Abstract
Wild birds are an important nonpoint source of fecal contamination of surface waters, but their contribution to fecal pollution is mostly difficult to estimate. Thus, to evaluate the relation between feces production and input of fecal indicator bacteria (FIB) into aquatic environments by wild waterfowl, we introduced a new holistic approach for evaluating the performance of FIB in six shallow saline habitats. For this, we monitored bird abundance, fecal pellet production, and the abundance of FIB concomitantly with a set of environmental variables over a 9-month period. For estimating fecal pellet production, a new protocol of fecal pellet counting was introduced, which was called fecal taxation (FTX). We could show that, over the whole range of investigated habitats, bird abundance, FTX values, and FIB abundance were highly significantly correlated and could demonstrate the good applicability of the FTX as a meaningful surrogate parameter for recent bird abundances and fecal contamination by birds in shallow aquatic ecosystems. Presumptive enterococci (ENT) were an excellent surrogate parameter of recent fecal contamination in these saline environments for samples collected at biweekly to monthly sampling intervals while presumptive Escherichia coli and fecal coliforms (FC) were often undetectable. Significant negative correlations with salinity indicated that E. coli and FC survival was hampered by osmotic stress. Statistical analyses further revealed that fecal pollution-associated parameters represented one system component independent from other environmental variables and that, besides feces production, rainfall, total suspended solids (direct), and trophy (indirect) had significant positive effects on ENT concentrations. Our holistic approach of linking bird abundance, feces production, and FIB detection with environmental variables may serve as a powerful model for application to other aquatic ecosystems.
Wild waterfowl are known to be an important nonpoint source of fecal contamination of surface waters (1, 8, 15, 25, 30). Wild birds have been reported to excrete large amounts of fecal indicator bacteria (FIB) (8, 25) and occasionally harbor enteric pathogens (20, 21). Especially for public swimming beaches, fecal contamination by wild birds can therefore impose a severe problem for the persons in charge to meet the legal limits for FIB. Locating the source of contamination, however, is often a tricky business, and the contribution of wild birds to the total contamination of surface waters is mostly difficult to estimate. Thus, it would be considerably helpful to better understand the relations between feces production by wild waterfowl and their input of FIB into aquatic environments.
We therefore monitored, over a 9-month period, the relations between bird abundances, their fecal pellet production, and three groups of FIB in six shallow, modestly saline aquatic environments. As the investigated saline pools are nearly exclusively visited by birds and no other animals and because they exhibit a steep gradient in the average number of wild birds present from single observations of a small number of individuals to frequently observed large flocks of waterfowl populations, we considered them to be ideal environments for the study of the relationship between these parameters. For estimating fecal pellet production, a new approach was introduced, quantifying the fecal pellets along the shoreline of the water. The use of fecal pellets to trace the abundance of wild animals has been used several times (11, 19, 33), but to our knowledge, no attempt has been made to use this method for birds. In addition, a set of environmental variables was monitored to better understand the relationship between bird abundance, fecal pellet production, and the abundance of FIB as well as to clarify which group of FIB is best suited for indicating fecal pollution by waterfowl in these modestly saline environments.
To our knowledge, this study is the first approach which simultaneously surveys the relationship between animal abundance, feces production, and the subsequent determination of FIB in aquatic ecosystems and which links their indicator performance with a set of important environmental variables.
MATERIALS AND METHODS
Study site and sampling.
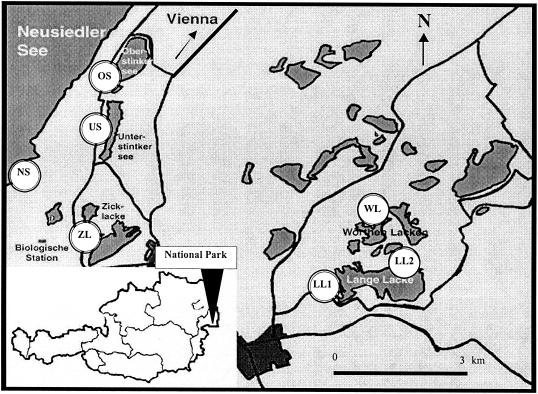
The study site, the national park Neusiedler See-Seewinkel, is located in Eastern Austria and represents one of the most important bird-protecting areas in middle Europe (Fig. 1). Large bird populations of a variety of species visit the national park for breeding, molting, and resting at the large number of ecologically different saltwater pools and the adjacent Lake Neusiedler See. A detailed description of these pools can be found in the work by Eiler et al. (7). Briefly, they are shallow (maximum depth, 0.70 m) hypertrophic ecosystems without inlet or outlet, and they exhibit broad gradients in salinity, in the concentration of total suspended solids (TSS), and in the abundances of feeding, resting, and breeding birds. Because of their shallowness, the water body is mostly mixed through the action of wind, and currents can be observed at high wind speeds. Samples were collected from five saline pools (Oberer Stinker [OS], Unterer Stinker [US], Zicklacke [ZL], Lange Lacke [LL], and Wörthenlacke [WL]) and one site within the reed belt of Lake Neusiedler See (NS) (Fig. 1) in biweekly intervals from May to September 2000 and once every 6 weeks from October 2000 to January 2001. Because of the large size of LL, two investigation sites were chosen for sampling (LL1 and LL2). Bird feces represents the overwhelming fecal pollution source in these ecosystems with the exception of WL, which has a significant input of cattle feces. Other sources of fecal pollution are negligible. An overview of their most important chemophysical characteristics is provided in Table 1. To avoid an influence of the time of day on our data set, the sampling sites were visited in rotating order on each sampling date.
FIG. 1.
Outline of the geographical position of the national park Neusiedler See-Seewinkel in Austria and locations of the 7 sampling sites: Neusiedler See (NS), Oberer Stinker (OS), Unterer Stinker (US), Zicklacke (ZL), Wörthenlacke (WL), and Lange Lacke 1 (LL1) and 2 (LL2).
