ABSTRACT
Breast cancer cells closely interact with different cell types of the surrounding adipose tissue to favor invasive growth and metastasis. Extracellular vesicles (EVs) are nanometer-sized vesicles secreted by different cell types that shuttle proteins and nucleic acids to establish cell-cell communication. To study the role of EVs released by cancer-associated adipose tissue in breast cancer progression and metastasis a standardized EV isolation protocol that obtains pure EVs and maintains their functional characteristics is required. We implemented differential ultracentrifugation as a pre-enrichment step followed by OptiPrep density gradient centrifugation (dUC-ODG) to isolate EVs from the conditioned medium of cancer-associated adipose tissue. A combination of immune-electron microscopy, nanoparticle tracking analysis (NTA) and Western blot analysis identified EVs that are enriched in flotillin-1, CD9 and CD63, and sized between 20 and 200 nm with a density of 1.076–1.125 g/ml. The lack of protein aggregates and cell organelle proteins confirmed the purity of the EV preparations. Next, we evaluated whether dUC-ODG isolated EVs are functionally active. ZR75.1 breast cancer cells treated with cancer-associated adipose tissue-secreted EVs from breast cancer patients showed an increased phosphorylation of CREB. MCF-7 breast cancer cells treated with adipose tissue-derived EVs exhibited a stronger propensity to form cellular aggregates. In conclusion, dUC-ODG purifies EVs from conditioned medium of cancer-associated adipose tissue, and these EVs are morphologically intact and biologically active.
KEYWORDS: aggregation, breast cancer, characterization, exosomes, function, isolation, proliferation
Introduction
Heterotypic cellular interactions are a prerequisite for primary tumor growth and metastasis.1 In breast cancer, adipose tissue is the main component of the tumor environment. Adipose tissue is no longer regarded as simply an inert store of excess energy, but as an endocrine organ that promotes interaction with cancer cells both through cell-cell and cell-matrix contacts as well as through secreted signaling molecules.2 It is an architecture of mature adipocytes, progenitor cells, endothelial cells, fibroblasts, macrophages and immune cells of which the relative composition changes due to obesity, inflammatory conditions and cancer. Multiple cellular elements of the adipose tissue and their products stimulate breast cancer cells toward further progression. Research into the communication between adipose tissue and cancer cells has mostly been focused on matrix contacts such as type VI collagen and soluble pro-inflammatory factors such as interleukin-6 and oncostatin M among others.3,4 In addition, emerging evidence suggests that extracellular vesicles (EVs) also play a role in tumor environment communications. EVs are nanometer-sized entities which contain numerous proteins, lipids and nucleic acids and are secreted by most cell types.5 A role of EVs in cell-to-cell communication was evidenced by the functional translation of EV mRNAs from a donor cell by target cells.6 Cancer patients have an increased number of EVs in the circulation and this number correlates with disease progression.7 However, it is not yet known whether these EVs are cancer cell- or host-specific.
Human abdominal adipose tissue explants produce EVs that modulate monocyte differentiation and alter insulin signaling in adipocytes and liver cells.8,9 Mouse visceral adipose tissue EVs mediate activation of macrophage-induced insulin resistance.10 Cultures of murine pre-adipocytes have also been shown to release EVs and these EVs stimulate fatty acid-oxidation dependent migration of melanoma cells.11 Deng et al., Kranendonk et al. and Lazar et al. have applied differential ultracentrifugation (dUC) to isolate EVs. dUC is a combination of centrifugation steps with increasing centrifugal strength to sequentially pellet cells (1500 g), larger EVs (10,000 g) and smaller EVs (100,000 g). Lazar et al. and Kranendonk et al. both washed the 100,000 g pellet in a large volume to reduce non-vesicular proteins, and centrifuged one last time at the same high speed. For some applications it may be advisable to include an extra purification step using density gradient centrifugation.12,13 This step eliminates more contaminants, such as proteins non-specifically associated with EVs, or large protein aggregates, which are sedimented by centrifugation but do not float on a density gradient.14 Kranendonk et al. further purified the dUC pellet by a sucrose density gradient to obtain high purity EVs.
The results of these pioneer studies warrant further investigation into the role of adipose tissue derived EVs in disease and cancer progression in particular. To be able to fully exploit the potential of EVs, standardized methodology for EV isolation and characterization is a crucial requirement. We prepared secretomes of cancer-associated adipose tissues derived from breast cancer patients and combined differential ultracentrifugation with a density gradient based floatation of EVs. Purified EVs were characterized for EV-enriched and non EV-enriched proteins, morphology, size distribution, number and functionality.
