Abstract
Lactobacillus sakei is a lactic acid bacterium widely represented in the natural flora of fresh meat. The aim of this study was to analyze the differences in protein expression during environmental changes encountered during technological processes in which L. sakei is involved in order to gain insight into the ability of this species to grow and survive in such environments. Using two-dimensional electrophoresis, we observed significant variation of a set of 21 proteins in cells grown at 4°C or in the presence of 4% NaCl. Six proteins could be identified by determination of their N-terminal sequences, and the corresponding gene clusters were studied. Two proteins belong to carbon metabolic pathways, and four can be clustered as general stress proteins. A phenotype was observed at low temperature for five of the six mutants constructed for these genes. The survival of four mutants during stationary phase at 4°C was affected, and surprisingly, one mutant showed enhanced survival during stationary phase at low temperatures.
Lactobacillus sakei, formerly Lactobacillus sake (49), is a lactic acid bacterium belonging to the natural flora of fresh meat and is the predominant flora of vacuum-packed meat stored at low temperatures. Moreover, some L. sakei strains produce bacteriocins active against various bacterial species, including Listeria monocytogenes (43). For these reasons, it is one of the main starters used in Europe, in association with micrococci, for the production of fermented sausage (18). Current knowledge about this species has recently been reviewed (5). A genetic map of the chromosome of L. sakei 23K, our model strain, has been established (10), and its sequence is being determined (4).
During technological processes, L. sakei, like many other lactic acid bacteria involved in food technology, is exposed to several stress conditions such as the presence of salt or cold temperatures. Little is known about its ability to respond to such environmental parameters, but recent studies have been focused on the impact of these factors on bacteriocin production (27, 28). The heat shock genes hrcA, groEL, dnaK, and dnaJ have also been identified, and their transcription has been shown to be induced by heat shock, salt, and ethanol (44). The 5′ end of a gene putatively encoding a ClpX protein has been described, but no experimental data showing its function has been reported (45).
Proteomics has been revealed to be a powerful tool for investigation of the responses of microorganisms to various environmental changes. For lactic acid bacteria, cold adaptation and salt stress challenge have been illuminated by this approach (6). Generally, proteins induced under stress conditions are clustered into three groups: (i) proteins involved in DNA or protein repair, referred to as chaperons, (ii) proteins of the general metabolism, and (iii) proteins specific for a given stress. In a proteomic analysis study of stress response in Enterococcus faecalis, 27 spots have been characterized (14). These proteins could be classified into three categories: proteins known as stress proteins with a protective function such as DnaK and GroEL; proteins involved in various metabolic pathways, such as enzymes of catabolic pathways or proteins under carbon-catabolite repression; and proteins of unknown function.
The induction of general and specific stress response proteins, but mainly that of heat shock proteins, has been well studied in Escherichia coli and Bacillus subtilis (20, 54). Large protective mechanisms induced by various stress conditions have been described for Lactococcus lactis (38), and some proteins are cross-induced by different stresses. When carbohydrate starved during stationary phase, L. lactis IL1403 becomes resistant to acid, ethanol, heat, oxidative, and osmotic-stresses (19). In L. lactis MG1363, an overlap between the heat and salt stress responses has been reported, demonstrating that all salt stress-induced proteins were also induced by heat-stress (23). In Streptococcus mutans, 52 proteins were overexpressed by salt but only 10 were specifically induced by salt stress (47). In the group of cold-induced proteins are also found low-molecular-weight proteins called cold shock proteins, induced in a wide range of bacteria including lactic acid bacteria. These proteins are involved in mRNA folding during protein synthesis (53).
No detailed study of cold or salt adaptation in L. sakei is yet available. The aim of the present study was to determine by a proteomic approach (29) the main proteins which are affected either by the presence of salt or by growth at suboptimal temperatures. Among 21 spots whose intensity varied on two-dimensional (2D) gels, 6 were identified and further studied. One was previously identified as 6-phosphate-fructokinase (PFK) (30), and five were identified by data mining against the partial DNA sequence of the L. sakei 23K chromosome. One, glycerol-3-phosphate dehydrogenase (G3PDH; encoded by glpD), is involved in glycerol metabolism, and four can be classified as general stress proteins by their similarity to Asp, Usp, MsrA, or Ohr proteins described in other bacterial species. Analysis of mutants with mutations in the corresponding genes revealed that five mutants had a modified survival rate during stationary phase at 4°C, suggesting their involvement in adaptation to growth at low temperatures in L. sakei.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli TGI (42) was used for plasmid propagation. Cloning and sequencing experiments were performed with the pBluescript phagemid SK(+) (Stratagene). L. sakei mutants were constructed with the integrative vector pRV300 (26) and were all derived from the wild-type strain L. sakei 23K (2).
Media, growth conditions, and electrotransformation.
E. coli strains were grown on Luria-Bertani medium at 37°C (42). The complex MRS medium (Difco) described by De Man et al. (7) was used for L. sakei propagation at 30°C under aerobiosis without shaking. Physiological studies concerning growth and survival under various conditions were performed in the chemically defined medium MCD (25) supplemented with 0.5% glucose. E. coli and L. sakei electrocompetent cells were prepared and transformed by the methods of Dower et al. (9) and Berthier et al. (2), respectively. Electroporation was carried out with a Gene Pulser and a Pulse controller apparatus (Bio-Rad). Ampicillin (50 mg liter−1 for E. coli) or erythromycin (5 mg liter−1 for L. sakei) was added to the medium for the selection and propagation of transformants.
Cell growth and viability were monitored by measuring the optical density at 600 nm on a Novaspec II visible spectrophotometer (Pharmacia) and by determination of CFU per milliliter. Viable cell counts were determined by serial dilutions of samples and plating on MRS agar. Plates were incubated under aerobiosis at 30°C for 30 h. All the measurements were performed in at least three independent assays.
2D electrophoresis conditions and N-terminal sequencing.
