ABSTRACT
PHOTOSYNTHESIS AFFECTED MUTANT71 (PAM71) is an integral thylakoid membrane protein that functions in manganese uptake into the lumen. Manganese is needed in the thylakoid lumen to build up the inorganic Mn4CaO5 cluster, the catalytic center for water oxidation, and is hence indispensable for oxygen evolution. A recent study revealed that PAM71 is well conserved in plants and shares homology to GCR1 DEPENDENT TRANSLATION FACTOR1 (GDT1) and TRANSMEMBRANE PROTEIN 165 (TMEM165) in Saccharomyces cerevisiae and Homo sapiens, respectively. In most eukaryotes only single members of this family, designated “Uncharacterized Protein Family 0016” (UPF0016), are present; however, plant genomes contain genes for several UPF0016 proteins. In Arabidopsis thaliana, this protein family comprises 5 members, which mainly differ in their N-terminal regions. PAM71 and its closest homolog PAM71-HL possess chloroplast transit peptides at their N-terminus. Two of the remaining 3 members are derived from a segmental chromosomal duplication event and lack an N-terminal extension. Thus, plants have evolved UPF0016 members residing in various compartments of the cell, whereas in non-plant eukaryotes just a Golgi localization occurs. The identification of PAM71 as a candidate Mn2+ transporter opens the question on the function of the remaining plant members. Here we resume briefly our current knowledge of UPF0016 members in Arabidopsis in comparison to their yeast and human UPF0016 members.
KEYWORDS: Arabidopsis, GDT1, integral membrane protein, manganese transport, PAM71, thylakoid membrane, TMEM165, UPF0016 family
Members of the Uncharacterized Protein Family 0016 (UPF0016; Pfam accession number, PF01169) are found in nearly all organisms. They are present in all eukaryotes and in many prokaryotes with only a few exceptions (e.g. Lactobacillales and Bacillales).1 The most prominent representative members of this family are ScGDT1 from yeast,2,3 HsTMEM165 from human4-10 and AtPAM71 ( = AtCCHA1) from Arabidopsis.11,12 ScGDT1 (GCR1 DEPENDENT TRANSLATION FACTOR 1) contributes to Ca2+ homeostasis in yeast via an uncharacterized Ca2+ transport pathway localized in the Golgi apparatus.2,3 It was shown that gdt1 mutant strains have a growth defect in the presence of high concentrations of calcium chloride, but behaved like wild-type under normal conditions. It was speculated that ScGDT1 could be a Golgi localized Ca2+/H+ antiporter regulating Ca2+ homeostasis in acidic Ca2+ stores. Interestingly the sensitivity to high concentrations of calcium could be partially rescued by expression of a truncated version of the human TMEM165.2 Defects in human TMEM165 cause a rare inherited disease, which results in severe growth and psychomotor retardations in affected patients.4,5 More specifically, the patients possess a glycosylation defect in the Golgi, the so-called Congenital Disorder of Glycosylation type II (CDG-II). Recent data support a role for HsTMEM165 in Golgi Mn2+ homeostasis, since Mn2+ is a cofactor of galactosyltransferases.9 Mn2+ dependent galactosyltransferases transfer galactose residues from UDP-Gal to the N-glycans of proteins. Interestingly it was shown that the N-glycosylation defects in HsTMEM165 depleted mammalian cells could be rescued by the addition of MnCl2 or MnSO4. Furthermore, it was shown that the yeast gdt1 strain was affected in N-glycosylation under high calcium concentrations, and that 1 mM MnCl2 was sufficient to completely suppress the glycosylation deficiency seen under this condition. 3,9