Material and methods
Conditioned medium of cancer-associated adipose tissue
Cancer associated adipose tissue (CAAT) was obtained from breast cancer patients undergoing mastectomy at Ghent University Hospital in accordance with local ethics committee and written informed consent was obtained from all subjects. The breast adipose tissue was devoid of fibrosis, washed in sterile phosphate-buffered saline (PBS), cut into pieces of approximately 1–2 mm3 and placed in DMEM/F12 culture medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml fungizone and 0.5% Bovine Serum Albumin (BSA) (Sigma-Aldrich), further called ‘control medium’, in 6-well plates at a ratio of 250 mg of adipose tissue per ml control medium. The 6-well plates were placed on a nutating mixer at 20 rpm (for constant hydration and maximal oxygen supply) (VWR International, Radnor, PA) in an incubator at 37°C and 5% CO2. After 24 h, the conditioned medium (CM) containing factors of cancer-associated adipose tissue (CMCAAT) was harvested and centrifuged for 10 min at 500 g and 4°C. The lipids floating on top of the CM were removed with a Pasteur pipette and the CM above the small pellet with debris was further centrifuged for 15 min at 1500 g, 30 min at 10,000 g and filtered through a 0.2 µm filter. CMCAAT was stored at −80°C until experimentation.3
Antibodies and reagents
The following primary and secondary antibodies were used for immunostaining: mouse monoclonal anti-Alix (1:1,000) (2171, Cell Signaling, Danvers, MA, USA), rabbit polyclonal anti-prohibitin (1:500) (NBP1–40505, Novus Biologicals), rabbit polyclonal anti-calreticulin (1:1,000) (2891, Cell Signaling), antiphospho-CREB (1:1000) (9198, Cell Signaling), anti-CREB (Cell Signaling), rabbit monoclonal anti-CD9 (1:1000) (D3H4P, Cell Signaling), mouse monoclonal anti-flotillin-1 (1:1000) (610820, BD Biosciences, Franklin Lakes, NJ, USA), mouse monoclonal anti-GM130 (1:500) (610822, Becton Dickinson, Franklin Lakes, NJ, USA), rabbit polyclonal anti-HSP70 (1:1,000) (EXOAB-HSP70A-1, System Biosciences, Mountain View, CA, USA). Secondary antibodies coupled to horseradish peroxidase were obtained from Amersham Pharmacia Biotech (Diegem, Belgium). OptiPrep™ was purchased from Axis-Shield PoC (Oslo, Norway).
Ultracentrifugation
Seventy-five ml of CMCAAT was transferred to open top polyallomer centrifuge tubes (Beckman Coulter, Fullerton, CA) and centrifuged for 2 h at 100,000 g and 4°C in a swinging bucket rotor (Optima XPN- 80, SW 55 Ti rotor, Beckman Coulter). The pellet was resuspended in 15 ml of PBS and centrifuged again for 2 h at 100,000 g. The resulting pellet was resuspended in 1 ml of PBS and further purified by OptiPrep™ density gradient centrifugation.
OptiPrep™ density gradient centrifugation
A discontinuous iodixanol gradient was used as described by Van Deun et al.13 Solutions of 5%, 10%, 20% and 40% iodixanol were made by mixing appropriate amounts of a homogenization buffer (0.25 M sucrose, 1 mM EDTA, 10 mM Tris-HCL, [pH 7.4]) and an iodixanol working solution. This working solution was prepared by combining a working solution buffer (0.25 M sucrose, 6 mM EDTA, 60 mM Tris-HCl, [pH 7.4]) and a stock solution of OptiPrep™ (60% (w/v) aqueous iodixanol solution). The gradient was formed by layering 4 ml of 40%, 4 ml of 20%, 4 ml of 10% and 3.5 ml of 5% solutions on top of each other in a 16.8 ml open top polyallomer tube (Beckman Coulter). 1 ml of resuspended EV pellet was overlaid onto the top of the gradient which was then centrifuged for 18 h at 100,000 g and 4°C (SW 32.1 Ti rotor, Beckman Coulter). Gradient fractions of 1 mL were collected from the top of the gradient, diluted to 16 ml in PBS and centrifuged for 3 h at 100,000 g and 4°C. The resulting pellets were resuspended in 100 µl PBS and stored at −80°C. The purity of the EV preparations was assessed according to the MISEV guidelines.15
Western blot analysis
To measure protein concentration of isolated EVs, 5 µl sample was mixed with 5 µL of Laemmli lysis buffer (0.125 M Tris–HCl [pH 6.8], 10% glycerol, 2.3% sodium dodecyl sulfate [SDS]). Protein concentration was determined using the Bio-Rad DC Protein Assay (Bio-Rad, Hercules, USA). For protein analysis, 10 µg of EV protein was lysed in reducing sample buffer (1 M Tris–HCl [pH 6.8], 30% glycerol, 6% SDS, 3% 2-mercaptoethanol, 0.005% bromophenol blue) and boiled at 95°C during 5 min. Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Hercules, California, USA). After blocking the membranes, blots were incubated overnight with primary antibodies. Incubation with secondary antibodies was performed after extensive washing of the membranes in PBS with 0.5% Tween20. After final extensive washing, chemiluminescence substrate (WesternBright Sirius, Advansta, Menlo Park, California, USA) was added and imaging was performed using Proxima 2850 Imager (IsoGen Life Sciences, De Meern, The Netherlands). Quantification of protein bands was performed using Image J software.