TABLE 1.
Basic environmental parameters of investigated saline watersa
| Parameter | Result for b:
|
|||||
|---|---|---|---|---|---|---|
| OS | US | ZL | LL | WL | NS | |
| Area (km2) | 0.49 | 0.19 | 0.7 | 2.24 | 0.29 | 0.1 |
| Temp (°C) | 3.3-29.5 | 3.7-32.3 | 4.3-34.3 | 1.2-32.9 | 0.1-32.2 | 1.5-31.7 |
| Salinity (g liter−1) | 8.3 (4.2-39) | 4.8 (2.8-17.4) | 6.8 (2.7-17.8) | 3.8 (2.4-10.4) | 5.9 (2.4-30) | 2.7 (2.3-4.2) |
| pH | 9.8 (9.4-10.1) | 9.7 (9.0-10.2) | 10.0 (9.4-10.9) | 9.5 (9.1-9.9) | 9.3 (8.9-9.8) | 8.9 (8.5-9.4) |
| DOC (mg liter−1) | 72 (41-205) | 54 (31-142) | 66 (14-424) | 40 (20-117) | 43 (25-120) | 23 (16-60) |
| TSS (g liter−1) | 1.77 (0.32-7.0) | 0.84 (0.02-3.7) | 0.08 (0.02-0.5) | 0.30 (0.08-2.1) | 0.31 (0.04-2.4) | 0.03 (0.02-0.1) |
| PTOT (mg liter−1) | 3.3 (0.9-11.7) | 1.1 (0.2-3.9) | 0.5 (0.2-2.4) | 1.2 (0.3-4.4) | 1.3 (0.2-4.9) | 0.3 (0.16-1.9) |
| CHLA (μg liter−1) | 64 (4-133) | 72 (3-402) | 6.6 (0-48) | 199 (21-1,100) | 72 (0-135) | 2.5 (0.6-16) |
| BN (109 liter−1) | 72 (11-268) | 28 (4-232) | 10 (0.8-66) | 37 (7-195) | 22 (2.3-172) | ND |
| BP (μg of C liter−1 h−1) | 106 (2-738) | 89 (3-434) | 60 (5-348) | 143 (3-626) | 64 (1-376) | ND |
Abbreviations: OS, Oberer Stinker; US, Unterer Stinker; ZL, Zicklacke; LL, Lange Lacke; WL, Wörthenlacke; NS, Lake Neusiedler See; PTOT, total phosphorus; CHLA, chlorophyll a; BN, bacterial numbers; BP, heterotrophic bacterial production; ND, not determined.
Values are medians and ranges for the period from May 2000 to June 2001.
Estimation of bird abundance and feces input.
At each site and sampling time, the actual bird abundance of the whole area was estimated from 2 locations by observation with field glasses within a 30-min interval (23). At LL, birds at the two sampling sites were counted in areas of an approximate size of 0.25 km2. In the reed belt of Lake Neusiedler See, the estimated bird abundances are most probably underestimations, as mostly up-flying and shouting birds could be identified. A new protocol was introduced, quantifying the fecal pellets along the shore line of the water, which was called fecal taxation (FTX). Counting fecal pellets is a common method for estimating the abundance of fleeing animals (11, 19, 33), but to our knowledge, it has not been applied to wild birds. Along the current shore lines of the pools, fecal pellets were counted within a 100-m-long and 1-m-wide strip. Accumulations of feces which obviously represented a single deposit from 1 bird were counted as 1. Fecal pellets of the four dominant bird groups—ducks, geese, gulls, and peeps (Limicola)—were registered, which can be crudely distinguished from each other. In preliminary investigations, when fecal pellets were counted in fixed squares, no correlation could be found between bird abundance and fecal contamination because of the varying position of the shore line.
Indicators of fecal pollution.
The abundance of FIB was determined via the membrane filtration technique. Cellulose nitrate membrane filters (45-mm diameter, 0.45-μm pore size; Sartorius, Vienna, Austria) were used. Membrane filtration has usually been considered impracticable in highly turbid environments, but preliminary experiments, comparing membrane filtration with the most-probable-number method, showed that membrane filtration was appropriate for quantifying FIB in our highly contaminated environments. Samples were collected by using waders at a distance of about 10 m from the shore line at a depth of about 20 cm in sterile 250-ml glass bottles fixed to a long pole. The samples were brought to the laboratory in cooled plastic containers (<8°C). The maximum time that passed from sampling to laboratory analysis was 16 h for the samples taken in the early morning, the minimum time for the samples taken in the late evening was 2 h (median, 8 h). Duplicate serial dilutions were used to gain the optimal number of CFU (30 to 100) on the agar plates. mFC agar (Biomerieux, Lyon, France) was used to detect fecal coliforms (FC) (16). The agar plates were incubated for 24 h at 44.5°C in a water bath. No preincubation at lower temperature to recover potentially damaged or fragile cells was performed for two reasons. First, we wanted to follow the commonly used standard procedure. Second, preliminary experiments and the data from this study showed a nearly perfect correlation between Escherichia coli and FC for these ecosystems, which makes it unlikely to assume a significant underestimation of FC due to a high initial incubation temperature. For the detection of presumptive E. coli, Chromocult coliform agar (Merck, Darmstadt, Germany) was used (3). The agar plates were incubated for 48 h at 37°C. Presumptive enterococci (ENT) were enumerated by using m-Enterococcus agar (Merck) according to the method of the International Organisation of Standardisation (17). The plates were incubated for 44 h at 37°C.
Environmental variables.