Bacterial-extract preparation, electrophoresis, and N-terminal protein sequencing were performed by standard methods as previously described (29). Gels were repeated three times and analyzed by Image Master software (Amersham Pharmacia Biotech) after silver staining. N-terminal sequences were determined on spots extracted from Coomassie-stained gels after transfer to a polyvinylidene difluoride membrane and were then searched against the coding sequences (CDS) deduced from genome sequencing of the L. sakei chromosome. Amino acid sequencing was performed by the automated P. Edman method on an Applied Biosystems 494 Procise Protein Sequenator (Foster City, Calif.).
Nucleic acid manipulation and DNA sequence analysis
DNA extractions and manipulations, and PCR amplification reactions, were performed by standard methods as previously described (45). PCR experiments were performed on a Perkin-Elmer 9600 apparatus using Taq DNA polymerase from Fermentas. PCR fragments corresponding to an internal part of the genes were amplified and cloned into the integrative plasmid pRV300 (26). The primers used to amplify internal fragments of genes to be mutated are listed in Table 1. EcoRI or KpnI restriction sites were added at the 5′ ends of primers in order to facilitate further cloning. Recombinant plasmids were then integrated into the chromosome of L. sakei 23K by homologous recombination, leading to knockout mutants. Mutants with mutations in glpD, pfk, msrA, asp-2, ohr, and usp were designated RV4047, RV4043, RV4048, RV4045, RV4044, and RV4046, respectively. The genetic structure of mutated genes was checked by PCR in all mutants.
TABLE 1.
Oligonucleotides used to amplify internal fragments of genes for mutant construction
| Primer | Sequencea |
|---|---|
| 1 | 5′-GGGGTACC GCA AGA TTT TGC TGA AGG-3′ (KpnI) |
| 1rev | 5′-GGGGTACC GAC GAA GTT TGT CCG ACG-3′ |
| 17 | 5′-CGGAATTC AAG ATG GAT CCA ATT ACG-3′ (EcoRI) |
| 17rev | 5′-CGGAATTC CAT GAC GCC TTC TGC CAA-3′ |
| 5 | 5′-CGGAATTC CAG GTG GGT GTT TCT GGT-3′ |
| 5rev | 5′-CGGAATTC GCT CGC CAA GGT TCC TTT-3′ |
| 20 | 5′-CGGAATTC AAT GGA TAA GAT GAT GGA-3′ |
| 20rev | 5′-CGGAATTC AGT CAA CAA GTC AGA AAC-3′ |
| 7 | 5′-CGGAATTC AAC GAT GAT TAA TGA AGG-3′ |
| 7rev | 5′-CGGAATTC TTC GTG GGC TTT TTC GAG-3′ |
| 3 | 5′-CGGAATTC TTT AGT TGG ATT AGA CGG-3′ |
| 3rev | 5′-CGGAATTC ATG AGC AAT CGT TCA ATA-3′ |
Restriction sites added to the 5′ ends of primers are underlined.
The genes and their surrounding regions were annotated with the Artemis annotation tool from the Sanger Center (41; http://www.sanger.ac.uk/Software/Artemis). DNA sequences were analyzed with GCG programs (Wisconsin package, version 10.0; Genetics Computer Group, Madison, Wis.) via the genome server from the Institut National de la Recherche Agronomique (INRA).
Nucleotide sequence accession numbers
GenBank accession numbers corresponding to genes described in this article are AY58823, AY588238, AY588239, AY588240, AY588241, and AY588242.
RESULTS
Identification of the main proteins affected by growth at 4°C or in the presence of NaCl.
In a recent study, we demonstrated that L. sakei was able to grow and survive at low temperatures (4°C) or in the presence of 4% NaCl. Both the doubling time and the final cell density were affected, but long-term survival was enhanced under these two conditions (31). In order to detect proteins putatively involved in the response of L. sakei to cold temperatures or NaCl, we compared protein patterns of cells harvested in mid-log phase during growth at 4°C or with 4% NaCl. The optimal growth temperature, 30°C, with omission of NaCl in the culture medium, was used as the standard condition.
Twenty-one proteins whose intensities were affected by one of these two parameters were detected (Table 2). Seventeen proteins were affected during growth at 4°C, of which 14 were overexpressed and only 3 were detected in lower quantities at low temperatures. Among 11 spots whose intensities varied with NaCl concentration, 9 were induced and 2 were detected in smaller amounts. Finally, 7 spots were affected by both conditions, 5 being overexpressed and 2 being underexpressed.
TABLE 2.
Quantitative variation of protein expression levels during growth at 4°C or at 4% NaCla
| Fold change in expression level
|
pIb | Molecular mass (kDa)b | Spot no. | N-terminal sequence | |
|---|---|---|---|---|---|
| 4°C vs 30°C | 4% NaCl vs 30°C | ||||
| >10 | — | 5.18 | 73.32 | 1 | TFSLESRQQAIENLK |
| >10 | — | 5.8 | 42.60 | 2 | Blocked |
| >10 | >5-10 | 6.4 | 15.80 | 3 | MLEYHNILVGLDGSELADSA |
| >5-10 | — | 6.13 | 42.48 | 4 | ND |
| >5-10 | — | 5.20 | 22.58 | 5 | ATETAIFA |
| >5-10 | — | 5.74 | 24.85 | 6 | ND |
| >5-10 | >5-10 | 5.08 | 14.48 | 7 | MAKQYETTMINEGGRNXFVYDPEKS |
| >2-5 | — | 5.18 | 58.59 | 8 | ND |
| >2-5 | — | 4.73 | 47.29 | 9 | ND |
| >2-5 | — | 6.35 | 41.83 | 10 | ND |
| >2-5 | — | 5.04 | 21.29 | 11 | ND |
| >2-5 | >2-5 | 5.03 | 67.63 | 12 | ND |
| >2-5 | >2-5 | 4.92 | 44.79 | 13 | ND |
| >2-5 | >2-5 | 4.92 | 44.11 | 14 | ND |
| <0.4-0.5 | — | 5.25 | 31.14 | 15 | ND |
| <0.4-0.5 | <0.4-0.5 | 4.93 | 27.40 | 16 | ND |
| <0.4-0.5 | <0.4-0.5 | 5.63 | 39.57 | 17 | MKRIGILT |
| — | >2-5 | 4.71 | 21.24 | 18 | ND |
| — | >2-5 | 5.10 | 40.52 | 19 | ND |
| — | >2-5 | 4.61 | 20.74 | 20 | MDXPNQXMDKEMD |
| — | >2-5 | 4.98 | 39.57 | 21 | ND |
Twenty-one proteins showed changes in their expression levels depending on temperature or NaCl concentration. Results are means from at least three independent gels. —, no detectable effect. ND, not determined (not enough material).