Nanoparticle tracking analysis (NTA)
Aliquots of isolated particles were used for NTA using NanoSight LM10 microscope (NanoSight Ltd, Amesbury, UK) equipped with 405 nm laser. For each sample, 3 videos of 60 seconds were recorded and analyzed with overall camera level 13 and detection threshold 5 in standard mode. The measurements were performed at ambient temperature which monitored and did not exceed 25°C. Recorded videos were analyzed with NTA Software version 3.1. For optimal measurements, samples were diluted with PBS until particle concentration was within the concentration range of NTA Software (between 3*108 and 5*108 particles/ml).16
Immune-electron microscopy
Isolated EVs were deposited and incubated on Formvar carbon-coated, glow-discharged grids as described previously.13 After 20 min, the grids were incubated in a blocking serum containing 1% BSA in PBS. Antibodies and gold conjugates were diluted in 1% BSA in PBS. The grids were exposed to the primary anti-CD63 antibody (clone H5C6) (557305, Becton Dickinson) for 20 min, followed by secondary antibody to rabbit anti-mouse IgG (Zymed, San Francisco, CA, USA) for 20 min and protein A-gold complex (10 nm size17) (CMC Utrecht, The Netherlands) for 20 min. The efficiency of blocking was controlled by performing the labeling procedure in the absence of the primary antibody. The grids were stained with neutral uranylacetate and embedded in methylcellulose/uranyl acetate and examined in a Tecnai Spirit transmission electron microscope (FEI, Eindhoven, The Netherlands). Images were captured by Quemesa charge-coupled device camera (Olympus Soft Imaging Solutions GMBH, Munster, Germany).
Functional assays
MCF-7 cells and ZR75.1 cells were obtained from the ATCC (http://www.lgcstandards-atcc.org). Cells were maintained in ‘culture medium’ which is DMEM supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 mg/ml streptomycin (Invitrogen).
To analyze the impact of CAAT-derived EVs on phosphorylation of CREB, semi-confluent ZR75.1 cells (1×106 cells) were treated with 1×108 EVs (corresponding to a dose of 100 EVs/cell) in 2,5 ml control medium and 48 h later lysates were prepared and analyzed by Western blot analysis.
To analyze tumor sphere formation, viable, single MCF-7 cells (5×104 cells/well) were transferred in a well of an ultra-low adherence 96-well plate (Corning, Avon, France) in 200 µl of culture medium supplemented or not with 5×109 EVs (corresponding to a dose of 10000 EVs/cell). The plate was placed into an IncuCyte™ FLR imaging system (Essen Biosciences, Welwyn Garden City, UK) within a regular cell culture incubator (37°C, 95% humidity, 5% CO2). Tumor spheres were allowed to grow during 4 d and spheroid formation was monitored every 3 h by IncuCyte™. Spheroid size increase was measured by IncuCyte™ software.