Local precipitation data were provided by the government of the Province of Burgenland (Amt der burgenländischen Landesregierung, DI Sinowatz). The presented data represent the sum of precipitation in the week before each sampling event. Water temperature and conductivity were measured in situ with a LF 330 conductivimeter (WTW, Weilheim, Germany), and pH was measured with a GHM pH meter (Kappl-Seibold, Vienna, Austria) three times along 20-m transects toward the centers of the pools. Along the transects, three 1-liter water samples were collected and integrated for analysis of the other chemophysical variables. Ion concentrations were measured by ion chromatography after 1:10 dilution with aqua dest. All columns and chemicals were supplied by DIONEX (Sunnyvale, Calif.). For anions, AS-4A columns (PN 46074) with AG-4A precolumns (PN 37041) were used, and for cations, CS-12A columns (PN 46073) with CG-12A precolumns (PN 46074) were used. Total salinity was calculated as the sum of all measured cations and anions plus the estimated carbonate and bicarbonate concentrations (for details, see reference 7). For the determination of TSS, a defined volume of sample water was filtered through premuffled glass fiber filters (GF/C; Whatman, Springfield Mill, England) and dried to constant weight. Total phosphorus was determined photometrically after dissolution of the unfiltered sample with potassium peroxydisulfate by the molybdenum blue method as described by Strickland and Parsons (32). Chlorophyll a was extracted with 90% ethanol (1 h at 80°C) and was measured spectrophotometrically with a Hitachi U-2000 spectrophotometer (INULA, Vienna, Austria) (24). For the determination of bacterial numbers, 15-ml samples were preserved with formalin (4% [vol/vol] final concentration) for direct epifluorescence microscopy. Each sample consisted of three 5-ml subsamples collected along the 20-m transects. Cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI, 0.01% [vol/vol] final concentration) as described in detail by Eiler et al. (7). Bacterial numbers were counted with a Leitz Diaplan microscope (UV excitation, 340 to 380 nm; barrier filter, 430 nm; Leica, Solms, Germany). To measure bacterioplanktonic production, the [3H]leucine incorporation method was used and a protocol adapted from Kirschner and Velimirov (18) was followed (7). Briefly, four 1-ml samples and 2 blanks were collected along the 20-m transects and incubated with 180 μM [3H]leucine (ARC, St. Louis, Mo.). Proteins were precipitated with 5% trichloroacetic acid, and radioactivity was counted with a 1900 TR Packard scintillation counter (Canberra-Packard, Schwadorf, Austria). [3H]leucine incorporation rates were converted to carbon production (heterotrophic bacterial production) according to the method of Simon and Azam (28).
Statistical analysis.
Data were analyzed according to the method of Zar (35). For principal component analysis (varimax rotated with Kaiser normalization), multiple linear stepwise regression, and Pearson correlation, all data not meeting the requirements of homoscedasticity and normal distribution (Kolmogorov-Smirnov test) were log10 transformed after adding 1 to the variable. Nonparametric Spearman rank correlations were calculated for correlating median values. For all statistical analyses, SPSS, version 10.0, software for Windows was used.
RESULTS
Correlation between bird abundance, feces input, and FIB.
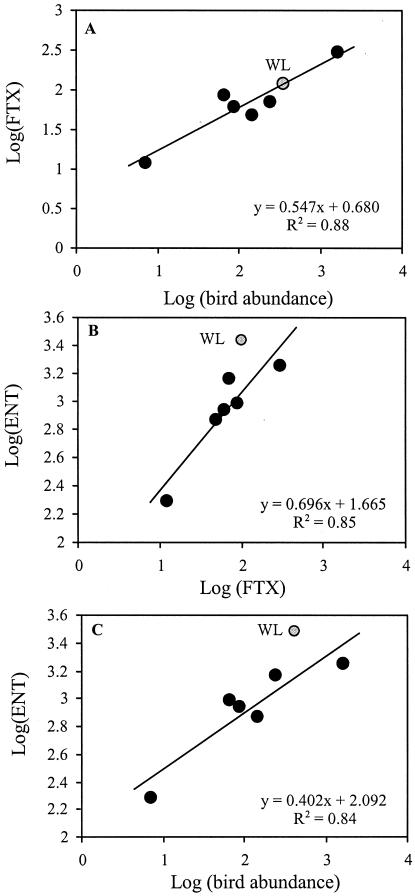
Within the area of the national park, the bird populations were unevenly distributed. Birds preferred LL and WL, where the highest median bird abundances (1,650 and 350 individuals per counting area, respectively) were observed (Table 2). The lowest median abundances were found at NS. This distribution pattern was also reflected by the median FTX values. The highest median (300 fecal pellets per 100 m2) was observed at LL2, and the lowest (11 fecal pellets per 100 m2) was observed at NS (Table 2). Also, the highest median values for FC and E. coli were found at LL2 (1,800 and 1,200 CFU 100 ml−1, respectively), whereas the highest median ENT numbers were observed at WL (2,650 CFU 100 ml−1). The lowest median numbers of FC and E. coli were detected at Zicklacke, and the lowest median ENT were detected at NS (Table 2). When log-transformed data were subject to correlation analysis, all investigated variables were significantly positively intercorrelated (Table 3). Bird abundance was highly significantly correlated with FTX (P < 0.001) and still significantly correlated with FC, E. coli, and ENT (P < 0.05). The FTX even showed a highly significant correlation with all 3 investigated FIB (P < 0.001). To account for the special characteristics of each environment, relations between the log-transformed median values of bird abundance, FTX, and ENT, pooled for each ecosystem, were plotted (Fig. 2). All regressions were highly significant, and their degrees of explanation, in all cases, were over 80%. The median values from WL deviated significantly from the regression lines of ENT on FTX and bird abundance (Fig. 2B and C) and were thus not included in the calculation of the regressions. ENT values were higher than predicted from the regressions due to the fact that at WL feces from cattle are also introduced to the water. Interestingly, all slopes were markedly lower than 1, ranging from 0.4 to 0.7 and indicating an increasing underestimation of FTX with increasing bird abundance and of ENT to FTX and bird abundance. Regressions of E. coli and FC were not appropriate to predict the abundance of these FIB groups from FTX and bird abundance (r < 0.5; P > 0.05).
