ABSTRACT
Cells from bacteria to man release extracellular vesicles (EVs) that contain signaling molecules like proteins, lipids, and nucleic acids. The content, formation, and signaling roles of these conserved vesicles are diverse, but the physiological relevance of EV signaling in vivo is still debated. Studies in classical genetic model organisms like C. elegans and Drosophila have begun to reveal the developmental and behavioral roles for EVs. In this review, we discuss the emerging evidence for the in vivo signaling roles of EVs. Significant effort has also been made to understand the mechanisms behind the formation and release of EVs, specifically of exosomes derived from exocytosis of multivesicular bodies and of microvesicles derived from plasma membrane budding called ectocytosis. In this review, we detail the impact of flies and worms on understanding the proteins and lipids involved in EV biogenesis and highlight the open questions in the field.
KEYWORDS: behavior, C. elegans, development, Drosophila, ectosome, exosome, extracellular vesicle, genetics, microvesicle, signaling
Introduction
Extracellular vesicles (EVs) are membrane-wrapped structures that are released by cells. All cell types examined so far release EVs, ranging from bacteria to fungi and from germ cells to neurons in metazoans.1-4 EVs carry nucleic acids, proteins, and lipids both as internal cargo and on their surface, and these molecules account for the immense signaling potential of EVs. EVs can mediate communication between cells, either on the cell surface or after uptake by a target cell.5,6 EVs are thought to carry signals between cells during normal development and homeostasis.6,7 They have also been implicated in a number of diseases like cancer as well as infections.8-11 Studies have also shown the importance of EVs as clinical biomarkers after extracting them from human biofluids like blood, spinal fluid, or urine.12-14 For these reasons, EVs are of broad interest as a new mode of cell signaling.
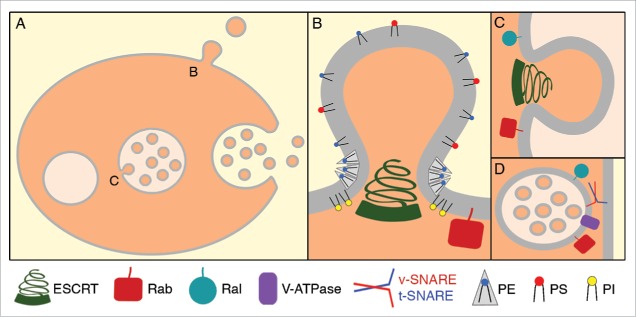
EVs can be broadly separated into 2 classes: exosomes and microvesicles.5 Exosomes are derived from the internal intraluminal vesicles (ILV) of multivesicular bodies (MVB), formed by the inward budding of the limiting endosomal membrane of the MVB (Fig. 1A, C). Fusion of an MVB with the plasma membrane (i.e. exocytosis) releases ILVs as exosomes outside the cell (Fig. 1A). Both ILVs and exosomes are 30–100 nm in diameter. The second class of EVs is called microvesicles, which are typically bigger than exosomes (>90 nm). Microvesicles form by direct budding of the plasma membrane into the extracellular space (Fig. 1A-B), a process called ectocytosis. Although we use the term microvesicle throughout this review, we would like to highlight the alternative term ectosome, which describes their origin (ectocytosis) rather than their larger size. The processes that cause the plasma membrane to release microvesicles are diverse. They range from budding the plasma membrane away from the cytoplasm (ectocytosis) to generate vesicles 90–500 nm in diameter, to asymmetric cytokinesis to release midbodies and polar bodies 1–20 µm in diameter, to blebbing driven by a loss in membrane tension to generate 100 nm–5 µm vesicles.15-18 Exosomes and microvesicles contain distinct cargos reflecting their origin (endosome vs. plasma membrane), but also contain many common cargos since they both bud from the cytoplasm (Fig. 1A-C).19 Consequently, there are a variety of EVs released by cells with numerous potential functions.
Figure 1.
