Abstract
Cancer exosomes are gaining considerable amount of attention in basic and applied clinical research for their established role in the modulation of the tumor niche, and their broad-range contribution to tumor-host cross-talk. Supporting evidence to their role in tumorigenesis comes from the observation that exosome secretion, composition and functional effects are all altered as tumors become more aggressive. At the molecular level, the mechanisms underlying exosome biogenesis and uptake are far from being understood. Recent work has highlighted the critical role for the small intracellular adaptor protein syntenin in the biogenesis of a subset of exosomes and loading of cargo. Here, we review this recent work and some unpublished data that further highlight the possible implications of syntenin and the syndecan (SDC) heparan sulphate proteoglycans during exosome uptake, suggesting a supporting role for this pathway in the entire life cycle of cancer exosomes.
Keywords: cancer; exosome; syndecans; syntenin
Secreted vesicles in general and exosomes in particular (50–100 nm extracellular vesicles of endocytic origin) are known to transfer biologically active material between cells, with the ability of reprogramming their target cells. While highly heterogeneous, vesicle compositions reflect those of the parental cells, providing a circulating traceable signature of particular interest in pathological settings. At a functional level, evidence is growing that exosome-mediated transfer of signaling proteins and various forms of RNA contributes to the development of cancer, neurodegenerative disorders, inflammation and other pathological conditions. For instance, cancer-derived exosomes have been reported to participate in multiple mechanisms that support tumorigenesis. These include the remodelling of the extracellular matrix and the promotion of angiogenesis, thrombosis and tumor cell proliferation. Exosomes are also found to travel to distant sites to promote and establish a pro-tumorigenic metastatic niche, in part via the transfer of oncoproteins and other cargo. On the other hand, early evidence has accumulated implicating cancer exosomes in the modulation of the anti-tumoral immune response; with exosomes found to display both immune stimulatory and suppressive effects. However, as we get better insights into the function of cancer exosomes, it now appears that cancer exosomes likely interfere with the induction of an efficient immune response via several mechanisms including triggering T cell suppression mechanisms, attenuating NK cell cytotoxicity, and engaging pro-metastatic inflammatory processes (reviewed in ref. 1). Cancer exosomes seem then to be arbitrating the generation of an immunosuppressive environment favoring tumor engraftment, escape from the immune system and, eventually, treatment failure. Finally, evidence is accumulating in support of a role for stroma-derived exosomes in the mediation of carcinogenesis (Fig. 1A).
Figure 1.
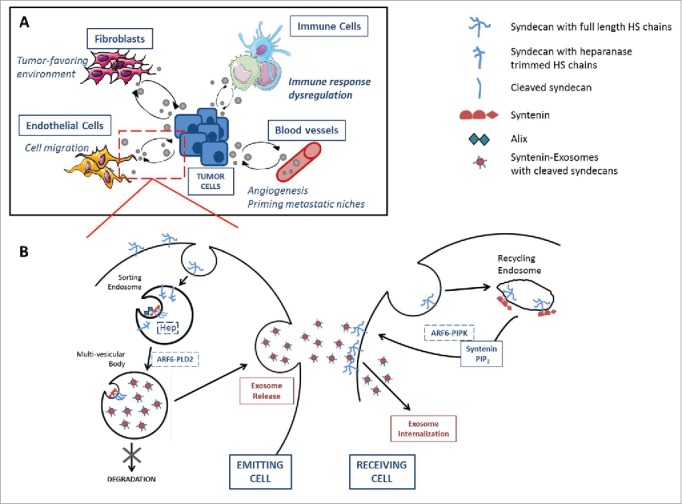
The syndecan-syntenin-ALIX pathway in intercellular exosomal communication. (A) Exosomes are mediators of intercellular communication between tumor and host cells including fibroblasts, endothelial cells, blood vessels and immune cells, to either favor or suppress the tumorigenic process depending on the cancer setting. Both host and tumor cells secrete and uptake exosomes and this multidirectionality seems to be regulated in part by the syndecan-syntenin pathway as detailed in (B). Syntenin binds to the cytosolic tail of syndecans, which are internalized in sorting endosomes along with their intact heparan sulfate (HS) chains. Endosomal heparanase (Hep) processes the HS chains to allow the clustering of syndecans for recruitment by syntenin to the ALIX/ESCRT machinery. Exosomal syndecans consist mainly of cleaved forms, i.e. C-terminal fragments of these molecules.6 Along with ARF6 and phospholipaseD2 (PLD2), they trigger the formation of multi-vesicular bodies, containing several endosomes, later released as exosomes. On the target cell, syntenin is also involved in maintaining a pool of HSPGs at the cell membrane, by stimulating their recycling, in their intact full form, via its direct interaction with phosphatidylinositol 4,5 bisphosphate (PIP2), depending on ARF6-phosphatidylinositol 4-phosphate 5-kinase (PIPk) activation. HSPGs presence is essential for efficient exosome internalization and function.