TABLE 2.
Median and range (in parentheses) of bird abundance, feces input, and investigated FIB at the 7 sampling sitesa
| Site | No. of birds/surveillance area | FTX (feces 100 m−2) | FC (CFU 100 ml−1) | E. coli (CFU 100 ml−1) | ENT (CFU 100 ml−1) |
|---|---|---|---|---|---|
| OS | 65 (0-250) | 87 (0.0-297) | 42 (0-2,300) | 52 (0-1,900) | 870 (20-17,000) |
| US | 142 (44-490) | 47 (0-600) | 88 (0-18,000) | 70 (0-13,000) | 640 (0-60,000) |
| ZL | 85 (34-1,290) | 60 (2-167) | 0 (0-1,040) | 4 (0-620) | 780 (28-10,000) |
| LL1 | 240 (50-1,800) | 70 (12-378) | 410 (0-2,400) | 210 (10-1,760) | 1,380 (290-6,100) |
| LL2 | 1,650 (120-5,000) | 300 (90-600) | 1,800 (100-12,000) | 1,200 (50-3,900) | 1,700 (250-20,000) |
| WL | 350 (18-500) | 123 (9-1,700) | 1,150 (0-8,400) | 1,000 (0-10,400) | 2,650 (0-38,000) |
| NS | 6 (0-70) | 11 (0-300) | 110 (0-720) | 54 (4-900) | 96 (4-340) |
Abbreviations: OS, Oberer Stinker; US, Unterer Stinker; ZL, Zicklacke; LL1 and LL2, two sampling stations at Lange Lacke; WL, Wörthenlacke; NS, Lake Neusiedler See.
TABLE 3.
Pearson correlation between log-transformed data of bird abundance, feces input, and investigated FIB
| Parameter (n) | Correlation (r) with:
|
|||
|---|---|---|---|---|
| FTX | FC | E. coli | ENT | |
| Bird abundance (79) | 0.569a | 0.255b | 0.317c | 0.237d |
| FTX (80) | 0.479a | 0.481a | 0.473a | |
| FC (85) | 0.976a | 0.729a | ||
| E. coli (85) | 0.739a | |||
P = 0.000.
P = 0.024.
P = 0.004.
P = 0.035.
FIG. 2.
Regressions between bird abundance and FTX (A), FTX and ENT (B), and bird abundance and ENT (C). Data represent log-transformed median values for each ecosystem. Grey points represent the data from Wörthenlacke (WL), which were not included in the calculation of the regressions in panels B and C.
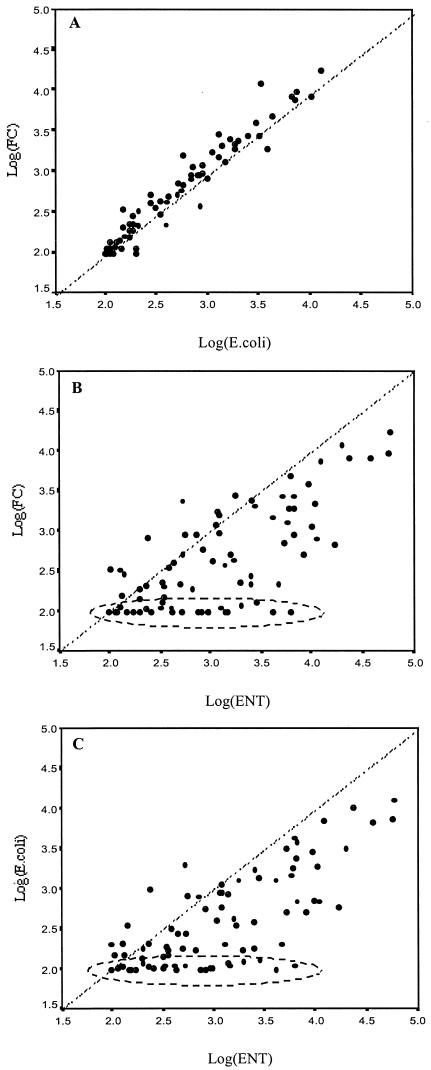
Among the FIB, the strongest correlation could be found between FC and E. coli, with an r of 0.976; the correlation of FC and E. coli to ENT was weaker, albeit still highly significant (r = 0.729 and 0.739, respectively) (Table 3). Figure 3 shows the plots of the correlations between the log-transformed FC, E. coli, and ENT data. It becomes obvious that, in many cases, FC and E. coli were not detected, whereas ENT were abundantly present. In addition, from the 1:1 diagonal, nearly only downward scatter towards lower y axis values is observed, indicating the die-off of E. coli and FC in saline waters.
FIG. 3.
Plots of the correlations between log-transformed data of E. coli and FC (A), ENT and FC (B), and ENT and E. coli (C). Dashed areas denote situations where ENT were abundant while FC and E. coli could not be detected.
Dependence on environmental variables.