Mechanisms of extracellular vesicle release. (A) Extracellular vesicles can be released by direct budding of the plasma membrane to form microvesicles. Extracellular vesicles can also be released by the fusion of multivesicular bodies (MVBs) with the plasma membrane to release exosomes. To form MVBs, endosomes must first bud vesicles into their lumen, called intraluminal vesicles (ILVs). (B) Plasma membrane budding away from the cytoplasm requires Rab GTPases and the ESCRT complex. Lipids also play an important role in microvesicle budding, with phosphatidylinositols recruiting membrane-sculpting proteins and cone-shaped phosphatidylethanolamine inducing membrane curvature. (C) The budding of ILVs into MVBs also requires Rab and Ral GTPases and the ESCRT complex. (D) The fusion of MVBs with the plasma membrane to release exosomes requires vesicle tethering and fusion factors, such as Rab and Ral GTPases, SNAREs, and the V-type ATPase.
The strength of genetic model organisms to unravel EV release and signaling
The in vivo signaling roles of EVs are debated because most studies demonstrate the signaling potential of EVs using purified vesicles on cultured cells. Researchers have begun to inject purified EVs into model organisms to demonstrate their in vivo roles. For example, injecting purified exosomes derived from specific cell types can redirect tumor metastases to different tissues in mice.20 These results are an exciting demonstration of the signaling potential of EVs in cancer, but it is unclear how physiological the levels of EVs are in this study. Another recent study showed that purified EVs released from mouse embryonic stem cells are able to influence implantation after injection into embryos.21 However, the authors did not show that embryonic cells release significant numbers of EVs in vivo. Thus, although it is clear that EVs have great signaling potential, it is unclear how often EVs actually send signals or transmit intracellular cargo like miRNAs in a living animal.
Invertebrate genetic model organisms like Drosophila melanogaster and Caenorhabditis elegans can serve as invaluable systems to study the signaling function of EVs in multicellular organisms. Research using flies and worms has contributed significantly to our understanding of cell physiology over the last century. For example, Nobel prize-winning research in Drosophila identified the first morphogens that regulate embryonic patterning conserved from flies to humans.22 Similarly, Nobel prize-winning work in C. elegans demonstrated that cells normally die during development and established the conserved mechanisms of programmed cell death and their subsequent uptake by phagocytosis.23 Transgenesis and live imaging are also well established in Drosophila and C. elegans animals, allowing the in vivo tracking of EVs. For example, EVs can be labeled with GFP fusion proteins such as CD63:GFP to allow live tracking.24 Thus, studies in Drosophila and C. elegans can serve as invaluable in vivo systems to establish whether EV signaling can change the development, behavior, or disease state in metazoans. In addition, the genetic tools available in flies and worms can also help us define the molecular mechanisms of EV release. In this review, we discuss the evidence for the functional roles of EVs in Drosophila and C. elegans followed by the mechanistic insights into EV formation drawn from these studies.
EVs and development
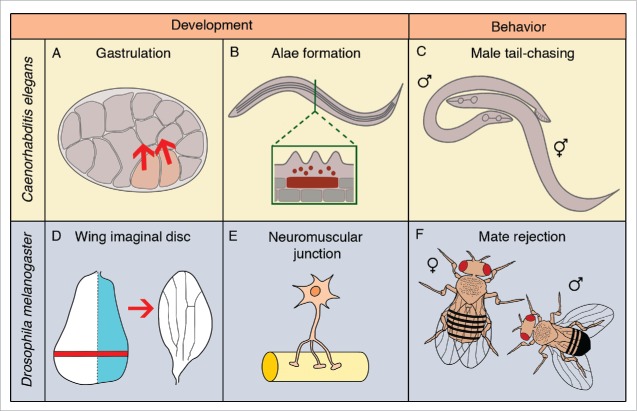
One example of EVs playing a role in normal development is shown in the C. elegans cuticle (Fig. 2B). The cuticle consists primarily of glycosylated and lipid-modified proteins secreted by underlying epithelial cells, including the seam cells.25,26 How lipid-modified proteins could be trafficked away from the plasma membrane of a cell was unclear. Work from Michel Labouesse's lab showed that the proper development of the cuticle requires the release of lipidated morphogens on exosomes.27 Seam cells release EVs of 50–100 nm in diameter carrying lipid-modified Hedgehog-related peptides. Seam cell EVs also contain the transmembrane protein CHE-14/Dispatched involved in Hedgehog secretion. Disrupting exosome secretion by depleting subunits of the V-ATPase (discussed below) results in MVB accumulation in the cytoplasm and traps hedgehog-related peptides intracellularly in MVBs. Depleting V-ATPase subunits also blocks the development of longitudinal ridges on the larval and adult cuticles called alae. This finding suggests that no alternative secretion pathway for hedgehog-related peptides exists in C. elegans larvae. Thus, EVs can provide a unique solution for the trafficking of lipid-modified or transmembrane proteins during development.