Values determined on 2D gels.
Among these 21 spots, only 6 N-terminal sequences could be determined. One polypeptide was blocked at its N-terminal extremity, and the remainder were present in amounts too small to obtain an N-terminal sequence long enough to be used. The six proteins whose N-terminal sequences were determined were further studied.
Analysis of gene clusters encoding the proteins involved in low-temperature or salt adaptation.
Because the genome sequence of L. sakei 23K was partially determined in our laboratory, we used the N-terminal sequences of the proteins to identify the corresponding genes in order to gain information on their genetic neighborhood. This allowed us to identify spot 17 as the PFK by its identity score to PFKs of other lactobacilli (30). A PCR fragment was cloned, sequenced, and used to construct a mutant and to clone the pfk gene. Further BLAST searches against the partial sequence of the L. sakei chromosome allowed the identification of a contig encompassing pfk and its surrounding region. Downstream from pfk, a pyk gene encoding pyruvate kinase (PYK) was observed (Fig. 1A). The two genes are separated by only 31 bp, and a rho-independent transcription terminator was detected downstream from pyk but not between the two genes. Moreover, no clear-cut promoter sequence could be detected upstream from pyk. This suggests that both genes might be expressed in a polycistronic mRNA. Since we observed that PFK was underexpressed at 4°C and in the presence of salt, we carefully examined whether a protein similar to PYK was similarly regulated. No spot in the pI and molecular mass range of PYK whose intensity was varying could be observed. Furthermore, measurement of PYK activity in cells grown under various conditions did not show PYK activity variations (data not shown), in contrast to what was previously observed for PFK activity (30). It seems, thus, that if protein expression level and PFK activity could be correlated to growth at low temperatures or in the presence of salt, this could not be observed for PYK, indicating either that the two genes do not belong to the same operon or that PFK regulation by temperature and salt acts at a posttranscriptional level.
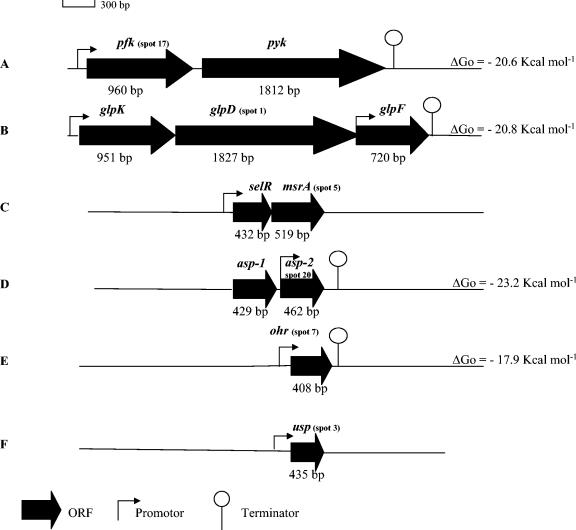
FIG. 1.
Schematic organization of genes encoding the proteins whose expression is regulated by salt or low temperatures in L. sakei. Spots 17, 1, 5, 20, 7, and 3 are encoded by the pfk, glpD, msrA, asp-2, ohr, and usp genes, respectively.
The sequence of the 15 N-terminal amino acids of spot 1 did not match that of any protein from the databases; however, it could be aligned with amino acids 2 to 16 of one CDS deduced from the L. sakei chromosome partial sequence. This CDS was 608 codons long, and the pI (5.01) and molecular mass (66.7 kDa) deduced from the DNA sequence were in accordance with those measured for spot 1 on 2D gels (Table 2). The protein, deduced from the DNA sequence, was 61% identical to the G3PDH of Lactobacillus plantarum (Q88ZF0) and also showed significant homology scores with other bacterial G3PDH proteins. Upstream from glpD, encoding G3PDH, we observed the glycerol kinase gene glpK, which was previously mapped onto the L. sakei chromosome (10). Downstream from glpD, a gene putatively encoding the putative glycerol facilitator GplF was observed. GlpF was 66% identical to GlpF of L. plantarum (Q88ZE9). A single transcription terminator was observed downstream from glpF, and several putative promoters were observed (Fig. 1B). The presence of these three genes in L. sakei, their organization, and evidence for expression of GlpD demonstrate that this species is able to use glycerol as a carbon source, as was previously reported (46). The gene order glpK, glpD, glpF in L. sakei is similar to the organization observed in L. plantarum but different from that of other gram-positive bacteria. In L. lactis, for example, the gene order is glpF, glpD, glpK (3). In B. subtilis, GlpP (absent in the L. sakei cluster), whose gene is located upstream from glpK, regulates the transcription and stability of the glpD transcript (21, 40, 16). Moreover, transcription terminators involved in gene regulation were observed between glpK and glpD (22). We cannot yet conclude that such a complex regulation of the glp operon exists in L. sakei also.