Results
Analysis of (non)-EV enriched proteins in EVs isolated from CMCAAT by dUC-ODG
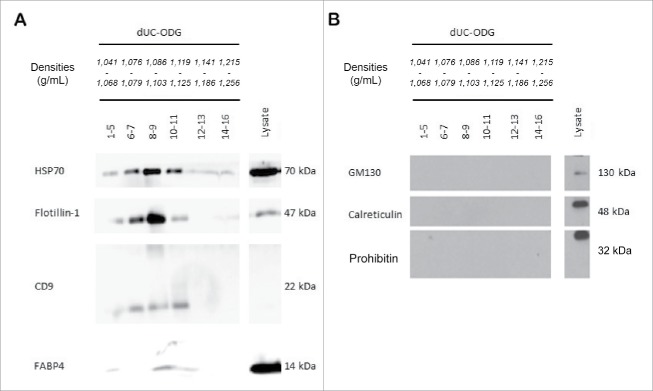
EVs were prepared from the secretome of ex vivo-cultivated breast cancer-associated adipose tissue (CMCAAT). The short, 24 h ex vivo cultivation did not affect adipose tissue integrity, metabolic activity and viability as evidenced by H&E staining, MTT uptake and monitoring lactate dehydrogenase(LDH) release (data not shown[3]).3 CMCAAT was pre-purified by short-term low-speed centrifugation steps and subsequently purified by a combination of dUC with ODG (dUC-ODG) (Fig. 1). Western blot demonstrated presence of the EV-enriched proteins HSP70, flotillin-1 and tetraspanin CD9 in lysates from the EVs obtained by the dUC-ODG protocol (Fig. 2A). These EV-enriched proteins are detected in different density fractions (1.076–1.125 g/ml). The adipocyte-specific fatty-acid binding protein 4 (FABP-4) is uniquely present in EV-enriched density fractions. All EV preparations are clear from contaminating cell organelles as indicated by the absence of proteins of the Golgi apparatus (GM130), the mitochondria (prohibitin) or the endoplasmic reticulum and apoptotic bodies (calreticulin) (Fig. 2B).
Figure 1.
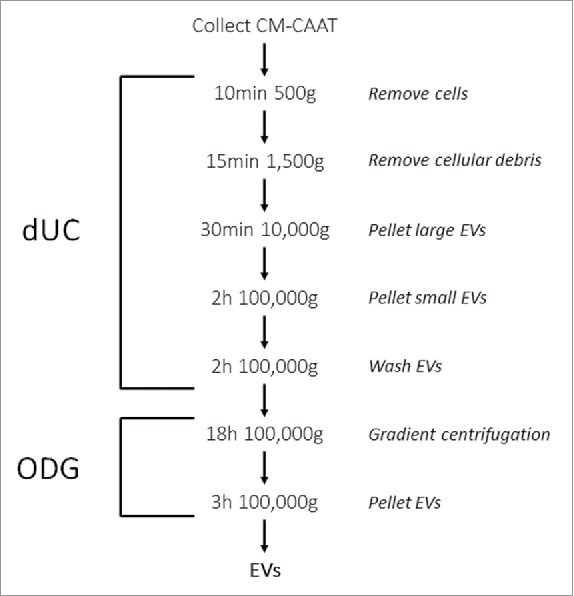
Schematic overview of the dUC-ODG protocol to isolate EVs from cancer-associated adipose tissue-derived conditioned medium (CMCAAT). Approximately 21 g of CAAT was ex vivo cultivated in control medium. CMCAAT was harvested, centrifuged and used for further isolation by a combination of differential ultracentrifugation followed by Optiprep density gradient centrifugation.
Figure 2.
Protein analysis of (non) EV-enriched proteins. EVs were isolated from the conditioned medium of cancer-associated adipose tissue by the dUC-ODG protocol. Western blot analysis of (A) EV-enriched proteins (flotillin-1, CD9 and HSP70) and adipocyte-specific protein FABP-4 and (B) cell organelle and apoptotic body proteins (GM130, prohibitin and calreticulin).
Determination of EV size, number and purity
EV preparations of ODG fractions 8–9 with a corresponding density of 1.086–1.103 g/ml were loaded onto carbon-coated grids and analyzed by immune-electron microscopy (Fig. 3A). This revealed a typically heterogeneous EV population consisting of a few CD63-positive and abundant CD63-negative EVs (33 EVs out of 300 EVs counted on EM images were CD63 positive) with a size range between 20 and 200 nm in diameter. The EV preparations are most enriched in EVs sized smaller than 70 nm. Contaminating protein aggregates were not detected. The dUC-ODG isolation protocol retrieved 2.2×109 EVs/g cancer-associated adipose tissue as quantified by nanoparticle tracking analysis (NTA). NTA revealed that the modus, i.e. the highest number of particles with similar size, of EV preparations obtained by dUC-ODG is 116 nm (Fig. 3B). This indicates that the most abundant EVs in the preparations sized smaller than 70 nm as analyzed by electron microscopy are not measured by NTA. As such the quantification of the number of particles released/g adipose tissue is likely to be underestimated.