Figure 2.
In vivo functions of extracellular vesicles in genetic model organisms. (A) In C. elegans embryos, excessive microvesicle release disrupts gastrulation movements. (B) In C. elegans larvae and adults, seam cells (brown rectangle) release exosomes to build the alae, longitudinal ridges on the cuticle. (C) In C. elegans adult males, ciliated neurons release EVs important for male mating behavior. (D) In Drosophila larvae, the wing imaginal disc is patterned by morphogens carried on EVs that induce the wing axes. (E) The neuromuscular junction also releases morphogens on EVs that are important for synaptic development. (F) Drosophila adult males release exosomes important for female mating behavior.
A second example of a role for EVs during development is in Drosophila larvae, where EVs are important for synaptic growth at the neuromuscular junction (NMJ) (Fig. 2E).28,29 Neurons at the NMJ release EVs carrying the glycosylated and lipidated morphogen Wingless (Wg), orthologous to mouse Wnt proteins. Wg is sorted onto EVs containing the transmembrane protein Evi/Wntless. Evi is important for Wnt trafficking and is found on both ILVs and the plasma membrane of the pre-synaptic neuron.28,29 NMJ EVs are 30–400 nm in diameter, suggesting that Evi is required for Wg secretion onto both exosomes and microvesicles. Evi mutants fail to release Wg from the NMJ, resulting in defects in synaptic growth and differentiation. Thus, Wg trafficking through EVs is thought to be required for signaling at the synapse. The post-synaptic muscle cell takes up Evi-containing EVs, but it is not clear whether EVs need to be taken up by the muscle for Wg signaling to occur. It remains possible that Wg binds its receptor and initiates signaling while outside the cell on EVs. It is interesting to note that the same neurons release the same signaling molecules on both exosomes and microvesicles. Therefore, cells may use redundant pathways to secrete regulators important for development.
EV formation is also required for long-range Wg and Hedgehog (Hh) signaling in the developing wing in Drosophila (Fig. 2D).30-32 The wing imaginal disc is a single-layered epithelium in fly larvae that is the precursor to the adult wing.33 The imaginal disc is polarized by morphogens to establish the cardinal axes of the wing. Hh in the posterior half of the imaginal disc is secreted and induces graded fates in the anterior cells of the disc. Similarly, Wg is secreted from the dorsal-ventral (DV) boundary of the imaginal disc and is required to pattern the DV axis as well as induce the outgrowth of the wing. Given that lipidation should limit the diffusion of Wg and Hh, it was challenging to explain how these morphogens were able to signal to distant cells in the epithelium. A first insight into long-range mechanisms of morphogen secretion came when Tom Kornberg's group showed that cells were connected over long distances by thin filopodia-like protrusions called cytonemes.34 More recently, EVs have also been shown to be required for morphogen transport in Drosophila.30-32 EVs even travel along cytonemes in vivo.32 Similarly, filopodia in Xenopus embryos have been shown to act as superhighways for microvesicles in vivo,35 suggesting that there may be a conserved link between cellular extensions and EVs. The budding or breaking of filopodia has also been proposed to be a source of EVs, but it is unknown whether Wg- and Hh-carrying EVs are released from cytonemes or filopodia in Drosophila. In the wing imaginal disc, EVs are likely to be derived from both the plasma membrane and ILVs. Hh is found both places and the imaginal disc EVs are 30–550 nm in diameter, indicating that the EVs are a mixture of exosomes and microvesicles.31,32 Inhibiting a number of different trafficking proteins required for EV release (discussed below) disrupts patterning of the wing, resulting in typical Hh or Wg mutant phenotypes.30-32 Thus, EVs carry morphogens across many cells to regulate development.