A recent study in Arabidopsis characterized the function of a plant member of the UPF0016 family, AtPAM7.11 The pam71 mutant plants were found impaired in photosynthesis with a primary defect in photosystem II (PSII). PSII is a multiprotein-pigment complex that functions as a light-driven water:plastoquinone oxidoreductase in the thylakoid membrane and binds all essential redox components.13,14 PSII subunits provide the ligands for the Mn4CaO5 cluster that is the catalytic center for water oxidation and oxygen evolution. The Mn4CaO5 cluster is also called the oxygen-evolving complex (OEC) and provides, together with its protein vicinity,15 the optimal environment for this process. It was concluded that reduced Mn2+ import into the thylakoid lumen of pam71 chloroplast leads to decreased oxygen-evolving complex formation (Fig. 1), which in turn affects PSII efficiency. Reduced PSII efficiency finally results in less reducing power and ATP for CO2 assimilation. In consequence, fewer carbohydrates are available for plant growth and development. The pam71 mutants have pale green leaves along with compromised growth. Interestingly, growth retardation and PSII defects could be restored by exogenous treatment with Mn2+.11 Based on this findings and homology to human TMEM165 it was postulated that AtPAM71 functions as a Mn2+ importer into the thylakoid lumen. Because pam71 mutant plants were able to grow photoautotrophally, the existence of low affinity systems located at the thylakoid membrane is emphasized (Fig. 1), which compensate the reduced Mn2+ import into the lumen. These low affinity systems were postulated to drive massive cation uptake into the thylakoid lumen causing an imbalance in calcium homeostasis in pam71 mutant plants.11 The impact of AtPAM71 on PSII function, levels of thylakoid proteins, nonphotochemical quenching, PSI redox kinetics and calcium homeostasis was verified in an independent study.12 In that study At1g64150 was named AtCCHA1 for putative CHLORPLAST-LOCALIZED CA2+/H+ ANTIPORTER1 based on its homology to yeast ScGDT1.12
Figure 1.
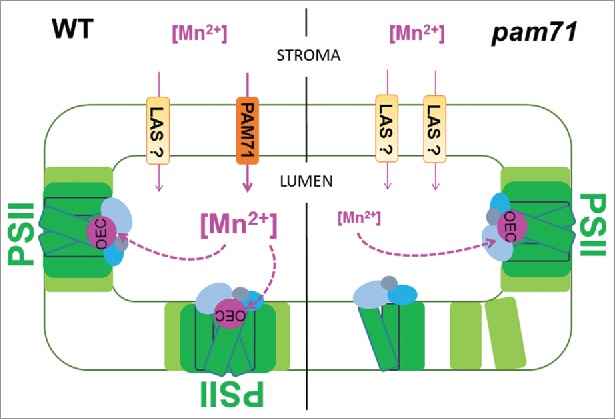
Model for the role of AtPAM71 in Mn2+ homeostasis in chloroplasts. The left part of the scheme shows the wild-type situation where AtPAM71 tentatively functions as Mn2+ transporter. Here, sufficient Mn2+ is delivered into the thylakoid lumen to ensure optimal oxygen-evolving complex (OEC) formation and hence PSII assembly. The right part of the scheme illustrates the situation in the pam71 mutant. Here, a limited amount of Mn2+ import into the thylakoid lumen may occur through the action of Low Affinity Systems (LAS) resulting in decreased oyxgen evolving complex formation. This senario finally results in less photosystem II (PSII) assembly and functionality. PSII subunits are colored in green and light green, the Mn4CaO5 cluster (OEC) in pink, the protein environment of the OEC in blue, light blue and gray, transport systems (PAM71 and LSA) in brown and light brown.
Sequence similarity of AtPAM71 with other UPF0016 family members
The founder of the UPF0016 family in plants is called AtPAM71 ( = AtCCHA1), named after the mutant phenotype of the respective loss-of-function mutation of gene At1g64150. The closest homolog of AtPAM71 is named AtPAM71-HL (or AtGDT1-Like2 as annotated at NCBI), which is the gene product of At4g13590 (Fig. 2). Both proteins share 49% identity over their entire length. This protein pair is strictly conserved in the green lineage, because we identified always for each of the 2 proteins one homolog in other plant species and in green algae.11 Accordingly, loss-of-function of CrCGLD1 in the green algae Chlamydomonas reinhardtii resulted in reduced PSII function16 similar to that observed in the Arabidopsis pam71 mutant. Furthermore, this defect could be partially compensated by the addition of MnCl2 to the growth medium.11 Therefore, the function of PAM71 and maybe also that of PAM71-HL is presumably conserved in the green lineage. In this context, it is important to note that PAM71-HL and PAM71 are not redundant within the plant, because these proteins reside in different membrane systems within the chloroplast (see below).
Figure 2.