Figure 3.
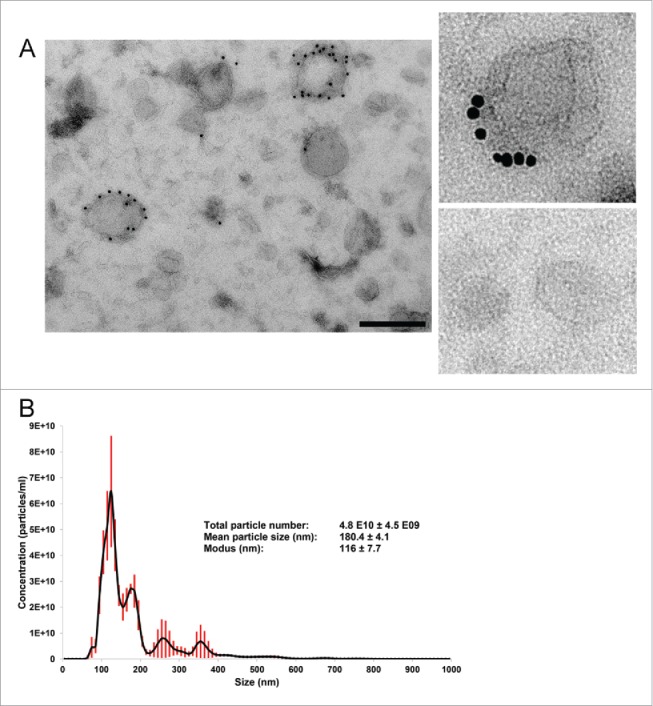
Morphological characterization and quantification of EV preparations by electron microscopy (EM) and nanoparticle tracking analysis (NTA). EVs were isolated from the conditioned medium of cancer-associated adipose tissue by the dUC-ODG protocol. (A) Left: Wide-field EM picture of EVs of fractions 8–9 fsrom the density gradient corresponding to the density of 1.086–1.103 g/ml. Scale bar: 200 nm. Right: Zoom in on CD63-positive and negative EVs. (B) The calculated size distribution of EVs analyzed by NTA depicted as a mean (black line) with standard error (red shaded area). Total particle number, mean particle size and modus are shown.
Assessment of the functional activity of dUC-ODG isolated EVs
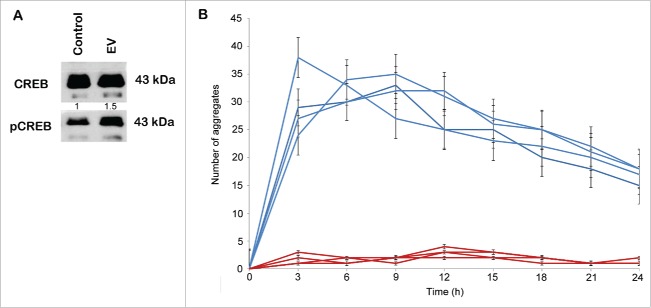
Next, we determined whether CMCAAT EVs obtained by dUC-ODG show functional activity. ZR75.1 breast cancer cells were treated with 1 × 108 EVs, as determined by NTA, supplemented in DMEM containing 0.5%BSA. After 48 h of incubation with either control medium or control medium supplemented with CMCAAT EVs, lysates were prepared and analyzed by Western blot. ZR75.1 showed a higher phosphorylation of CREB serine residue 133 after CMCAAT EV treatment suggesting that EVs contribute to the proliferative effect of adipose tissue on breast cancer cells (Fig. 4A).
Figure 4.
Stimulation of CREB transcription factor phosphorylation and sphere formation by EVs. EVs were isolated from the conditioned medium of cancer-associated adipose tissue by the dUC-ODG protocol. (A) Western blot analysis of phospho-CREB and total CREB from lysates of ZR75.1 cells under control conditions or treated by 1×108 EVs. (B) The number of aggregates formed by MCF7 single cells seeded in ultra-low attachment plates under control conditions or treated by 5×109 EVs for 24h. Each experiment was performed in quadruplicate.