In addition to EV release being important for development, studies in C. elegans have shown that overproducing EVs can also disrupt development.15 During embryogenesis, cells divide, change their shapes, and migrate to attain their proper location in the body (Fig. 2A). We previously found that loss of the PE flippase TAT-5 (discussed below) disrupts C. elegans embryogenesis because of an overproduction of microvesicles. Both wild type and tat-5 mutant embryonic cells released EVs 90–400 nm in diameter by plasma membrane budding, but the microvesicles accumulated between cell contacts in tat-5 mutant embryos. The extra EVs disrupted the normally tight cell-cell adhesion necessary for morphogenesis and gastrulation movements, resulting in embryonic lethality. Thus, EV release needs to be tightly regulated to produce the correct number of EVs at the appropriate time.
EVs and animal behavior
EVs also play a role in adult physiology, as shown by behavior-modifying EVs released from cilia. Cilia are conserved structures from algae to mammals that can function as sensory organelles. Cilia have been shown to release EVs in many organisms, including single-celled organisms like Chlamydomonas and multicellular organisms from C. elegans to mammals.36 One example of a behavioral role for ciliary EVs is in Dictyostelium, where cilia release EVs containing a transmembrane protein important for sexual reproduction.37 Similarly, C. elegans ciliated sensory neurons release EVs carrying transmembrane proteins that mediate male mating behavior (Fig. 2C).38,39 The mechanism of ciliary EV release is unknown in C. elegans neurons, but their size is consistent with a mix of both exosomes and microvesicles (on average 50–150 nm diameter). The EVs accumulate outside the base of the cilia in a lumenal pocket and are released into the environment via an opening in the worm's cuticle. The released EVs contain polycystins known to be responsible for male mating behaviors.40,41 To demonstrate that the polycystins released into the environment via EVs could modify male behavior, Maureen Barr and colleagues took advantage of a mutant in the Kinesin-III protein KLP-6 that reduced EV release.38 In klp-6 mutants, EVs accumulate in the lumenal pocket between the neuron and the sheath cell, with fewer EVs released into the environment. KLP-6 is not required for EV biogenesis, but the trafficking of some polycystins into EVs is disrupted in klp-6 mutants. Purified EVs from klp-6 mutant males failed to induce the tail-chasing behavior induced by wild-type EVs. This suggests that proteins presented by ciliary EVs are able to influence the behavior of other worms. It would be interesting to see whether the natural release of EVs would also be able to induce male mating behaviors.
EV release has also been shown to play a role in mating behavior in Drosophila, this time with male exosomes altering female behavior (Fig. 2F). Large accessory gland cells in Drosophila males secrete ∼40 nm exosomes from giant MVBs into the gland lumen.42 Exosomes are later secreted into the seminal fluid and associate with sperm or with female epithelial cells in the reproductive tract after mating. Reducing exosome secretion by 80% specifically in the male accessory gland resulted in altered behavior in females after mating, increasing remating by females three fold. This study dramatically demonstrates the signaling roles of exosomes in vivo. It also establishes a new system for visualizing exosome biogenesis in relatively large cells and is likely to contribute significantly to our understanding of the mechanisms of MVB formation and exosome release.