The 8-amino-acid N-terminal sequence from spot 5 matched a CDS encoding a protein 76% identical to L. plantarum MsrA2 (TrEMBL accession number Q88W34) and 72% identical to L. plantarum MsrA4 (TrEMBL accession number Q88VQ9), both of which are described as putative MsrA, a peptide methionine sulfoxide reductase catalyzing the reduction of methionine sulfoxide to methionine. This enzyme is present in all living organisms and is involved in the repair of damage caused by protein oxidation. Analysis of the nucleotide sequence showed that the putative L. sakei msrA gene encodes a 172-amino-acid protein with a calculated molecular mass of 19.5 kDa and a pI of 5.12, both in accordance with values measured for spot 5 on the gels (Table 2). Only 3 bp upstream from the start codon of msrA, we observed a 143-codon CDS encoding a protein 71% identical to the MsrA3 protein (MsrB) of L. plantarum (TrEMBL accession number Q88W33) and 74% identical to E. faecalis MsrB (TrEMBL accession number Q9XB38). All these proteins contain a selR domain previously known as DUF25. No Rho-independent transcription terminator could be detected between the two L. sakei genes (Fig. 1C). Two kinds of Msr proteins have been described in the literature: the MsrB domain (selR domain) is stereospecific for the R isomer of the sulfoxide of methionine sulfoxide, whereas the MsrA domain is stereospecific for the S isomer. In some organisms these two proteins are fused in a single protein. This is the case for Neisseria meningitidis (TrEMBL accession number Q9JWMS) and Campylobacter fetus (TrEMBL accession number Q93KF3). In L. sakei the two domains are encoded by two distinct genes, certainly belonging to the same operon. This organization is similar to that observed for L. plantarum, in which genes were named msrA3 and msrA4, while the paralogs msrA1 and msrA2 were located elsewhere on the chromosome. The two L. sakei genes were named selR and msrA. Because selR and msrA seem to be transcribed from a polycistronic mRNA, one could expect that the expression level of SelR should also vary on 2D gels. Nevertheless, we could not observe such a variation in the relevant zone of 2D gels.
N-terminal sequencing of spot 20 gave information on 13 amino acids. This sequence matched a CDS in the L. sakei genome database encoding a protein 47% identical to L. plantarum Asp-1 (TrEMBL accession number Q88Y61), 33.6% identical to Staphylococcus aureus Asp-23 (TrEMBL accession number Q53485), induced by alkaline shock (24), and 31% identical to the general stress protein Gls-24 of E. faecalis (TrEMBL accession number O32593), which is induced by glucose starvation and various stresses. The putative L. sakei Asp protein contains 153 residues, and its calculated molecular mass (17 kDa) and pI (4.68) are in accordance with those measured for spot 20 (Table 2). Upstream from this L. sakei asp gene, we observed a paralog, encoding a protein also similar to Asp (Fig. 1D). The second protein is 41 and 37% identical to L. plantarum Asp-1 and E. faecalis Gls-24, respectively. In E. faecalis, a similar genetic organization with two paralog operons was also observed in the gls-24 region (13). The presence of three gls-24 paralogs (Ef0604, ymG, and ytgH) was recently demonstrated in E. faecalis (15), and analysis of the complete L. lactis IL1403 genome also revealed the presence of several paralog genes encoding proteins from the same family (3). Some of these genes encode proteins induced under several stress conditions, and a common origin has been argued for three paralogs. Proteins encoded by the two adjacent genes of L. sakei are 44% identical and were therefore named Asp-1 and Asp-2 as in L. plantarum (Fig. 1D).
The 25 N-terminal amino-acids of spot 7 matched a CDS of the L. sakei chromosome, encoding a protein sharing 60% identity with Lin2302 of L. monocytogenes (TrEMBL accession number Q929H4). Lin2302 is a protein belonging to the Ohr/OsmC family. Phylogenetic analysis revealed that proteins encoded by ohr and osmC homologs cluster into two related subfamilies that are functionally and structurally distinct, each defined by a specific motif. The conserved amino acid motif VCPY around position 125, specific for the Ohr family and absent in members of the OsmC family (1), was present at position 117 in the L. sakei protein identified as spot 7. The corresponding gene was thus named ohr. The L. sakei Ohr protein is 135 amino acids long, with a calculated molecular mass of 14.8 kDa and a pI of 5.13, both in agreement with the values of spot 20 (Table 2). Putative promoter and terminator motifs were observed upstream and downstream from ohr (Fig. 1E), suggesting a monocistronic organization as has been observed for Pseudomonas aeruginosa and Deinococcus radiodurans (1).
Finally, analysis of the L. sakei CDS matching the first 21 amino acids from the N terminus of spot 3 showed identity scores with proteins belonging to the universal stress protein (USP) family. The highest score (36% identity) was obtained with the conserved hypothetical protein YxbE of L. lactis (TrEMBL accession number Q9CDI4). Several genes encoding USP-like proteins, the physiological and molecular assignment of which have not yet been determined, are present in the L. lactis genome. The calculated molecular mass (15.6 kDa) and pI (5.93) were in accordance with those of spot 3, whose size was 144 amino acids (Table 2). Analysis of the nucleotide sequence of the region surrounding the L. sakei gene, named usp (Fig. 1F), showed that the putative usp gene did not belong to an operon, as was described for the monocistronic uspA gene of E. coli (33).
Construction of mutants.
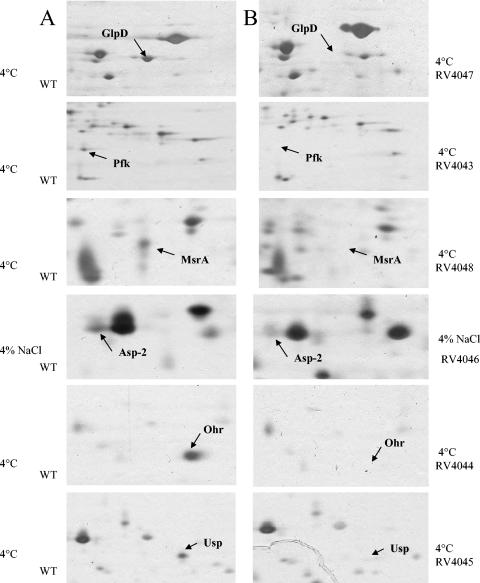
Mutants were constructed for each of the six genes described above, coding for proteins whose expression was modified by growth at 4°C or with NaCl, and disappearance of the corresponding spots was verified by 2-D electrophoresis (Fig. 2). Mutants were grown in MCD medium under the standard condition, at 4°C, or in the presence of 4% NaCl. As mentioned above, growth of the wild-type strain L. sakei 23K at 4°C or with NaCl was impaired, but long term survival was enhanced. Thus, the mutants were tested for their ability to grow and survive under these conditions.