Exosomes most probably vary in ontogeny,2-4 yet the complexity of the mechanisms driving exosome biogenesis on one hand, and cargo sorting into exosomes5 on the other hand, are only beginning to come to light. In recent work, the syndecan-syntenin pathway has emerged as a major player controlling the formation of endosomal intraluminal vesicles that get released as exosomes, and the sorting of cargo in these vesicles. Syntenin binds to the cytosolic tail of syndecans, which by virtue of their extracellular heparan sulfate chains interact with a plethora of signaling and adhesion molecules. Syntenin also binds with ALIX via motifs used by some viruses to egress from cells by budding. ALIX thereby connects the syntenin-syndecan cargo to the ESCRT machinery known to play a role in membrane budding and scission at the endosome.6 Other syntenin-associated regulators involved during this process include the small GTPase ARF6 and the lipid-modifying enzyme PLD2, which have been reported to also play a role during the formation of intraluminal vesicles.7 More recently, the endoglycosidase heparanase was identified as a stimulator of the syndecan-syntenin-ALIX pathway. Indeed, endosomal heparanase, by trimming the heparan sulfate side chains of syndecans, allows the clustering of syndecans, for subsequent recruitment by syntenin to the ALIX-ESCRT sorting machinery at endosomes. The study further indicates that heparanase controls the selection of specific cargo to exosomes8 (Fig. 1B).
Considerable evidence exists to support the concerted roles of heparanase and syndecan in promoting tumor growth and angiogenesis both in host and tumor cells. Heparanase has been found on exosomes of cancer patients, suggesting that its delivery to recipient cells could further accentuate exosomal release via the described syndecan-syntenin-ALIX pathway9 (reviewed in ref. 10). Syntenin's expression is now reported to be upregulated in several types of cancer, like melanoma and breast cancer, and found to favor tumor growth and metastasis in mouse xenograft tumor models (reviewed in ref. 11). Consistently, we have shown that syntenin plays a supporting role in the migration, growth and proliferation of tumor cells (melanoma, colon cancer and breast cancer), and further controls the G1/S cycle transition in these cells.12 Ongoing work in our lab, using syntenin knockout mice provides further evidence for a role for syntenin in the host organism in promoting a pro-carcinogenic environment. In our hands, loss of syntenin within the tumor niche correlates with reduced cancer engraftment and spreading, and an altered immune profile in the host mice [unpublished data]. While these results are highly encouraging, it remains to be determined whether the observed phenotypes are a direct effect of an alteration in the exosomal release pathway.