Proteins required for EV formation
In order to demonstrate the functional roles of EVs in vivo, it is necessary to understand how they are formed. Proteins are needed at various stages of EV release. First, membrane-sculpting proteins are required to induce membrane curvature either at the endosomal membrane for ILV budding or to curve the plasma membrane for microvesicle budding. After the formation of a curved membrane, proteins are needed for scission to pinch off the ILVs or microvesicles. Since these membrane curvature and scission events would have the same topology (budding away from the cytoplasm), they are likely to be carried out by the same proteins (Fig. 1B-C). Protein regulators that are more likely to be specific for exosome release include proteins required to traffic MVBs to the plasma membrane, to tether MVBs to the plasma membrane, and to fuse MVBs with the plasma membrane. However, membrane proteins also require vesicular trafficking to reach the plasma membrane, so trafficking factors may also have a role in microvesicle formation. Here, we summarize the function of known proteins in exosome and microvesicle formation, emphasizing where the same proteins are required for both exosome and microvesicle formation.
Endosomal sorting complex required for transport (ESCRT)
The best-studied factor in EV biogenesis is the Endosomal Sorting Complex Required for Transport (ESCRT), which is known for its role in forming ILVs in MVBs. ESCRT is one of the few membrane sculpting complexes known to bend membranes away from the cytoplasm.43 The ESCRT machinery is subdivided into 4 subcomplexes, which function at subsequent stages in budding the endosomal membrane of MVBs to form ILVs. ESCRT-0 engages and clusters ubiquitinated cargo and recruits ESCRT-I and ESCRT-II to the bud site. ESCRT-I and ESCRT-II curve the membrane and recruit ESCRT-III. Finally, the ESCRT-III complex forms a coil that is thought to pull the bud neck together, leading to scission and release of the ILV into the MVB lumen (Fig. 1C). The disassembly factor VPS4 is an ATPase responsible for the disassociation of ESCRT subcomplexes from the endosomal membrane.44 ESCRT proteins are also found in released EVs, suggesting that at least some ESCRT proteins are trapped in the vesicle during scission.5
Vincent Hyenne and colleagues recently used the formation of C. elegans alae on the adult cuticle in a candidate screen of over 1000 RNAi clones to identify proteins involved in exosome biogenesis.45 They identified 10 ESCRT proteins important for alae formation, including an ESCRT-0 subunit (HGRS-1), ESCRT-I subunits (TSG-101, VPS-28, VPS-37), ESCRT-II subunits (VPS-22, VPS-25, VPS-36), ESCRT-III subunits (VPS-20, VPS-32), and VPS-4. These findings support the role of ESCRT in exosome biogenesis in C. elegans seam cells. In Drosophila, the ESCRT-0 subunit Hrs and accessory factor ALiX were found to be required for exosome secretion from male gland cells.42 Hence, ESCRT plays a role in exosome biogenesis in worms and flies, as in mammalian cells.5,43,46
In addition to playing a role in exosome biogenesis, the ESCRT machinery also acts at the plasma membrane to release microvesicles (Fig. 1B). In C. elegans embryos, at least ESCRT-I (TSG-101) and ESCRT-III (VPS-32) proteins are recruited to the plasma membrane where microvesicles form.15 Depleting proteins from the ESCRT-0 (HGRS-1, STAM-1) or ESCRT-I (TSG-101, VPS-28) subcomplexes significantly suppressed microvesicle release in embryos, demonstrating that ESCRT proteins are required for plasma membrane budding. Similarly, VPS4 and the ESCRT-I subunit TSG101 are required for microvesicle budding in cultured mammalian cells and TSG101 is trapped in released microvesicles purified from the supernatant.47 In the Drosophila wing imaginal disc, knockdown of ESCRT-0 Hrs, ESCRT-I TSG101, ESCRT-II Vps22, ESCRT-III subunits (Chmp1, Vps24), Vps4, or Alx inhibits the release of microvesicles carrying Hh.31,48 Several ESCRT-I and ESCRT-III proteins were also found in purified imaginal disc microvesicles.31 Viruses can also bud from the plasma membrane like microvesicles and also require the ESCRT machinery for budding.43 Thus, the ESCRT pathway not only functions in ILV budding and thereby exosome formation, but also in plasma membrane budding to release microvesicles.