FIG. 2.
Portions of 2D gels showing proteins expressed in the wild type (WT) at 4°C or with 4% NaCl (A) and lack of protein expression in mutants at 4°C or with 4% NaCl (B).
The survival of the pfk mutant is affected at 30°C, at 4°C, and with 4% NaCl.
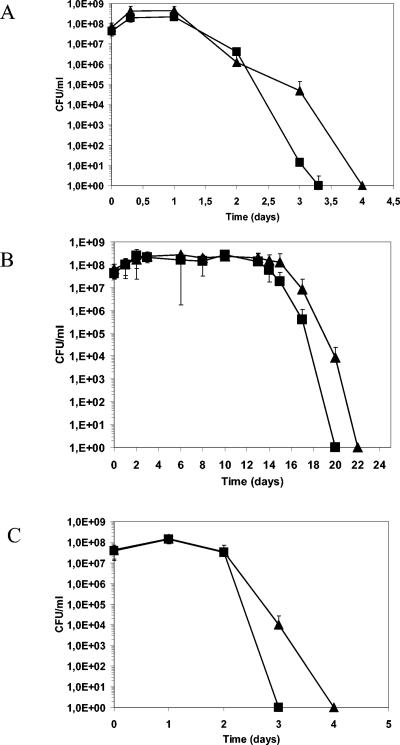
The pfk mutant (RV4043) is not able to grow with glucose as the carbon source. Thus, the study was performed with MCD medium containing ribose, which is not used by the glycolytic pathway. Under these conditions, the growth of the mutant is identical to that of the wild-type strain both at 30 and at 4°C. At 30°C, with or without NaCl, and at 4°C, survival of the mutant was affected (Fig. 3). Thus, PFK could play a role, direct or not, in cell survival.
FIG. 3.
Survival of the wild-type strain (triangles) and RV4043 (squares) at 30°C (A), at 4°C (B), and with 4% NaCl (C) in MCD with 0.5% ribose. Values are means from three independent experiments. Error bars, standard deviations.
The long-term survival of the glpD mutant (RV4047) is enhanced.
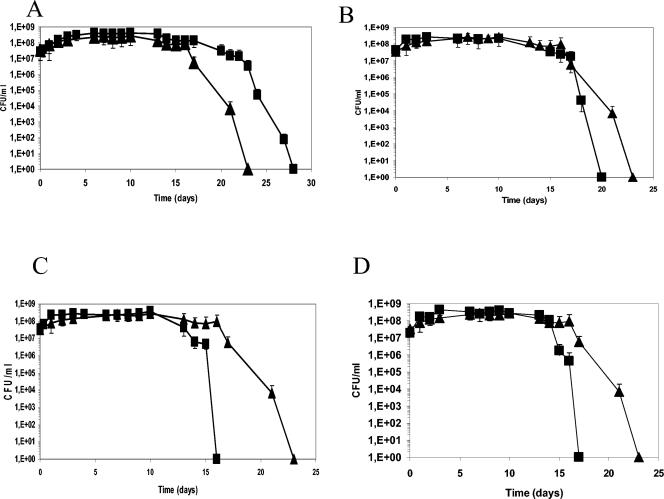
At 30°C, with or without NaCl, and at 4°C, growth of the mutant was not affected in glucose-containing MCD. However, at 4°C, the mutant showed enhanced survival during stationary phase compared to the wild-type strain (Fig. 4A).
FIG. 4.
Growth and survival of the wild-type strain (triangles) and various mutants (squares) at 4°C in MCD with 0.5% glucose. (A) RV4047 (ΔglpD); (B) RV4046 (Δusp); (C) RV4045 (Δasp-2); (D) RV4048 (ΔmsrA). Values are means from three independent experiments. Error bars, standard deviations.
Long-term survival at low temperatures is affected in msrA (RV4048), asp-2 (RV4045), and usp (RV4046) mutants.
Growth of the three mutants was not modified. However, long-term survival was reduced after growth at 4°C (Fig. 4B, C, and D), indicating that the putative proteins MsrA, Asp-2, and Usp play a role in adaptation of L. sakei to low temperatures. Such a phenotype was not observed in cells grown in the presence of NaCl.
No phenotype could be observed for the ohr mutant (RV4044).
Mutant RV4044 grew and survived at 30°C, at 4°C, and in the presence of 4% NaCl, as did the wild-type strain (data not shown).
DISCUSSION
By 2D electrophoresis we detected 21 proteins, expression of which was modified during growth at 4°C or with 4% NaCl. In S. mutans, 52 proteins, 10 of which were specific proteins, were induced by NaCl stress (47). In E. faecalis, 13 proteins were induced by salt (37). In L. lactis, analysis of 2D gels allowed identification of the general stress proteins DnaK, GroEL, and GroES, but no specifically NaCl-induced protein could be detected (23). After a cold stress, the number of proteins affected differs with the species but also with the methods that are used. In fact, in L. lactis IL1403, 12 proteins are induced even though 22 proteins are induced in L. lactis MG1363 (35, 50, 51, 52). In Streptococcus thermophilus, 24 versus 2 proteins were induced in strains CNRZ302 and PB, respectively (50, 36). By 2D electrophoretic analysis, 11 proteins were shown to be induced after a cold stress in E. faecalis (35). Concerning repressed proteins, in L. lactis MG1363, most of them are repressed by salt and about 30 are repressed after a cold shock (53). In L. sakei only three proteins are repressed, two by salt and one by low temperatures only.
In the present study, we analyzed six proteins which are affected during growth of L. sakei at low temperatures or in the presence of salt. Two are proteins belonging to carbon metabolic pathways, and four are general stress proteins.