The formation of spherical cellular aggregates (spheres) of MCF-7 cells was monitored via the IncuCyte system over a 5-day time course, allowing comparison of control condition and EV treatment (Fig. 4B and Supplementary Movies 1 and 2). Freshly dissociated single MCF-7 cells exhibit a strong and fast propensity to aggregate in culture on ultra-low attachment plates, with the formation of one large sphere being evident as early as 9 h after seeding. This large aggregate becomes more compact over time. Under treatment with 5 × 109 EVs, multiple small spheres are apparent 3 hours after dissociation and treatment. These smaller spheres coalesce with each other to form a large, continuously expanding sphere that becomes larger in size compared with control conditions.
Discussion
To accurately define EV-specific content, and thus understand the functional significance of intercellular EV communication, there is a growing need for standardized and validated isolation methods to obtain pure EVs. We combined dUC as a pre-purification method (13, 14), with ODG, a floatation-based separation method, and analyzed the EV samples for the presence of (non) EV-enriched proteins, morphology, size and number.
Characteristics of the dUC-ODG protocol are listed in Table 1. We found that the protocol was able to isolate EVs from conditioned medium of cancer-associated adipose tissue from breast cancer patients, as illustrated by Western blot analysis, immune electron microscopy and NTA. A practical advantage of dUC as a pre-enrichment method is the use of large volumes and the EV-containing pellet can be resuspended in a volume of choice, which in general depends on the next step. Clustering or aggregation of EVs may be induced during dUC18 but is not apparent from electron microscopy analysis which is performed after dUC followed by ODG. Kranendonk et al. combined the dUC method with a sucrose density gradient. While both sucrose and iodixanol separate EVs based on density, reports in literature encourage the use of iodixanol-based gradients for improved separation of EVs from viruses and small apoptotic bodies.19 Also, unlike sucrose, iodixanol is capable of forming iso-osmotic solutions at all densities, thus better preserving the size of EVs in the gradient.20
Table 1.
Characteristics of the dUC-ODG protocol implemented to isolate EVs from CMCAAT.
| dUC-ODG | |
|---|---|
| Sample volume limitation | No |
| Aggregation of EVs | No |
| Protein interference | No |
| Yield | 2.2*109 EVs/g adipose tissue |
| Functional activity | Yes |
Adipose tissue has been shown to secrete several cytokines potentially affecting breast cancer cells but the functional effect of adipose tissue-derived EVs is less clear. Our study demonstrates that highly purified EVs induce phosphorylation of CREB at Serine residue 133. CREB is a bZIP transcription factor that activates target genes through cAMP response elements. CREB is able to mediate signals from numerous physiologic stimuli, resulting in regulation of a broad array of cellular responses. CREB promotes cellular survival and is activated by various signaling pathways including Erk, Ca2+ and stress signaling. Some of the kinases involved in phosphorylating CREB at Serine residue 133 are p90RSK, MSK, CaMKIV, and MAPKAPK-2. The increased phosphorylation of CREB in ZR75.1 breast cancer cells by treatment with adipose tissue-derived EVs is in accordance with our previous findings where conditioned medium of cancer-associated adipose tissue from breast cancer patients stimulates CREB phosphorylation in MCF-7 and T47D breast cancer cells and induces the differential transcription of CREB target genes (unpublished). This underlines the functional activity of adipose tissue-derived EVs and supports the idea of EVs as an additional player in cell-cell communication.
Real-time imaging on ultra-low attachment plates revealed effects of adipocyte tissue derived EVs on the aggregation of cancer cells. Consistently with the functional effect of CREB phosphorylation, aggregates from breast cancer cells treated with EVs were larger in size compared with the control conditions. In addition, live-imaging revealed that in the presence of EVs the cancer cells formed multiple small cellular aggregates before merging together in one single cellular aggregate. The effect on aggregation was already prominent in the first 24 h of the experiment excluding that the difference in aggregation is just a reflection of the difference in proliferation considering that the population doubling time of MCF-7 cells is 24h. This indicates that EVs might change the expression of surface proteins to enhance aggregation, but also that EVs might be scaffolds to capture and stimulate the growth of cancer cells. In cancer, the activation of endothelial cells by EVs also induces the adhesion of platelets and the formation of platelet aggregates.21 This is probably due to an increased expression of platelet adhesion molecules in endothelial cells after EV uptake. The effect of EVs on protein aggregation has been studied in neurologic diseases. EVs catalyze the aggregation of α-synuclein in Parkinson disease22 and presumably act as a seed for amyloid plaque nucleation in Alzheimer disease.23 Similarly EVs might induce the formation of protein aggregates as scaffolds for cancer cells to adhere. Also, the detachment of adherent cells induces a rapid and substantial secretion of EVs, which then concentrate on the cell surfaces and mediate adhesion to various extracellular matrix proteins.24 Cancer-associated adipose tissue derived EVs may also concentrate on the cancer cell surface to enhance adhesion. Fluorescent labeling of EVs to follow their destination (cell surface or cytosol) might improve our understanding of the role of EVs in aggregation of breast cancer cells.