Although the ESCRT machinery plays a significant role in EV release, there is also evidence that both exosomes and microvesicles can be formed by ESCRT-independent mechanisms. For example, ciliary EVs in C. elegans do not require the non-essential ESCRT subunits for their release,38 although it would be interesting to test whether essential ESCRT subunits are required using genetic tricks to knock ESCRT protein levels down specifically in neurons. In addition, the depletion of ESCRT proteins did not completely suppress exosome or microvesicle release in Drosophila gland cells or C. elegans embryos.15,42 In cultured Drosophila S2 cells, knocking down the ESCRT-0 subunit Hrs, ESCRT-I Vps28, Vps4, and accessory factor ALiX does not disrupt EV release, although several ESCRT proteins are found in purified EVs.29,49 Furthermore, not all released EVs contain ESCRT proteins in C. elegans embryos (AW, unpublished data). Viruses can also bud independently of VPS4, instead depending on a Rab11-binding protein.50 Thus, depleting ESCRT proteins is likely to eliminate only a subset of EVs, incompletely defining EV functions.
Another caveat in using ESCRT depletion to define the in vivo roles of EVs is that ESCRT is required in many other processes from the nuclear membrane to the plasma membrane. For example, ESCRT plays an important role during cytokinesis, with ESCRT-III coils thought to bring the plasma membrane close together during abscission.51 Interestingly, the intercellular bridge between dividing cells is cut on both sides at the end of cytokinesis to release the midbody remnant as an EV of 1−2 µm in size.52 In addition, microvesicles are also released from intercellular bridges during cell division,53 suggesting that membrane sculpting to thin out a structure may be another function of microvesicles. These observations demonstrate that cytokinetic bridges are another source of EVs, which are likely to be released by distinct mechanisms in comparison to EVs from non-dividing cells. In summary, ESCRT plays a role in many membrane-remodeling processes. Consequently, inferring a role for EVs based solely on disrupting ESCRT proteins can be problematic.
Rab family GTPases
Small Rab family GTPases are also required for exosome and microvesicle formation.5 Rab GTPases are universal vesicle trafficking regulators, acting at every step of membrane trafficking.54 There are over 60 Rab GTPases in humans, each associated with specific lipid-containing organelles from the ER to the plasma membrane. In C. elegans, 8 different Rab proteins have been associated with exosome secretion based on the alae screen.45 However, whether all 8 Rabs are required for exosome biogenesis remains to be tested. For 5 of these Rabs (RAB-2, RAB-7, RAB-11, RAB-27, and RAB-35), there is evidence from other systems that they have a role in EV biogenesis. For example, Golgi/ER-associated Rab2 has also been shown to have a role in EV release in cultured mammalian cells,55 but has not been closely studied.
Rab27 is a Rab GTPase that regulates the fusion of lysosome-related organelles like melanosomes with the plasma membrane.56 Rab27 localizes to late endosomes and lysosome-related organelles, including MVBs.54 MVB fusion and exosome release are decreased after Rab27A/B knockdown in mammalian cells.55 Hence, Rab27 knockdown is frequently used to test the role of exosomes in mammalian studies. The identification of RAB-27 in the C. elegans alae screen suggests that it will also have a role in exosome formation.45 In contrast, knocking down Rab27 only mildly disrupted CD63:GFP-positive exosome release from male gland cells in Drosophila.42 Knocking down Rab27 also did not disrupt EV release in cultured Drosophila S2 cells.29 Rab27 has recently been shown to regulate the trafficking of lipid regulators like PI4KIIa to the plasma membrane via endosome fusion.57 Rab27 is thereby required for viruses to assemble on the plasma membrane at phosphoinositide-rich microdomains. These data could indicate a role for Rab27 in microvesicle budding, but to date Rab27 is only thought to regulate exosome release.