One of the two proteins involved in the metabolic pathway is the PFK. It is underexpressed either at low temperatures or in the presence of NaCl. PFK catalyzes the conversion of fructose-6-phosphate into fructose-1,6-bisphosphate in the glycolytic pathway, the central and constitutive route of carbohydrate catabolism, and its role for catabolic repression is known (8). Our results suggest that the L. sakei PFK activity, and therefore the flux through the glycolytic pathway, decreases at 4°C or in the presence of NaCl. In B. subtilis, low temperatures induce glyceraldehyde-3-phosphate dehydrogenase (GAPDH), catalyzing one step of glycolysis, and HPr, involved both in sugar uptake and in catabolite repression (17). However, it has been demonstrated for L. lactis that GAPDH is not induced, whereas both HPr and CcpA are induced, by low temperatures (52). It is possible that in L. sakei, CcpA and HPr are induced, as in B. subtilis, by glucose 6-P (11) and thus can control the transcription of genes involved in cold or salt adaptation in L. sakei.
The second protein for which expression varied with temperature or salt, and which is also involved in carbon metabolism, is G3PDH, belonging to the glycerol catabolic pathway. It is known that glycerol metabolism is linked to membrane properties. G3P can be converted to phosphatidic acid, which leads to membrane phospholipid synthesis. In the glpD mutant, G3P catabolism into dihydroxyacetone phosphate is no longer possible, and all glycerol metabolism might then be redirected into phosphatidic acid synthesis, which becomes available for phospholipid biosynthesis, used to maintain membrane integrity. This might explain the enhanced survival of the mutant at 4°C. However, it is contradictory to the fact that GlpD is overexpressed at low temperatures. It has been shown for E. coli that membrane permeability was reduced in a glpF mutant, leading to better resistance to ethanol (48). We can hypothesize that modification of glycerol metabolism in the glpD mutant might confer such modified membrane properties, leading to the enhanced survival observed. Mutants with mutations in the other four genes encoding general stress proteins (msrA, asp-2, usp, and ohr), like the glpD mutant, reveal no difference during growth at 30°C, at 4°C, or with 4% NaCl. Nevertheless, three of the four proteins seem to play a role during survival, since the survival of mutants was affected. These results demonstrate that proteins expressed during growth can play an important role in survival during stationary phase.
It is known that in other bacteria, MsrA is involved in the repair of oxidative damage of proteins, and its function seems to be related to protection of the cell against oxidative stress. Our results demonstrate that in L. sakei, MsrA is induced at low temperatures and is necessary for optimal survival during stationary phase.
One of the proteins induced by cold or NaCl presents homology to Asp-23 of S. aureus and Gls-24 of E. faecalis. In S. aureus, Asp-23 is induced by alkaline shock and is under the control of σB (12). In E. faecalis, Gls-24 is induced by different stresses and by glucose starvation. As in E. faecalis, the L. sakei gls-24 gene is duplicated. In E. faecalis, a gls-24 mutant showed a longer doubling time than the wild-type strain, but its survival was identical under conditions of glucose starvation or heat, hydrogen peroxide, CdCl2, acid pH, basic pH, or ethanol shock. A reduction in survival was observed only in the presence of 0.3% NaCl. Such results were not observed for the mutant with a mutation of the second copy of gls-24, a phenotype which was similar to that of the wild-type strain (15). In L. sakei, the growth of the asp-2 mutant is not affected by salt, but survival is reduced at 4°C. In that case we cannot exclude a polar effect of the mutation. The asp-1 and asp-2 genes are probably both involved in the stress response.
We also identified an Usp homolog protein. In E. coli, UspA has been proposed to have a general protective function related to the growth arrest state. Its induction results from transcriptional activation of a single σ70-dependent promoter (33). Growth of an uspA mutant of E. coli is not affected, but increased sensitivity to complete and prolonged starvation conditions, or to various stresses, including osmotic shock, has been reported (34). The phenotype of the L. sakei usp mutant is similar to that observed for the E. coli usp mutant with regard to survival, except that it does not seem to be involved in an osmotic stress response.
The last protein identified was Ohr. Ohr is known to be involved in organic peroxide protection (1, 32). Osmotic stress has not yet been reported to induce expression of the ohr homologs. In L. sakei, the homologous Ohr protein is induced by low temperatures and salt. In E. faecalis, an ohr gene encodes a general stress protein named Gsp-65, induced by different stresses (heat, pH, SDS, bile salts, ethanol, NaCl, tBOOH [tert-butylhydroperoxide]) (39). The gsp-65 mutant had no clear phenotype relative to the wild-type strain regarding response to these stresses. Only a higher sensitivity to tBOOH, an oxidative agent, was observed (39). Survival of the gsp-65 mutant was not significantly affected by NaCl, heat shock, pH, detergent, or H2O2, but the mutant was less resistant to the oxidative stress generated with tBOOH and ethanol. This might be explained by the involvement of another protein(s) which compensates for the Ohr deficiency. Like those of the E. faecalis gsp-65 mutant, the growth and survival of L. sakei RV4044 are not affected in the presence of salt or low temperatures.
This work provides an initial insight into the mechanisms of adaptation of L. sakei to its environment and illuminates the role of key proteins indispensable for survival of this species. This complex regulation should be further elucidated by analysis of protein patterns in the mutant strains and further identification of other proteins.
Acknowledgments
We thank V. Davant for technical assistance.
This work was supported by financial aid from Rhodia-Food.