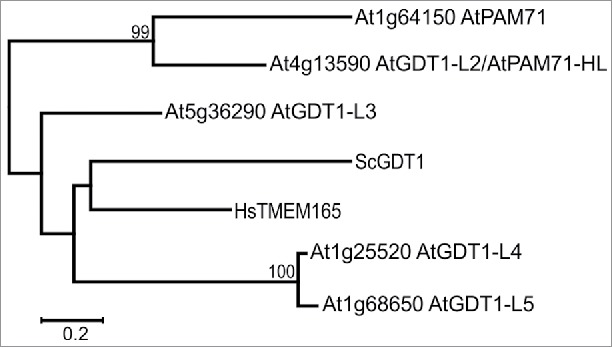
Phylogenetic tree based on selected members of the UPF0016 family, including all members of Arabidopsis, yeast and human. The phylogenetic tree was constructed with MEGA725,26 and the evolutionary history was inferred by using the Maximum Likelihood method based on the General Reversible Chloroplast model.27 The tree is drawn to scale with branch lengths measured in the number of substitutions per site, the scale bar corresponds to 0.2 substitutions per site. Sequence data were obtained from NCBI and accession numbers were reported.11 AtGDT1-L2 to AtGDT1-L5 correspond to AtGDT1-Like2 to AtGDT1-Like5 in the main text.
The Arabidopsis genome contains 3 additional genes encoding proteins that belong to the UPF0016 family, namely At5g36290 (AtGDT1-Like3), At1g25520 (AtGDT1-Like4) and At1g68650 (AtGDT1-Like5) (Fig. 2). The proteins share 38% (AtGDT1-Like3), 32% (AtGDT1-Like4) and 32% (AtGDT1-Like5) identical amino acids with AtPAM71 when applying blastp at NCBI.17 The gene pair At1g25520 and At1g68650 was found in a duplicated region encompassing 57 gene loci on chromosome 1 of Arabidopsis (http://chibba.agtec.uga.edu/duplication).18 Thus, this gene pair presumably originated from a segmental chromosomal duplication event in a progenitor of Arabidopsis and accordingly this duplication is also present in Arabidopsis lyrata and Capsella rubella. Interestingly, genomes of Brassica oleracea and Brassica rapa have acquired extra gene copies encoding GDT1-Like 4/GDT1-Like5 proteins: Brassica oleracea contains 3 genes encoding GDT1-Like4/GDT1-Like5 proteins and Brassica rapa contains 5 genes encoding GDT1-Like4/GDT1-Like5 proteins, some arose from segmental chromosomal duplication events. Thus, and different to PAM71/PAM71-HL11, GDT1-Like4/GDT1-Like5 is not strictly conserved as a pair in plants.
In contrast to plants, only single members of the UPF0016 family were identified in human and yeast (Fig. 2) and other eukaryotes and prokaryotes.1
Structural similarity of AtPAM71 with other UPF0016 family members
The overall structure of eukaryotic members of the UPF0016 family is characterized by 2 clusters of 3 transmembrane domains (numbered TM1 to TM3 and TM4 to TM6), separated by a central loop1 (Fig. 3). Although the central loops vary in length between 31 amino acids (in AtPAM71) and 61 amino acids (in ScGDT1), they all contain several acidic residues.1,9,11 Two highly conserved E-x-G-D-(KR)-(TS) motifs are found in transmembrane domains TM1 and TM4, each which 2 negatively charged acidic residues. Together, these features presumably provide a suitable environment for the binding and transport of cations. So far there is evidence for AtPAM71 to function as Mn2+ transporter, and a broader specificity for other divalent cations cannot be excluded.11,12 In addition, ScGDT1 and HsTMEM165 were postulated as Ca2+/H+ exchangers,2,3 although some recent experimental data suggest a role for HsTMEM165 as Mn2+ transporter canditate.9 Taken together the amino acid analysis as well as experimental data strongly support the idea that the UPF0016 family represents a new family of cation transporters and we anticipate the remaining plant homologs to act also as Mn2+ (and/or Ca2+) transporters. The mode of Mn2+ transport remains enigmatic at present, perhaps a Mg2+/H+ exchange mode is imaginable for AtPAM71. The acidic pH inside the thylakoid lumen could drive Mg2+ uptake from the chloroplast stroma into the lumen via AtPAM71, resembling the transport mode of the K+/H+ antiporter KEA3.19,20 The later one was shown to drive luminal K+ uptake using the pH gradient of the thylakoid membrane to dissipate a high ΔpH.19,20
Figure 3.