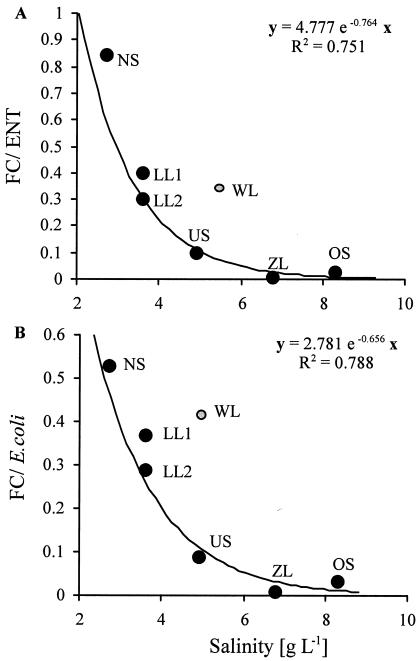
The survival of E. coli and FC is obviously hampered in saline environments (Fig. 3), whereas ENT seem to be better adapted to saline conditions. We therefore plotted the median FC/ENT and E. coli/ENT ratios against the median salinity values for each investigated environment (Fig. 4). Highly significant negative relationships (P < 0.001) were found, where the ratios decreased exponentially with increasing salinity. When the ratios were plotted against median conductivity values, similar curves were obtained (data not shown). The data from WL were not included in both regressions, as their FC/ENT and E. coli/ENT ratios were much higher than expected from their salinity values, most probably due to the influence of cattle feces in this pool.
FIG. 4.
Correlation of the median FC/ENT (A) and E. coli/ENT (B) ratios with median salinity values for each investigated environment. Grey points represent data from WL, which were not included in the calculation of the regression. Abbreviations are defined in the legend to Fig. 1.
Log-transformed FC and E. coli data showed weak but significant negative correlation with salinity (r = −0.31 and −0.30, respectively; P < 0.01; n = 72) and conductivity (r = −0.24 and −0.22, respectively; P < 0.05; n = 72). Significant negative correlations were also found with the major cations (Na+ and K+) and anions (SO42− and Cl−). ENT, on the other hand, showed no negative correlation to any salinity-associated variable. Interestingly, positive correlations to several variables were found for ENT. Log-transformed ENT data showed highly significant correlations with chlorophyll a (r = 0.476; P < 0.001; n = 70), total phosphorus concentrations (r = 0.343; P < 0.01; n = 71), bacterial production (r = 0.389; P < 0.01; n = 62), pH (r = 0.306; P < 0.01; n = 71), carbonate (r = 0.343; P < 0.01; n = 72), TSS (r = 0.436; P < 0.001; n = 72), and rain (r = 0.471; P < 0.001; n = 84). A weak positive correlation could be found with dissolved organic carbon (DOC) (r = 0.243; P = 0.05; n = 62). With the exception of rain, FC and E. coli did not show any significant positive correlation with any of the investigated environmental variables. FTX values (r = 0.290; P < 0.05; n = 68) showed significant positive correlations only to chlorophyll a values.
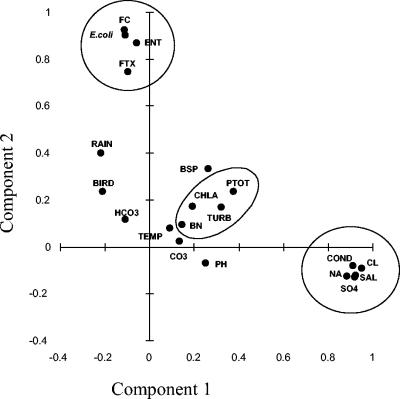
By principal component analysis, it became obvious that the fecal parameters represent one independent component in the investigated saline ecosystems (Fig. 5). Component 2 consisted of all feces-related parameters (FC, E. coli, ENT, and FTX) and explained 22.7% of the total variance. Salinity-related variables described component 1 and explained 30.2% of the total variance of the system. A third component, consisting of all particulate parameters (bacterial numbers, chlorophyll a, total phosphorus, and TSS) and explaining 12.1% of the total variance, and a fourth component, including temperature and bacterial production (8.8% of total variance), could also be extracted.
FIG. 5.
Two-dimensional plot of principal component analysis (varimax rotation, Kaiser normalization). Circles enclose all variables which are contained in the respective component. TEMP, temperature; BSP, bacterial production; CHLA, chlorophyll a; TURB, turbidity; PTOT, total phosphorus; BN, bacterial numbers; COND, conductivity; SAL, salinity.
To predict the abundance of FIB, preferably ENT, in the saline environments, we also tried to establish multiple linear regression equations. Two combinations of factors were statistically significant predictors of ENT abundance, although the degree of explanation was only 46.7% for regression 1 (r = 0.683; P < 0.001) and 47.2% for regression 2 (r = 0.689; P < 0.001). In addition to the amount of feces (FTX), rain was included in both regressions. In regression 1, chlorophyll a was the third independent parameter explaining the variation of ENT concentration, and TSS was the third independent parameter in regression 2.
DISCUSSION
Fecal contamination by waterfowl.
Over the whole range of investigated habitats, bird abundance and feces production were highly significantly correlated to the abundance of FIB in the water (Table 3). Wild birds, including gulls, geese, and ducks, are well known to be important nonpoint sources of fecal contamination of surface waters (1, 15, 25, 30). Feces of gulls (1, 20, 25) and geese (15, 25) contain up to 107 and 104 CFU of FC per gram (wet weight) of feces, respectively. The amount of ENT was reported to be lower (105 and 103 g−1 for swans and geese, respectively) than that of FC and depended on the diet of the birds (15). Because the bird populations observed in this study consisted mainly of geese, ducks, and gulls, similar high contamination with FIB can be assumed for the fecal pellets in our investigated environments. Maximum concentrations of the measured FIB in the water were rather high, with values up to 1.3 × 104, 1.8 × 104, and 6 × 104 CFU per 100 ml for E. coli, FC, and ENT, respectively (Table 2). Bird-caused contaminations with FC of shallow aquatic environments were reported to be in the range of 102 to 103 CFU per 100 ml of surface water and 104 CFU per 100 ml of sediment (15). This comparison shows that some of the shallow saline pools are heavily contaminated with FIB by the bird populations.
Integration of environmental variables to assess the indicator performance of FIB.