A limitation of this study is the lack of an irrelevant EV control such as EVs from different origin to further strengthen the functional observations of CAAT EVs on proliferation and aggregation. Indeed, the default state of the breast cancer cell cultures is exposure to EVs from fetal bovine serum (FBS) for many passages. Preparation of conditioned medium after depleting FBS results in diminished growth of the cultured cells.25 Adding another source of EVs, especially in the excess as used in the aggregation experiment (a dose of 10000 EVs/cell), might displace or outcompete the FBS EVs almost entirely and influence aggregation of the MCF-7 cells. A second limitation is the use of different doses in the phosphorylation experiments versus the aggregation experiments. Future work should compare dose response effects of purified EVs on functional and biochemical cellular activities. In addition, to further understand the functional role of EVs in cell culture experiments it is important to report the experimental parameters of the functional assays.26 Current work shows the ex vivo secretion of EVs by adipose tissue but it doesn't allow the identification of the cell types responsible for EV production. FABP-4, an adipocyte specific protein, is present in fractions containing EV-enriched proteins suggesting the presence of adipocyte-specific EVs. Although adipocyte EVs are identified, it is speculative to suggest that adipocyte-derived EVs activate a pro-survival pathway in breast cancer cells based on current experimentation. Other cell types such as inflammatory cells, endothelial cells and fibroblasts are part of the stroma vascular fraction in adipose tissue and may contribute to the presence of EVs in adipose tissue-conditioned medium. The relative contribution of each of these cell types to the CAAT-derived EV cocktail is a subject of further investigation. Cell type-specific EV proteins combined with flow cytometry may enable the identification and relative contribution of adipose tissue-derived EVs. Alternatively, insight in the protein, RNA and lipid content of EVs from cancer-associated adipose tissue can potentially inform us about the different cell types that home the adipose tissue and as such broaden our understand of this complex tumor environment.
In conclusion, we validated the combined use of dUC and ODG centrifugation to isolate EVs from a complex ex vivo-prepared biofluid. CAAT secretes EVs positive for FABP-4 that stimulate CREB activation and sphere formation of breast cancer cells.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank the staff of the Electron Microscopy Laboratory (Biocenter Oulu, University of Oulu, Finland) and Sofie De Geyter, Glenn Wagemans and Davy Waterschoot for excellent technical assistance.
Funding
This study was supported by Fund for Scientific Spearheads of the Ghent University Hospital, Concerted Research Actions from Ghent University, the National Cancer Plan (KPC_29_012), Kom op tegen Kanker, a doctoral grant (JVD) from Fund for Scientific Research-Flanders, a postdoctoral grant and Krediet aan Navorsers (AH) from Fund for Scientific Research-Flanders.
