, CNDA, FCA, FNOSAD, GDS, SNA, SPMF, and UNAF) that provided help.
This work was supported by a grant from the EEC and the French Ministère de l'Agriculture et de la Pêche (Règlement CE no. 1221/97, convention no. 2207).
REFERENCES
- 1.Allen, M., and B. V. Ball. 1996. The incidence and world distribution of honey bee viruses. Bee World 77:141-162. [Google Scholar]
- 2.Allen, M. F., B. V. Ball, R. F. White, and J. F. Antoniw. 1986. The detection of acute paralysis virus in Varroa jacobsoni by the use of a simple indirect ELISA. J. Apic. Res. 25:100-105. [Google Scholar]
- 3.Anderson, D. L., and A. J. Gibbs. 1988. Inapparent virus infections and their interactions in pupae of the honey bee (Apis mellifera L.) in Australia. J. Gen. Virol. 69:1617-1625. [Google Scholar]
- 4.Anderson, D. L. 1995. Viruses of Apis cerana and Apis mellifera, p. 161-170. In The Asiatic hive bee: apiculture, biology, and role in sustainable development in tropical and subtropical Asia. Enviroquest, Ltd., Cambridge, Ontario, Canada.
- 5.Anderson, D. L., and J. W. H. Trueman. 2000. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24:165-189. [DOI] [PubMed] [Google Scholar]
- 6.Bailey, L., B. V. Ball, and J. N. Perry. 1981. The prevalence of viruses of honey bee in Britain. Ann. Appl. Biol. 97:109-118. [Google Scholar]
- 7.Bailey, L., B. V. Ball, and J. N. Perry. 1983. Association of viruses with two protozoal pathogens of the honey bee. Ann. Appl. Biol. 103:13-20. [Google Scholar]
- 8.Bailey, L., and B. V. Ball. 1991. Honey bee pathology, Harcourt Brace Jovanovich Sidcup, Kent, United Kingdom.
- 9.Bakonyi, T., R. Farkas, A. Szendroi, M. Dobos-Kovacs, and M. Rusvai. 2002. Detection of acute bee paralysis virus by RT-PCR in honey bee and Varroa destructor field samples: rapid screening of representative Hungarian apiaries. Apidologie 33:63-74. [Google Scholar]
- 10.Ball, B. V., and M. Allen. 1988. The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Ann. Appl. Biol. 113:237-244. [Google Scholar]
- 11.Ball, B. V. 1989. Varroa jacobsoni as a virus vector, p. 241-244. In R. Cavalloro (ed.), Present status of varroatosis in Europe and progress in varroa mite control. Office for Official Publications of the European Communities, Luxembourg.
- 12.Benjeddou, M., N. Leat, M. Allsopp, and S. Davison. 2001. Detection of acute bee paralysis virus and black queen cell virus from honeybees by reverse transcriptase PCR. Appl. Environ. Microbiol. 67:2384-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonmatin, J. M., I. Moineau, R. Charvet, C. Fleche, M. E. Colin, and E. R. Bengsch. 2003. A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants, and in pollens. Anal. Chem. 75:2027-2033. [DOI] [PubMed] [Google Scholar]
- 14.Bowen-Walker, P. L., S. J. Martin, and A. Gunn. 1999. The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invertebr. Pathol. 73:101-106. [DOI] [PubMed] [Google Scholar]
- 15.Charvet, R., M. Katouzian-Safadi, M. E. Colin, P. A. Marchand, and J. M. Bonmatin. 2004. Systemic insecticides: new risk for pollinator insects. Ann. Pharm. Fr. 62:29-35. [DOI] [PubMed] [Google Scholar]
- 16.Colin, M. E., J. M. Bonmatin, I. Moineau, C. Gaimon, S. Brun, and J. P. Vermandere. 2004. A method to quantify and analyze the foraging activity of honey bees: relevance to the sublethal effects induced by systemic insecticides. Arch. Environ. Contam. Toxicol. 47:387-395. [DOI] [PubMed] [Google Scholar]