Which of the different types of FIB is the best predictor of a recent fecal contamination in an aquatic ecosystem depends to a large extent on the specific environmental conditions. Thus, to evaluate the indicator performance of the mainly used FIB groups, we linked the abundance of FIB not only to feces input and abundance of feces-producing animals but also to a variety of environmental variables. To our knowledge, this is the first approach applying such an integral strategy, as earlier investigations mostly focused on the influence of single environmental stressors on the survival of FIB. Saltwater was shown to inhibit the survival of FC and E. coli in several studies (5, 34) while ENT seem to be less sensitive to osmotic stress (4, 13). In our saline ecosystems, the correlations between FC, E. coli, and ENT were all statistically highly significant (Table 3). However, when the individual correlation plots are observed (Fig. 3), it became obvious that E. coli and FC had a nearly perfect linear slope with an r value of 0.976, whereas the correlations of these groups with ENT showed a much higher scatter and r values of 0.739 and 0.729, respectively. Moreover, from a thought 1:1 diagonal, scatter is only observed towards lower values along the y axis. This is due to the fact that, on many occasions, ENT were abundant when E. coli and FC were reduced or could not be detected, suggesting that ENT are better indicators of recent fecal contamination in these saline environments when samples are collected at biweekly to monthly intervals. A similar observation was made by Rivilla and Gonzalez (26) for salty, alkaline environments in Spain. In their study, fecal streptococci proved to be the best FIB while total coliforms gave a false estimate of fecal pollution. We are aware that a long sample storage (up to 16 h) increases the probability of a significant underestimation of FIB, especially for E. coli and FC. However, on one hand, we could minimize the possible influence of unequally long sample storage on our data set due to the rotation used in sampling design (see above). On the other hand, a significant reduction for E. coli and FC, but not of ENT, again underlines the better suitability of ENT as an indicator of fecal pollution in alkaline, saline environments. E. coli and FC concentrations showed weak but significant negative correlations with conductivity and salinity values and with the major anions and cations while such relationships were not observed for ENT. In addition, highly significant negative exponential relationships between FC/ENT and E. coli/ENT ratios and salinity were found (Fig. 4), decreasing from median values of >0.3 in the least saline waters to median values of <0.05 in the most saline pools. The significance of FC/ENT ratios has been subject to several speculations. According to Geldreich and Kenner (8), FC/ENT ratios in fresh feces of <1.5 should indicate pollution by waterfowl while ratios of >4 should be typical for anthropogenic pollution. This concept was called into question by Hussong et al. (15), who reported that the FC/ENT ratio depended largely on the diet of the birds and that waterfowl feeding in the wild have ratios similar to those of humans. The low ratios of <1 observed in this study indicate waterfowl pollution in the sense of Geldreich and Kenner (8). However, we used these ratios to show that E. coli and FC populations decline more rapidly than ENT in the more saline pools due to osmotic stress. At WL, higher ratios were found than predicted from the regressions with salinity. This indicates that the ratios of the FIB in cattle feces may be different from that in bird feces and that the origin of the FIB may nevertheless have influenced the ratios observed in WL. Rozen and Belkin (27) reported in their review that the optimal survival of E. coli and FC was observed at a salinity of around 0.85% and decreased toward more saline conditions (seawater) as well as toward less saline conditions (distilled water). The median salinity values in our pools ranged from 0.83% down to 0.27%, and thus, more E. coli and FC could be expected to occur in the more saline waters. However, the opposite was observed in this study, with decreasing FC/ENT and E. coli/ENT ratios with increasing salinity and a significant negative correlation of FC and E. coli to salinity. One likely explanation could be the difference in the ion composition of seawater (predominantly NaCl) and the water in the investigated pools (predominantly NaHCO3).
Principal component analysis revealed (Fig. 5) that all fecal variables together represent one important component independent from the other environmental variables. However, ENT exhibited significant positive correlations with a series of different environmental variables, including trophic indicators like chlorophyll a, total phosphorus, and bacterial production, with chemical variables like pH, carbonate concentration, TSS, and rain. By multiple linear regression, we could show that FTX and rain in combination with either chlorophyll a or TSS explained 47% of the variation of ENT. Rain is included in both regressions, most probably because rainfall leads to increased surface runoff and transport of FIB from the fecal pellets at the shore line into the pools. Also, Stanridge et al. (30) reported that high counts of FC and ENT were always associated with weather events. Chlorophyll a represents a trophic component and is also positively correlated to bird abundance (see above). Thus, more birds are attracted by ecosystems with higher trophy where more food is available, they are producing more fecal pellets, and thus, more ENT can enter the ecosystem. TSS seem to have a positive effect on ENT concentrations in several ways. First, at high TSS concentrations, more sediment is resuspended in the water body. Sediments are more suitable environments for survival of FIB than the water itself (2, 31). Because of the higher organic content and the capability of glycine-betaine accumulation against osmotic stress, survival times of FIB are longer in marine sediments (9). Second, the extremely high TSS concentrations of some of the pools limits the penetration of solar radiation through the water body. Solar radiation is known to be a main factor for the removal of FIB in aquatic ecosystems (22, 29), and in the investigated ecosystems, light could be assumed to be a major regulator of the survival of FIB because of the shallowness of the pools, especially in combination with salinity (12). Thus, a reduction of radiation by high TSS concentrations may have a positive effect on the survival of FIB. Third, a weak, yet insignificant intercorrelation of TSS with rain (r = 0.19; P = 0.1) was observed. As during rainy weather there is usually more wind, this leads to a higher concentration of TSS of the water bodies of these flat saline environments, concomitant with the wash-in of feces-associated FIB from the shore into the pools. The positive correlation between ENT and production rates most probably reflects the indirect trophic influence on the abundance of FIB (see above). Because of the high production rates and bacterial numbers, high protistan numbers and predation pressure on FIB can also be assumed, especially on more sensitive species like E. coli (6). It is well known that the elimination of FIB by grazing is one major factor controlling the survival of FIB introduced to aquatic environments (10, 14). The influence of grazing in these ecosystems, however, is unclear, as no data exist on protistan grazing activities.