Rab7 is involved in trafficking between early endosomes and lysosomes and is associated with late endosomes and MVBs like Rab27.58 Rab7 has a number of binding partners, which are important for vesicle transport by motor proteins, tethering to lysosomes, and regulating phosphoinositide lipids (discussed below) in the endosome membrane. Given the localization of Rab7 to MVBs, it could be expected that Rab7 would be required for exosome biogenesis. In Drosophila, knocking down Rab7 or expressing a dominant-negative version of Rab7 in secretory gland cells disrupts exosome release.42 Rab7 is also required for the secretion of microRNAs in EVs from cultured mammalian cells.59 Thus, Rab7 plays a conserved role in exosome biogenesis in C. elegans, Drosophila, and mammals. However, the specific role of Rab7 and which Rab7 effectors are required for exosome biogenesis remain to be determined.
Rab11 is best known for its role in trafficking membrane cargo to and from recycling endosomes on the way to the plasma membrane.60,61 In cultured human cells, Rab11 was shown to be required for MVB tethering, docking, or fusion with the plasma membrane, thus regulating exosome release.62,63 In C. elegans and Drosophila, RAB-11 is required for microvesicle budding as well as exosome release. In Drosophila, Rab11 is required for EV release from wing imaginal discs and neurons as well as S2 cells in culture.29,32,49 Expressing a dominant-negative version of Rab11 disrupts exosome release from male gland cells.42 In C. elegans, depletion of RAB-11 disrupted alae formation in adults and dramatically reduced microvesicle release in embryos.15,45 Furthermore, RAB-11 is secreted in microvesicles.15 Viruses can also bud from the plasma membrane using a Rab11-binding protein.50 RAB-11 is required for the trafficking of many proteins as well as lipids to the plasma membrane, making it difficult to assign a role to EVs based on the pleiotropic nature of RAB-11 knockdown. In addition, whether RAB-11 regulates microvesicle formation by recycling lipids or proteins to the plasma membrane or whether it has a more direct role in budding is unclear. It will also be interesting to test whether RAB-11 acts at the level of MVB transport, tethering, or fusion in C. elegans alae and Drosophila secretory glands.
Rab35 is involved in a parallel recycling pathway to Rab11 and also helps traffic endosomes to the plasma membrane.64 Expressing dominant-negative Rab35 or knocking down Rab35 levels results in decreased exosome release in cultured mammalian cells, due to failed fusion of MVBs with the plasma membrane.65 This suggests Rab35 would have a role in MVB tethering, docking, or fusion. Similarly, in Drosophila, Rab35 RNAi disrupted exosome release from male gland cells.42 However, expressing a dominant-negative version of Rab35 in neurons or knocking down Rab35 in cultured S2 cells did not disrupt EV release.29,49 Thus, there is good evidence for Rab35 regulating exosome release, but no evidence yet for a role in microvesicle budding. As with the other Rabs, the precise role of Rab35 and identity of Rab35 effectors important for exosome release remains to be determined.
SNAP receptors (SNAREs)
In addition to Rab GTPases regulating vesicle tethering and fusion, SNARE proteins are well known regulators of vesicle docking and fusion.66 Specific SNAREs are found on endosomes and the plasma membrane that undergo heterotypic binding to dock the vesicle and allow fusion (Fig. 1D). SNAREs have also been implicated in EV release. For example, VAMP7 is a v-SNARE found on the MVB, which is required for MVB fusion with the plasma membrane to release exosomes from cultured human cells.67 In C. elegans adults, the t-SNARE SYX-5 was identified in the alae screen as an exosome regulator.45 In yeast and mammals, syntaxin 5 is better known for mediating vesicle fusion in ER-Golgi trafficking,68 but in C. elegans, SYX-5 also localizes to apical microdomains on the seam cell plasma membrane. SYX-5 is required for the fusion of MVBs with the plasma membrane to release exosomes.45 However, whether VAMP-7 or another v-SNARE on the MVB membrane pairs with SYX-5 is unknown in C. elegans. Different MVB subpopulations may also use distinct SNARE combinations for fusion with the plasma membrane. In summary, similar to other vesicle fusion events, MVBs also use SNARE-mediated fusion to release exosomes.