REFERENCES
- 1.Atichartpongkul, S., S. Loprasert, P. Vattanaviboon, W. Whangsuk, J. D. Helmann, and S. Mongkolsuk. 2001. Bacterial ohr and osmC paralogues define two protein families with distinct functions and patterns of expression. Microbiology 147:1775-1782. [DOI] [PubMed] [Google Scholar]
- 2.Berthier, F., M. Zagorec, M. Champomier-Vergès, S. D. Ehrlich, and F. Morel-Deville. 1996. Efficient transformation of Lactobacillus sake by electroporation. Microbiology 142:1273-1279. [DOI] [PubMed] [Google Scholar]
- 3.Bolotin, A., P. Wincker, S. Mauger, et al. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaillou, S., A. M. Dudez, and M. Zagorec. 2002. Genome analysis of Lactobacillus sakei 23K: towards new biological and genetic studies of the species. Sci. Aliments 22:55-58. [Google Scholar]
- 5.Champomier-Vergès, M. C., S. Chaillou, M. Cornet, and M. Zagorec. 2002. Lactobacillus sakei: recent developments and future prospects. Res. Microbiol. 153:115-123. [DOI] [PubMed] [Google Scholar]
- 6.Champomier-Vergès, M. C., E. Maguin, M. Y. Mistou, P. Anglade, and J. F. Chich. 2002. Lactic acid bacteria and proteomics: current knowledge and perspectives. J. Chromatogr. B 771:329-342. [DOI] [PubMed] [Google Scholar]
- 7.De Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 8.Deutscher, J., and M. H. Saier, Jr. 1983. ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 80:6790-6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudez, A. M., S. Chaillou, L. Hissler, R. Stentz, M. C. Champomier-Vergès, C. A. Alpert, and M. Zagorec. 2002. Physical and genetic map of the Lactobacillus sakei 23K chromosome. Microbiology 148:421-431. [DOI] [PubMed] [Google Scholar]
- 11.Fujita, Y., Y. Miwa, A. Galinier, and J. Deutscher. 1995. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol. Microbiol. 17:953-960. [DOI] [PubMed] [Google Scholar]
- 12.Gertz, S., S. Engelmann, R. Schmid, K. Ohlsen, J. Hacker, and M. Hecker. 1999. Regulation of σB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol. Gen. Genet. 261:558-566. [DOI] [PubMed] [Google Scholar]
- 13.Giard, J. C., A. Rincé, H. Capiaux, Y. Auffray, and A. Hartke. 2000. Inactivation of the stress- and starvation-inducible gls24 operon has a pleiotropic effect on cell morphology, stress sensitivity, and gene expression in Enterococcus faecalis. J. Bacteriol. 182:4512-4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giard, J. C., J. M. Laplace, A. Rincé, V. Pichereau, A. Benahour, C. Leboeuf, S. Flahaut, Y. Auffray, and A. Hartke. 2001. The stress proteome of Enterococcus faecalis. Electrophoresis 22:2947-2954. [DOI] [PubMed] [Google Scholar]
- 15.Giard, J. C., N. Verneuil, Y. Auffray, and A. Hartke. 2002. Characterization of genes homologous to the general stress-inducible gene gls24 in Enterococcus faecalis and Lactococcus lactis. FEMS Microbiol. Lett. 206:235-239. [DOI] [PubMed] [Google Scholar]
- 16.Glatz, E., R. P. Nilsson, L. Rutberg, and B. Rutberg. 1996. A dual role for the Bacillus subtilis glpD leader and the GlpP protein in the regulated expression of glpD: anti-termination and control of mRNA stability. Mol. Microbiol. 19:319-328. [DOI] [PubMed] [Google Scholar]
- 17.Graumann, P., K. Schröder, R. Schmid, and M. A. Marahiel. 1996. Cold shock stress-induced proteins in Bacillus subtilis. J. Bacteriol. 178:4611-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammes, W. P., A. Bantleon, and S. Min. 1990. Lactic acid bacteria in meat fermentation. FEMS Microbiol. Rev. 87:165-174. [Google Scholar]
- 19.Hartke, A., S. Bouché, J. M. Laplace, A. Benachour, P. Boutibonnes, and Y. Auffray. 1995. UV-inducible proteins and UV-induced cross-protection against acid, ethanol, H2O2 or heat treatments in Lactococcus lactis subsp. lactis. Arch. Microbiol. 163:329-336. [Google Scholar]
- 20.Hecker, M., W. Shumann, and U. Völker. 1996. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19:417-428. [DOI] [PubMed] [Google Scholar]
- 21.Holmberg, C., L. Beijer, B. Rutberg, and L. Rutberg. 1990. Glycerol catabolism in Bacillus subtilis: nucleotide sequence of the genes encoding glycerol kinase (glpK) and glycerol-3-phosphate dehydrogenase (glpD). J. Gen. Microbiol. 136:2367-2375. [DOI] [PubMed] [Google Scholar]
- 22.Holmberg, C., and L. Rutberg. 1992. An inverted repeat preceding the Bacillus subtilis glpD gene is a conditional terminator of transcription. Mol. Microbiol. 6:2931-2938. [DOI] [PubMed] [Google Scholar]
- 23.Kilstrup, M., S. Jacobsen, K. Hammer, and F. K. Vogensen. 1997. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl. Environ. Microbiol. 63:1826-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuroda, M., T. Ohta, and H. Hayashi. 1995. Isolation and the gene cloning of an alkaline shock protein in methicillin resistant Staphylococcus aureus. Biochem. Biophys. Res. Commun. 207:978-984. [DOI] [PubMed] [Google Scholar]
- 25.Lauret, R., F. Morel-Deville, F. Berthier, M. C. Champomier-Vergès, P. W. Postma, S. D. Ehrlich, and M. Zagorec. 1996. Carbohydrate utilization in Lactobacillus sake. Appl. Environ. Microbiol. 62:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leloup, L., S. D. Ehrlich, M. Zagorec, and F. Morel-Deville. 1997. Single-crossover integration in the Lactobacillus sake chromosome and insertional inactivation of the ptsI and lacL genes. Appl. Environ. Microbiol. 63:2117-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leroy, F., and L. De Vuyst. 1999. Temperature and pH conditions that prevail during fermentation of sausages are optimal for production of the antilisterial bacteriocin sakacin K. Appl. Environ. Microbiol. 65:974-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leroy, F., and L. De Vuyst. 1999. The presence of salt and a curing agent reduces bacteriocin production by Lactobacillus sakei CTC 494, a potential starter culture for sausage fermentation. Appl. Environ. Microbiol. 65:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marceau, A., T. Méra, M. Zagorec, and M. C. Champomier-Vergès. 2001. Protein expression under uracil privation in Lactobacillus sakei. FEMS Microbiol. Lett. 200:49-52. [DOI] [PubMed] [Google Scholar]