Applicability of FTX and ENT for monitoring bird-caused fecal contamination.
Because of the highly significant correlations among bird abundance, FTX values, and all investigated FIB, the method of feces quantification proposed in this study (FTX) and all FIB groups used seemed reasonable indicators of fecal contamination in the investigated saline ecosystems (Table 3). By going into more detail, it becomes obvious that ENT are better at predicting fecal contamination than FC and E. coli over the whole range of habitats for the sampling intervals used. Highly significant regressions of ENT on FTX and even on bird abundance were found when median values of each habitat were plotted (Fig. 2) while E. coli and FC showed no significant regressions. However, the slopes from all regressions were <1, indicating increasing underestimations of ENT concentrations with both increasing FTX values and bird abundances. In our protocol, we counted all avian fecal pellets irrespective of the time that had passed since defecation. When a great portion of the fecal pellets are older at the time of sampling and no fresh feces has entered the system, ENT would be underestimated because of the natural and continuous reduction of these organisms in water. Thus, a separation of old and fresh feces in future investigations should therefore be considered, which could be achieved by counting fecal pellets along the same line on a daily scale. In addition, the variable content of ENT concentrations in the feces of the present bird species and the different resistance capacities of the various ENT populations may influence the relation between FTX values and ENT concentrations in the water. Also, feces from other animals can be of relevance. At WL, ENT counts were significantly higher than predicted from the regression curve because of the entry of cattle feces at this pool. Even environmental factors have to be considered, e.g., differing weather situations may lead to variations of the transport of FIB into the pools.
We also observed a highly significant regression of FTX on bird abundance. As with ENT concentrations, the slope of the regression was <1, indicating increasing underestimations of the FTX values with increasing bird abundance. We counted accumulations of feces which obviously represented a single deposit as 1 pellet, as it seemed reasonable to assume that this originated from one bird. But when dense bird populations are present at the pools, FTX values may be underestimated because of the difficulty in separating the different deposits from each other.
Despite these uncertainties and the high diversity of the investigated systems, the highly significant correlations of FTX values with bird abundance as well as with all FIB groups demonstrate the good applicability of FTX as a meaningful surrogate parameter for recent bird abundances and fecal contamination by birds in shallow aquatic ecosystems. In addition, our holistic approach of linking bird abundance, feces production, and FIB detection with environmental key variables may serve as a powerful model for application to other aquatic ecosystems.
Acknowledgments
We thank Alexander Eiler for help with the bacterial number and production measurements, Claudia Hurban and Regina Krachler for measuring ion concentrations, and Franz Rauchwarter, Elfriede Müller, and Erich Boetsch for providing DOC and chlorophyll a data.
The study was financed by a grant of the administration of the national park Neusiedler See-Seewinkel (project no. NP-24; K. Kirchberger). In addition, A.H.F. was supported by a grant from the Austrian Academy of Sciences (APART 10794 [Austrian Programme for Advanced Research and Technology]).
REFERENCES
- 1.Alderisio, K. A., and N. DeLuca. 1999. Seasonal enumeration of faecal coliform bacteria from the feces of ring-billed gulls (Larus delawarensis) and Canada geese (Branta canadensis). Appl. Environ. Microbiol. 65:5628-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm, E. W., J. Burke, and A. Spain. 2003. Fecal indicator bacteria are abundant in wet sand at freshwater beaches. Water Res. 37:3978-3982. [DOI] [PubMed] [Google Scholar]
- 3.Alonso, J. L., K. Soriano, I. Amoros, and M. A. Ferrus. 1998. Quantitative determination of E. coli and faecal coliforms in water using a chromogenic medium. J. Environ. Sci. Health A 33:1229-1248. [Google Scholar]
- 4.Bordalo, A. A., R. Onrassami, and C. Dechskulwatana. 2002. Survival of faecal indicator bacteria in tropical estuarine waters (Bangpakong River, Thailand). J. Appl. Microbiol. 93:864-871. [DOI] [PubMed] [Google Scholar]
- 5.Carlucci, A. F., and D. Pramer. 1960. An evaluation of factors affecting the survival of Escherichia coli in seawater. II. Salinity, pH, and nutrients. Appl. Environ. Microbiol. 8:247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies, C. M., J. A. Long, M. Donald, and N. J. Ashbolt. 1995. Survival of fecal microorganisms in marine and freshwater sediments. Appl. Environ. Microbiol. 61:1888-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eiler, A., A. H. Farnleitner, T. C. Zechmeister, A. Herzig, C. Hurban, W. Wesner, R. Krachler, B. Velimirov, and A. K. T. Kirschner. 2003. Factors controlling extremely active procaryotic communities in shallow soda pools. Microb. Ecol. 46:43-54. [DOI] [PubMed] [Google Scholar]
- 8.Geldreich, E. E., and B. A. Kenner. 1969. Concepts of fecal streptococci in stream pollution. J. Water Pollut. Control Fed. 41:336-352. [PubMed] [Google Scholar]
- 9.Ghoul, M., T. Bernard, and M. Cournier. 1990. Evidence that Escherichia coli accumulates glycine betaine from marine sediments. Appl. Environ. Microbiol. 56:551-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez, J. M., J. Iriberri, L. Egea, and I. Barcina. 1992. Characterization of culturability, protistan grazing, and death of enteric bacteria in aquatic ecosystems. Appl. Environ. Microbiol. 58:998-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gossow, H. 1999. Wildökologie: Begriffe, Methoden, Ergebnisse, Konsequenzen, p. 269. N. Knessel Publ., Remagen-Oberwinter, Germany.