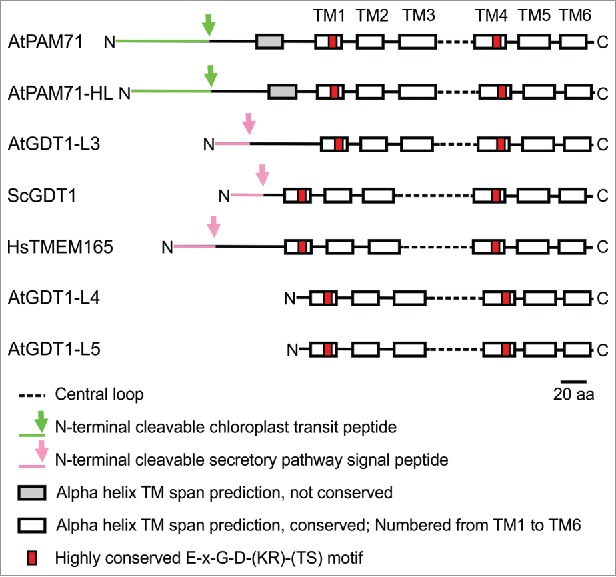
Motif diagram of selected members of the UPF0016 family, including all members of Arabidopsis, yeast and human. Transmembrane helix predictions were performed with TmHMM2.028 and adjusted manually afterwards. The subcellular localization based on the N-terminal amino acid sequence and the prediction of the respective cleavage site was performed with TargetP1.1.29 Signal peptides were discriminated from transmembrane regions using SignalP4.0.30 The scale bar represents 20 amino acids.
The highest diversity is found in the N-terminal region of UPF0016 proteins. Both proteins, ScGDT1 and HsTMEM165, are predicted to possess an N-terminal cleavable secretory pathway signal peptide (Fig. 3), which is occasionally also predicted as a transmembrane domain. Indeed, HsTMEM165 was found mainly in the trans-Golgi membranes in mammalian cells4 and ScGDT1 was localized in the cis- and medial Golgi of yeast.2 Out of the 5 Arabidopsis members, an N-terminal cleavable secretory pathway signal peptide is only predicted for AtGDT1-Like3 (Fig. 3). AtGDT1-Like4 and AtGDT1-Like5 lack N-terminal extensions nevertheless some prediction programs suggest that they are also substrates of the secretory pathway (http://aramemnon.botanik.uni-koeln.de/).21 AtPAM71 and AtPAM71-HL contain N-terminal cleavable chloroplast transit peptides of approximately 70 amino acids (Fig. 3). Accordingly, AtPAM71-HL was found enriched in the chloroplast envelope membrane fraction,22,23,24 and for AtPAM71 a thylakoid membrane localization was demonstrated.11 Therefore, it can be concluded that the N-terminal regions of UPF0016 eukaryotic members target the respective proteins to their subcellular destination. It remains to be determined, whether the N-terminus might also contribute to regulation and/or sensing of cation homeostasis and by that impacts on the adjustment to different cation requirements of the organelle.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Demaegd D, Colinet AS, Deschamps A, Morsomme P. Molecular evolution of a novel family of putative calcium transporters. PLoS One 2014; 9:e100851; PMID:24955841; http://dx.doi.org/ 10.1371/journal.pone.0100851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demaegd D, Foulquier F, Colinet AS, Gremillon L, Legrand D, Mariot P, Peiter E, Van Schaftingen E, Matthijs G, Morsomme P. Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc Natl Acad Sci USA 2013; 110:6859-64; PMID:23569283; http://dx.doi.org/ 10.1073/pnas.1219871110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colinet AS, Sengottaiyan P, Deschamps A, Colsoul ML, Thines L, Demaegd D, Duchene MC, Foulquier F, Hols P, Morsomme P. Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci Rep 2016; 6:24282; PMID:27075443; http://dx.doi.org/ 10.1038/srep24282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foulquier F, Amyere M, Jaeken J, Zeevaert R, Schollen E, Race V, Bammens R, Morelle W, Rosnoblet C, Legrand D, et al.. TMEM165 deficiency causes a congenital disorder of glycosylation. Am J Hum Genet 2012; 91:15-26; PMID:22683087; http://dx.doi.org/ 10.1016/j.ajhg.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeevaert R, de Zegher F, Sturiale L, Garozzo D, Smet M, Moens M, Matthijs G, Jaeken J. Bone dysplasia as a key feature in three patients with a novel congenital disorder of glycosylation (CDG) type II due to a deep intronic splice mutation in TMEM165. JIMD Rep 2013; 8:145-52; PMID:23430531; http://dx.doi.org/ 10.1007/8904_2012_172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosnoblet C, Legrand D, Demaegd D, Hacine-Gherbi H, de Bettignies G, Bammens R, Borrego C, Duvet S, Morsomme P, Matthijs G, Foulquier F. Impact of disease-causing mutations on TMEM165 subcellular localization, a recently identified protein involved in CDG-II. Hum Mol Genet 2013; 22(14):2914-2928; PMID:23575229; http://dx.doi.org/ 10.1093/hmg/ddt146 [DOI] [PubMed] [Google Scholar]