- 17.Dall, D. J. 1985. Inapparent infection of honey bee pupae by Kashmir and sacbrood bee viruses in Australia. Ann. Appl. Biol. 106:461-468. [Google Scholar]
- 18.Dandeu, J. P., M. Lux, M. E. Colin, J. Rabillon, and B. David. 1991. Etude immuno-chimique de l'hémolymphe d'abeille ouvrière adulte (Apis mellifera L.) saine ou infestée par Varroa jacobsoni Oud. Apidologie 22:37-42. [Google Scholar]
- 19.Grabensteiner, E., W. Ritter, M. J. Carter, S. Davison, H. Pechhacker, J. Kolodziejek, O. Boecking, I. Derakhshifar, R. Moosbeckhofer, E. Licek, and N. Nowotny. 2001. Sacbrood virus of the honeybee (Apis mellifera): rapid identification and phylogenetic analysis using reverse transcription-PCR. Clin. Diagn. Lab. Immunol. 8:93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung, A. C. F., H. Shimanuki, and D. A. Knox. 1996. Inapparent infection of acute bee paralysis virus and Kashmir bee virus in the U.S. honey bees. Am. Bee J. 136:874-876. [Google Scholar]
- 21.Hung, A. C. F., H. Shimanuki, and D. A Knox. 1996. The role of viruses in bee parasitic mite syndrome. Am. Bee J. 136:731-732. [Google Scholar]
- 22.Koppenhöfer, A. M., and H. K. Kaya. 2000. Interactions of a nucleopolyhedrosis virus with Azadirachtin and Imidaclopride. J. Invertebr. Pathol. 75:84-86. [DOI] [PubMed] [Google Scholar]
- 23.Martin, S., A. Hogarth, J. Van Breda, and J. Perrett. 1998. A scientific note on Varroa jacobsoni Oudemans and the collapse of Apis mellifera L. colonies in the United Kingdom. Apidologie 29:369-370. [Google Scholar]
- 24.Mayo, M. A. 2002. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 147:1655-1663. [DOI] [PubMed] [Google Scholar]
- 25.Nordström, S., I. Fries, A. Aarhus, H. Hansen, and S. Korpela. 1999. Virus infection in Nordic honey bee colonies with no, low or severe Varroa jacobsoni infestations. Apidologie 30:475-484. [Google Scholar]
- 26.Nordstrom, S. 2003. Distribution of deformed wing virus within honey bee (Apis mellifera) brood cells infested with the ectoparasitic mite Varroa destructor. Exp. Appl. Acarol. 29:293-302. [DOI] [PubMed] [Google Scholar]
- 27.Ribière, M., J. P. Faucon, and M. Pépin. 2000. Detection of chronic honey bee (Apis mellifera L.) paralysis virus infection: application to a field survey. Apidologie 31:567-577. [Google Scholar]
- 28.Ribière, M., C. Triboulot, L. Mathieu, C. Aurières, J. P. Faucon, and M. Pépin. 2002. Molecular diagnostic of chronic bee paralysis virus infection. Apidologie 33:339-351. [Google Scholar]
- 29.Shimanuki, H., N. W. Calderone, and D. A. Knox. 1994. Parasitic mite syndrome: the symptoms. Am. Bee J. 134:827-828. [Google Scholar]
- 30.Stoltz, D., X. R. Shen, C. Boggis, and G. Sisson. 1995. Molecular diagnostic of Kashmir bee virus infection. J. Apic. Res. 34:153-165. [Google Scholar]
- 31.Tentcheva, D., L. Gauthier, S. Jouve, L. Canabady-Rochelle, B. Dainat, F. Cousserans, M. E. Colin, B. V. Ball, and M. Bergoin. 2004. Polymerase chain reaction detection of deformed wing virus (DWV) in Apis mellifera L. and Varroa destructor. Apidologie 35:431-439. [Google Scholar]
- 32.Van Loveren, H., P. S. Ross, A. D. Osterhaus, and J. G. Vos. 2000. Contaminant-induced immunosuppression and mass mortalities among harbor seals. Toxicol. Lett. 15:319-324. [DOI] [PubMed] [Google Scholar]