- 12.Gourmelon, M., D. Touati, M. Pommepuy, and M. Cormier. 1997. Survival of Escherichia coli exposed to visible light in seawater: analysis of rpo-S dependent effects. Can. J. Microbiol. 43:1036-1043. [DOI] [PubMed] [Google Scholar]
- 13.Hanes, N. B., and R. Fragala. 1967. Effect of seawater concentration on survival of indicator bacteria. J. Water Pollut. Control Fed. 39:97-104. [PubMed] [Google Scholar]
- 14.Hartke, A., S. Lemarinier, V. Pichereau, and Y. Auffray. 2002. Survival of Enterococcus faecalis in seawater microcosms is limited in the presence of bacterivorous zooflagellates. Curr. Microbiol. 44:329-335. [DOI] [PubMed] [Google Scholar]
- 15.Hussong, D., J. M. Damaré, R. J. Limpert, W. J. L. Sladen, R. M. Weiner, and R. R. Colwell. 1979. Microbial impact of Canada geese (Branta canadensis) and whistling swans (Cygnus columbianus columbianus) on aquatic ecosystems. Appl. Environ. Microbiol. 37:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Organisation of Standardisation. 1990. Water quality—detection and enumeration of coliforms, thermotolerant coliform organisms and presumptive Escherichia coli. Part 1, 9308-1 (E). International Organisation of Standardisation, Geneva, Switzerland.
- 17.International Organisation of Standardisation. 2000. Water quality—detection and enumeration of intestinal enterococci. Part 2, 7899-2. International Organisation of Standardisation, Geneva, Switzerland.
- 18.Kirschner, A. K. T., and B. Velimirov. 1999. Modification of the 3H-leucine centrifugation method for determining bacterial protein synthesis in freshwater samples. Aquat. Microb. Ecol. 17:201-206. [Google Scholar]
- 19.Langbein, J., M. R. Hutchings, S. Harris, C. Stoate, S. C. Tapper, and S. Wray. 1999. Techniques for assessing the abundance of brown hares (Lepus europaeus). Mammal Rev. 29:93-116. [Google Scholar]
- 20.Levesque, B., P. Brousseau, P. Simard, E. Dewailly, M. Meisels, D. Ramsay, and J. Joly. 1993. Impact of the ring-billed gull (Larus delawarensis) on the microbiological quality of recreational water. Appl. Environ. Microbiol. 59:1228-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell, T. R., and T. Ridgwell. 1971. The frequency of salmonellae in wild ducks. J. Med. Microbiol. 4:359-361. [DOI] [PubMed] [Google Scholar]
- 22.Muela, A., J. M. Garcia-Bringas, I. Arana, and I. Barcina. 2000. The effect of simulated solar radiation on Escherichia coli: the relative roles of UV-B, UV-A and photosynthetically active radiation. Microb. Ecol. 39:65-71. [DOI] [PubMed] [Google Scholar]
- 23.Nichols, J. D., J. E. Hines, F. W. Fallon, J. E. Fallon, and P. J. Heglund. 2000. A double-observer approach for estimating detection probability and abundance from point counts. Auk 117:393-408. [Google Scholar]
- 24.Nusch, E. A. 1980. Comparison of different methods for chlorophyll and phaeopigment determination. Arch. Hydrobiol. Beih. Ergeb. Limnol. 14:14-36. [Google Scholar]
- 25.Ricca, D. M., and J. J. Cooney. 1998. Coliphages and indicator bacteria in birds around Boston Harbor. J. Industr. Microbiol. Biotechnol. 21:28-30. [Google Scholar]
- 26.Rivilla, R., and C. C. Gonzalez. 1989. Seasonal variation of pollution indicators in a wildfowl reserve (Donana National Park, Spain). J. Appl. Bacteriol. 67:219-223. [DOI] [PubMed] [Google Scholar]
- 27.Rozen, Y., and S. Belkin. 2001. Survival of enteric bacteria in seawater. FEMS Microbiol. Rev. 25:513-529. [DOI] [PubMed] [Google Scholar]
- 28.Simon, M., and F. Azam. 1989. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 51:201-213. [Google Scholar]
- 29.Sinton, L. W., R. K. Findlay, and P. A. Lynch. 1999. Sunlight inactivation of fecal bacteriophages and bacteria in sewage polluted seawater. Appl. Environ. Microbiol. 65:3605-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanridge, J. H., J. J. Delphino, L. B. Kleppe, and R. Butler. 1979. Effect of waterfowl (Anas platyrhynchos) on indicator bacteria populations in a recreational lake in Madison, Wisconsin. Appl. Environ. Microbiol. 38:547-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenström, T. A., and A. Carlander. 2001. Occurrence and die-off of indicator organisms in the sediment in two constructed wetlands. Water Sci. Technol. 44:223-230. [PubMed] [Google Scholar]
- 32.Strickland, J. D., and T. R. Parsons. 1968. A practical handbook of seawater analysis. Bull. Fish. Res. Board Can. 167:1-311. [Google Scholar]
- 33.Sullivan, B. J., G. S. Baxter, and A. T. Lisle. 2002. Low-density koala (Phascolarctos cinereus) populations in the mulgalands of south-east Queensland. I. Faecal pellet sampling protocol. Wildl. Res. 29:455-462. [Google Scholar]
- 34.Troussellier, M., J.-L. Bonnefort, C. Courties, A. Derrien, E. Dupray, M. Gauthier, M. Gourmelon, F. Joux, P. Lebaron; Y. Martin, and M. Pommepuy. 1999. Responses of enteric bacteria to environmental stress in seawater. Oceanol. Acta 21:965-981. [Google Scholar]
- 35.Zar, J. H. 1974. Biostatistical analysis, p. 620. Prentice-Hall, Inc., Englewood Cliffs, N.J.