- 7.Reinhardt TA, Lippolis JD, Sacco RE. The Ca(2+)/H(+) antiporter TMEM165 expression, localization in the developing, lactating and involuting mammary gland parallels the secretory pathway Ca(2+) ATPase (SPCA1). Biochem Biophys Res Commun 2014; 445:417-21; PMID:24530912; http://dx.doi.org/ 10.1016/j.bbrc.2014.02.020 [DOI] [PubMed] [Google Scholar]
- 8.Bammens R, Mehta N, Race V, Foulquier F, Jaeken J, Tiemeyer M, Steet R, Matthijs G, Flanagan-Steet H. Abnormal cartilage development and altered N-glycosylation in Tmem165-deficient zebrafish mirrors the phenotypes associated with TMEM165-CDG. Glycobiology 2015; 25:669-82; PMID:25609749; http://dx.doi.org/ 10.1093/glycob/cwv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potelle S, Morelle W, Dulary E, Duvet S, Vicogne D, Spriet C, Krzewinski-Recchi MA, Morsomme P, Jaeken J, Matthijs G, et al.. Glycosylation abnormalities in Gdt1p/TMEM165 deficient cells result from a defect in Golgi manganese homeostasis. Hum Mol Genet 2016; 25:1489-500; PMID:27008884; http://dx.doi.org/ 10.1093/hmg/ddw026 [DOI] [PubMed] [Google Scholar]
- 10.Dulary E, Potelle S, Legrand D, Foulquier F. TMEM165 deficiencies in congenital disorders of glycosylation type II (CDG-II): clues and evidences for roles of the protein in Golgi functions and ion homeostasis. Tissue Cell 2016; PMID:27401145; http://dx.doi.org/ 10.1016/j.tice.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 11.Schneider A, Steinberger I, Herdean A, Gandini C, Eisenhut M, Kurz S, Morper A, Hoecker N, Ruhle T, Labs M, et al.. The evolutionarily conserved protein PHOTOSYNTHESIS AFFECTED MUTANT71 is required for efficient manganese uptake at the thylakoid membrane in Arabidopsis. Plant Cell 2016; 28:892-910; PMID:27020959; http://dx.doi.org/ 10.1105/tpc.15.00812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Xu W, Jin H, Zhang T, Lai J, Zhou X, Zhang S, Liu S, Duan X, Wang H, et al.. A Putative Chloroplast-Localized Ca(2+)/H(+) Antiporter CCHA1 Is Involved in Calcium and pH Homeostasis and Required for PSII Function in Arabidopsis. Mol Plant 2016; 9:1183-1196; PMID:27302341; http://dx.doi.org/ 10.1016/j.molp.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 13.Nelson N, Junge W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu Rev Biochem 2015; 84:659-83; PMID:25747397; http://dx.doi.org/ 10.1146/annurev-biochem-092914-041942 [DOI] [PubMed] [Google Scholar]
- 14.Shi LX, Hall M, Funk C, Schroder W. Photosystem II, a growing complex: updates on newly discovered components and low molecular mass proteins. Biochim Biophys Acta 2012; 1817:13-25; PMID:21907181; http://dx.doi.org/ 10.1016/j.bbabio.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Suorsa M, Aro EM. Expression, assembly and auxiliary functions of photosystem II oxygen-evolving proteins in higher plants. Photosynth Res 2007; 93:89-100; PMID:17380423; http://dx.doi.org/ 10.1007/s11120-007-9154-4 [DOI] [PubMed] [Google Scholar]