Alternatively, such phenotypes could be due to another mechanism by which syntenin is impacting on syndecans. Indeed, syntenin has been shown to function as a rate limiting factor for the recycling of syndecans, allowing their escape from degradation (as full-length molecules). This function depends on the activation of ARF6, the ARF6-effector phosphatidylinositol 4-phosphate 5-kinase and the direct interaction of syntenin with phosphatidylinositol 4,5-bisphosphate.13 Consistently, mouse embryonic fibroblasts isolated from syntenin knock-out mice display lesser amounts of heparan sulfate proteoglycans (HSPGs) on their cell surfaces, which correlates with reduced uptake of retrovirus and cancer-cell derived exosomes (Kashyap et al., in preparation). Interestingly, Belting and co-workers highlighted a role for HSPGs in exosomal uptake. Indeed, HSPGs appear to function as internalizing receptors of cancer cell-derived exosomes, and to be required for their functional activity, namely in driving cancer cell migration.14 The full picture might thus be that syntenin impacts on HSPGs to boost the complete (biogenesis and uptake) exosome “life-cycle” (Fig. 1B).
Syntenin might thus represent a non-negligible target for the inhibition of exosome-mediated tumor development. This holds true for both host and tumor cells within the tumor microenvironment, where the syntenin route of intercellular communication seems to be working in interdependent and multidirectional loops.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Syn N, Wang L, Sethi G, Thiery JP, Goh BC. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol Sci 2016; 37(7):606-17; PMID:27157716; http://dx.doi.org/ 10.1016/j.tips.2016.04.006 [DOI] [PubMed] [Google Scholar]
- [2].Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319(5867):1244-7; PMID:18309083; http://dx.doi.org/22660413 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- [3].Hurley JH. ESCRTs are everywhere. EMBO J 2015; 34(19):2398-407; PMID: 26311197; http://dx.doi.org/22660413 10.15252/embj.201592484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Friand V, David G, Zimmermann P. Syntenin and syndecan in the biogenesis of exosomes. Biol Cell 2015; 107(10):331-41; PMID:26032692; http://dx.doi.org/22660413 10.1111/boc.201500010 [DOI] [PubMed] [Google Scholar]
- [5].Stoorvogel W. Resolving sorting mechanisms into exosomes. Cell Res 2015; 25(5):531-2; PMID:25828531; http://dx.doi.org/22660413 10.1038/cr.2015.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al.. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 2012; 14(7):677-85; PMID:22660413; http://dx.doi.org/ 10.1038/ncb2502 [DOI] [PubMed] [Google Scholar]
- [7].Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, Slavík J, Machala M, Zimmermann P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun 2014; 5:3477; PMID:24637612; http://dx.doi.org/ 10.1038/ncomms4477 [DOI] [PubMed] [Google Scholar]
- [8].Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res 2015; 25(4):412-28; PMID:25732677; http://dx.doi.org/ 10.1038/cr.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem 2013; 288(14):10093-9; PMID:23430739; http://dx.doi.org/ 10.1074/jbc.C112.444562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JL, Sanderson RD. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. FEBS J 2013; 280(10):2294-306; PMID:23374281; http://dx.doi.org/ 10.1111/febs.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kegelman TP, Das SK, Emdad L, Hu B, Menezes ME, Bhoopathi P, Wang XY, Pellecchia M, Sarkar D, Fisher PB. Syntenin Targeting tumor invasion: the roles of MDA-9/Syntenin. Expert Opin Ther Targets 2014; 19(1):97-112; http://dx.doi.org/ 10.1517/14728222.2014.959495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kashyap R, Roucourt B, Lembo F, Fares J, Carcavilla AM, Restouin A, Zimmermann P, Ghossoub R. Syntenin controls migration, growth, proliferation, and cell cycle progression in cancer cells. Front Pharmacol 2015; 6:241; PMID:26539120; http://dx.doi.org/ 10.3389/fphar.2015.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N'Kuli F, Courtoy PJ, David G. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell.2005 Sep;9(3):377-88. Erratum in: Dev Cell 2005; 9(5):721. PubMed PMID: 16139226.24101524 [DOI] [PubMed] [Google Scholar]
- [14].Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. PNAS 2013; 110(43):17380-5; PMID:24101524; http://dx.doi.org/ 10.1073/pnas.1304266110 [DOI] [PMC free article] [PubMed] [Google Scholar]