References
- [1].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144(5):646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- [2].Lapeire L, Denys H, Cocquyt V, De Wever O. When fat becomes an ally of the enemy: adipose tissue as collaborator in human breast cancer. Horm Mol Biol Clin Investig 2015; 23(1):21-38; PMID:26154196; http://dx.doi.org/ 10.1515/hmbci-2015-0018 [DOI] [PubMed] [Google Scholar]
- [3].Lapeire L, Hendrix A, Lambein K, Van Bockstal M, Braems G, Van Den Broecke R, Limame R, Mestdagh P, Vandesompele J, Vanhove C, et al.. Cancer-associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and Jak/STAT3 signaling. Cancer Res 2014; 74(23):6806-19; PMID:25252914; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0160 [DOI] [PubMed] [Google Scholar]
- [4].Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, et al.. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 2011; 71(7):2455-65; PMID:21459803; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-3323 [DOI] [PubMed] [Google Scholar]
- [5].Hendrix A, Hume AN. Exosome signaling in mammary gland development and cancer. Int J Dev Biol 2011; 55(7–9):879-87; PMID:22161843; http://dx.doi.org/ 10.1387/ijdb.113391ah [DOI] [PubMed] [Google Scholar]
- [6].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9(6):654-9; PMID:17486113; http://dx.doi.org/ 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- [7].Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al.. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18(6):883-91; PMID:22635005; http://dx.doi.org/ 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kranendonk ME, Visseren FL, van Balkom BW, Nolte-'t Hoen EN, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MH, et al.. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring, Md) 2014; 22(5):1296-308; PMID:24339422; http://dx.doi.org/ 10.1002/oby.20679 [DOI] [PubMed] [Google Scholar]
- [9].Kranendonk ME, Visseren FL, van Herwaarden JA, Nolte-'t Hoen EN, de Jager W, Wauben MH, Kalkhoven E. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring, Md) 2014; 22(10):2216-23; PMID:25045057; http://dx.doi.org/ 10.1002/oby.20847 [DOI] [PubMed] [Google Scholar]
- [10].Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, Wang J, Xiang X, Zhang S, Zhuang X, et al.. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009; 58(11):2498-505; PMID:19675137; http://dx.doi.org/ 10.2337/db09-0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lazar I, Clement E, Dauvillier S, Milhas D, Ducoux-Petit M, LeGonidec S, Moro C, Soldan V, Dalle S, Balor S, et al.. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res 2016; 76(14):4051-7; PMID:27216185 [DOI] [PubMed] [Google Scholar]
- [12].Zonneveld MI, Brisson AR, van Herwijnen MJ, Tan S, van de Lest CH, Redegeld FA, Garssen J, Wauben MH, Nolte-'t Hoen EN. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J Extracell Vesicles 2014; 3; PMID:25206958; http://dx.doi.org/ 10.3402/jev.v3.24215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles 2014; 3; http://dx.doi.org/ 10.3402/jev.v3.24858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006; Chapter 3:Unit 3:22.. [DOI] [PubMed] [Google Scholar]
- [15].Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al.. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014; 3:26913; PMID:25536934; http://dx.doi.org/ 10.3402/jev.v3.26913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gardiner C, Ferreira YJ, Dragovic RA, Redman CW, Sargent IL. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles 2013; 2; http://dx.doi.org/ 10.3402/jev.v2i0.19671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Slot JW, Geuze HJ. A new method of preparing gold probes for multiple-labeling cytochemistry. Euro J Cell Biol 1985; 38(1):87-93; PMID:4029177 [PubMed] [Google Scholar]
- [18].Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles 2015; 4:29509; PMID:26700615; http://dx.doi.org/ 10.3402/jev.v4.29509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cantin R, Diou J, Belanger D, Tremblay AM, Gilbert C. Discrimination between exosomes and HIV-1: purification of both vesicles from cell-free supernatants. J immunol Methods 2008; 338(1–2):21-30; PMID:18675270; http://dx.doi.org/ 10.1016/j.jim.2008.07.007 [DOI] [PubMed] [Google Scholar]
- [20].Dettenhofer M, Yu XF. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol 1999; 73(2):1460-7; PMID:9882352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Terrisse AD, Puech N, Allart S, Gourdy P, Xuereb JM, Payrastre B, Sié P. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J Thromb Haemos 2010; 8(12):2810-9; PMID:21029362; http://dx.doi.org/ 10.1111/j.1538-7836.2010.04088.x [DOI] [PubMed] [Google Scholar]
- [22].Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S. Acceleration of alpha-synuclein aggregation by exosomes. J Biol Chem 2015; 290(5):2969-82; PMID:25425650; http://dx.doi.org/ 10.1074/jbc.M114.585703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dinkins MB, Enasko J, Hernandez C, Wang G, Kong J, Helwa I, Liu Y, Terry AV Jr, Bieberich E. Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer's Disease Pathology and Improves Cognition in the 5XFAD Mouse. J Neurosci 2016; 36(33):8653-67; PMID:27535912; http://dx.doi.org/ 10.1523/JNEUROSCI.1429-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PloS One 2011; 6(9):e24234; PMID:21915303; http://dx.doi.org/ 10.1371/journal.pone.0024234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eitan E, Zhang S, Witwer KW, Mattson MP. Extracellular vesicle-depleted fetal bovine and human sera have reduced capacity to support cell growth. J Extracell Vesicles 2015; 4:26373; PMID:25819213; http://dx.doi.org/ 10.3402/jev.v4.26373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dhondt B, Rousseau Q, De Wever O, Hendrix A. Function of extracellular vesicle-associated miRNAs in metastasis. Cell Tissue Res 2016; 365(3):621-41; PMID:27289232; http://dx.doi.org/ 10.1007/s00441-016-2430-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.