- 16.Dent RM, Sharifi MN, Malnoe A, Haglund C, Calderon RH, Wakao S, Niyogi KK. Large-scale insertional mutagenesis of Chlamydomonas supports phylogenomic functional prediction of photosynthetic genes and analysis of classical acetate-requiring mutants. Plant J 2015; 2:337-51; PMID:25711437; http://dx.doi.org/10339815 10.1111/tpj.12806 [DOI] [PubMed] [Google Scholar]
- 17.Tatusova TA, Madden TL. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett 1999; 174 :247-50; PMID:10339815; http://dx.doi.org/ 10.1111/j.1574-6968.1999.tb13575.x [DOI] [PubMed] [Google Scholar]
- 18.Lee TH, Tang H, Wang X, Paterson AH. PGDD: a database of gene and genome duplication in plants. Nucleic Acids Res 2013; 41(Database issue):D1152-1158; PMID:23180799; http://dx.doi.org/ 10.1093/nar/gks1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armbruster U, Carrillo LR, Venema K, Pavlovic L, Schmidtmann E, Kornfeld A, Jahns P, Berry JA, Kramer DM, Jonikas MC. Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun 2014; 5:5439; PMID:25451040; http://dx.doi.org/ 10.1038/ncomms6439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunz HH, Gierth M, Herdean A, Satoh-Cruz M, Kramer DM, Spetea C, Schroeder JI. Plastidial transporters KEA1, -2, and -3 are essential for chloroplast osmoregulation, integrity, and pH regulation in Arabidopsis. Proc Natl Acad Sci U S A 2014; 111:7480-7485; PMID:24794527; http://dx.doi.org/12529511 10.1073/pnas.1323899111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwacke R, Schneider A, van der Graaff E, Fischer K, Catoni E, Desimone M, Frommer WB, Flugge UI, Kunze R. ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol 2003; 131:16-26; PMID:12529511; http://dx.doi.org/ 10.1104/pp.011577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferro M, Salvi D, Brugiere S, Miras S, Kowalski S, Louwagie M, Garin J, Joyard J, Rolland N. Proteomics of the chloroplast envelope membranes from Arabidopsis thaliana. Mol Cell Proteomics 2003; 2:325-45; PMID:12766230; http://dx.doi.org/ 10.1074/mcp.M300030-MCP200 [DOI] [PubMed] [Google Scholar]
- 23.Ferro M, Brugiere S, Salvi D, Seigneurin-Berny D, Court M, Moyet L, Ramus C, Miras S, Mellal M, Le Gall S, et al.. AT_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Mol Cell Proteomics 2010; 9:1063-84; PMID:20061580; http://dx.doi.org/ 10.1074/mcp.M900325-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simm S, Papasotiriou DG, Ibrahim M, Leisegang MS, Muller B, Schorge T, Karas M, Mirus O, Sommer MS, Schleiff E. Defining the core proteome of the chloroplast envelope membranes. Front Plant Sci 2013; 4:11; PMID:23390424; http://dx.doi.org/ 10.3389/fpls.2013.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol 2013; 30:1229-35; PMID:23486614; http://dx.doi.org/ 10.1093/molbev/mst012 [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol 2016; 33:1870-4; PMID:27004904; http://dx.doi.org/ 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adachi J, Waddell PJ, Martin W, Hasegawa M. Plastid genome phylogeny and a model of amino acid substitution for proteins encoded by chloroplast DNA. J Mol Evol 2000; 50:348-58; PMID:10795826; http://dx.doi.org/ 10.1007/s002399910038 [DOI] [PubMed] [Google Scholar]
- 28.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001; 305:567-80; PMID:11152613; http://dx.doi.org/ 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 29.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 2000; 300:1005-16; PMID:10891285; http://dx.doi.org/ 10.1006/jmbi.2000.3903 [DOI] [PubMed] [Google Scholar]
- 30.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 2011; 8:785-86; PMID:21959131; http://dx.doi.org/ 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]