- 30.Marceau, A., M. Zagorec, and M. C. Champomier-Vergès. 2002. Analysis of Lactobacillus sakei adaptation to its environment by a proteomic approach. Sci. Aliments 22:97-105. [Google Scholar]
- 31.Marceau, A., M. Zagorec, and M. C. Champomier-Vergès. 2003. Positive effects of growth conditions at suboptimal temperature and salt concentration on long term survival of Lactobacillus sakei. Res. Microbiol. 154:37-42. [DOI] [PubMed] [Google Scholar]
- 32.Mongkolsuk, S., W. Praituan, S. Loprasert, M. Fuangthong, and S. Chamnongpol. 1998. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J. Bacteriol. 180:2636-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyström, T., and F. C. Neidhardt. 1992. Cloning, mapping and nucleotide sequencing of a gene encoding a universal stress protein in Escherichia coli. Mol. Microbiol. 6:3187-3198. [DOI] [PubMed] [Google Scholar]
- 34.Nyström, T., and F. C. Neidhardt. 1994. Expression and role of the universal stress protein, UspA, of Escherichia coli during growth arrest. Mol. Microbiol. 11:537-544. [DOI] [PubMed] [Google Scholar]
- 35.Panoff, J. M., D. Corroler, B. Thammavongs, and P. Boutibonnes. 1997. Differentiation between cold shock proteins and cold acclimation proteins in a mesophilic gram-positive bacterium, Enterococcus faecalis JH2-2. J. Bacteriol. 179:4451-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrin, C., C. Guimont, P. Bracquart, and J. L. Gaillard. 1999. Expression of a new cold shock protein of 21.5 kDa and of the major cold shock protein of Streptococcus thermophilus after cold shock. Curr. Microbiol. 39:342-347. [DOI] [PubMed] [Google Scholar]
- 37.Pichereau, V., S. Bourot, S. Flahaut, C. Blanco, Y. Auffray, and T. Bernard. 1999. The osmoprotectant glycine betaine inhibits salt-induced cross-tolerance towards lethal treatment in Enterococcus faecalis. Microbiology 145:427-435. [DOI] [PubMed] [Google Scholar]
- 38.Rallu, F., A. Gruss, and E. Maguin. 1996. Lactococcus lactis and stress. Antonie Leeuwenhoek 70:243-251. [DOI] [PubMed] [Google Scholar]
- 39.Rincé, A., J. C. Giard, V. Pichereau, S. Flahaut, and Y. Auffray. 2001. Identification and characterization of gsp65, an organic hydroperoxide resistance (ohr) gene encoding a general stress protein in Enterococcus faecalis. J. Bacteriol. 183:1482-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutberg, B. 1997. Anti-termination of transcription of catabolic operons. Mol. Microbiol. 23:413-421. [DOI] [PubMed] [Google Scholar]
- 41.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M. A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 43.Schillinger, U., M. Kaya, and F. K. Lücke. 1991. Behaviour of Listeria monocytogenes in meat and its control by a bacteriocin-producing strain of Lactobacillus sake. J. Appl. Bacteriol. 70:473-478. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt, G., C. Hertel, and W. P. Hammes. 1999. Molecular characterisation of the dnaK operon of Lactobacillus sakei LTH681. Syst. Appl. Microbiol. 22:321-328. [DOI] [PubMed] [Google Scholar]
- 45.Stentz, R., R. Lauret, S. D. Ehrlich, F. Morel-Deville, and M. Zagorec. 1997. Molecular cloning and analysis of the ptsHI operon in Lactobacillus sake. Appl. Environ. Microbiol. 63:2111-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stentz, R., M. Cornet, S. Chaillou, and M. Zagorec. 2001. Adaptation of Lactobacillus sakei to meat: a new regulatory mechanism of ribose utilization? Lait 81:131-138. [Google Scholar]
- 47.Svensäter, G., B. Sjögreen, and I. R. Hamilton. 2000. Multiple stress responses in Streptococcus mutans and the induction of general and stress-specific proteins. Microbiology 146:107-117. [DOI] [PubMed] [Google Scholar]
- 48.Truniger, V., and W. Boos. 1993. Glycerol uptake in Escherichia coli is sensitive to membrane lipid composition. Res. Microbiol. 144:565-574. [DOI] [PubMed] [Google Scholar]
- 49.Trüpper, H. G., and L. De Clari. 1997. Taxonomic note: necessary correction of specific epithets formed as substantive (nouns) in apposition. Int. J. Syst. Bacteriol. 47:908-909. [Google Scholar]
- 50.Wouters, J. A., B. Jeynov, F. M. Rombouts, W. M. de Vos, O. P. Kuipers, and T. Abee. 1999. Analysis of the role of 7 kDa cold-shock proteins of Lactococcus lactis MG1363 in cryoprotection. Microbiology 145:3185-3194. [DOI] [PubMed] [Google Scholar]
- 51.Wouters, J. A., M. Mailhes, F. M. Rombouts, W. M. de Vos, O. P. Kuipers, and T. Abee. 2000. Physiological and regulatory effects of controlled overproduction of five cold shock proteins of Lactococcus lactis MG1363. Appl. Environ. Microbiol. 66:3756-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wouters, J. A., H. H. Kamphuis, J. Hugenholtz, O. P. Kuipers, W. M. de Vos, and T. Abee. 2000. Changes in glycolytic activity of Lactococcus lactis induced by low temperature. Appl. Environ. Microbiol. 66:3686-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wouters, J. A., F. M. Rombouts, O. P. Kuipers, W. M. de Vos, and T. Abee. 2000. The role of cold-shock proteins in low-temperature adaptation of food-related bacteria. Syst. Appl. Microbiol. 23:165-173. [DOI] [PubMed] [Google Scholar]
- 54.Yura, T., H. Nagai, and H. Mori. 1993. Regulation of the heat-shock response in bacteria. Annu. Rev. Microbiol. 47:321-350. [DOI] [PubMed] [Google Scholar]