There is also speculative evidence that SNAREs play a role in microvesicle release as well. In Drosophila wing discs and cultured human cells, the v-SNARE Ykt6 localizes on endosomes and plays a role in the secretion of Hh and Wg/Wnt on EVs.30,32 Hh-carrying EVs have also been shown to be microvesicle sized,31 so SNARE fusion may also be required for the delivery of lipids or proteins to the plasma membrane that are required for microvesicle budding. Drosophila syntaxin 1A is a plasma membrane t-SNARE known for its role in synaptic vesicle fusion.69 Syx1A is also required for the release of EVs from neurons and cultured S2 cells.29 Since Drosophila NMJ neurons also release both microvesicles and exosomes,28,29 it is possible that Syx1A has a role in microvesicle release. However, whether Syx1A and Ykt6 are important for microvesicle biogenesis is unclear. Another interesting untested possibility is that Syx1A and Ykt6 form heterocomplexes to fuse MVBs with the plasma membrane. Syx1A could also interact with VAMP7 or syntaxin 5 could interact with Ykt6 to release exosomes. Thus, the precise roles of SNARE proteins in EV biogenesis require further investigation.
V-ATPase
The original protein discovered to be important for exosome release during alae formation in C. elegans is VHA-5,27 part of the V0 complex of the vacuolar H+-ATPase (V-ATPase). The V-ATPase is an ATP-dependent proton pump conserved in all eukaryotes, consisting of the transmembrane V0-complex and cytoplasmic V1 complex.70 In knockdowns for three V0 subunits (vha-1, vha-4, vha-5), larval alae are not formed.27 Similarly, treating Drosophila imaginal discs ex vivo with bafilomycin, a V-ATPase inhibitor, dramatically reduced Wg secretion in EVs.30 In contrast, subunits of the cytoplasmic V1 complex are not required for alae formation in C. elegans larvae,27 although knocking down one V1 subunit mildly disrupted Wg-dependent wing development in Drosophila.30 The V0 and V1 complexes can dissociate from each other, but a complete V-ATPase containing both V0 and V1 is required for proton pumping, suggesting that the acidifying function of the V-ATPase is not required for exosome secretion. Consistent with this, the C-terminus of the V0 subunit VHA-5 is required for exosome release, while the N-terminus is required for proton pumping and interaction with the V1 complex.27 MVBs cannot fuse with the plasma membrane in VHA-5 depleted worms, resulting in an accumulation of MVBs and reduced exosome release. There is accumulating evidence implicating the V0 complex in endosome tethering or fusion,71 but its exact role in MVB fusion is unclear. Intriguingly, the V0-ATPase has been shown to bind to SNAREs in Drosophila,72 including Syx1A, which is required for EV release from neurons.29 Thus, it will be interesting to test whether SYX-1 or SYX-5 interact with the V0-ATPase in C. elegans seam cells to release exosomes.
RAL-1 and the exocyst
In a candidate screen for trafficking proteins involved in exosome formation, Hyenne et al. used the formation of C. elegans alae to identify 4 genes of the exocyst complex and the small GTPase RAL-1.45 RAL-1 and the exocyst complex are conserved regulators of vesicle tethering and fusion, with RAL-1 typically acting upstream of the exocyst complex.73,74 RAL-1 localizes to MVBs in mammalian cell culture and in C. elegans in vivo.45 Loss of RAL-1 results in fewer MVBs and fewer ILVs, presumably because proteins required for ILV budding are not recruited to the endosome membrane. MVBs are still able to tether to the plasma membrane when RAL-1 is depleted, but MVB fusion to release exosomes is hampered. This observation suggests that MVB fusion factors are also not recruited when RAL-1 is depleted.45 These data demonstrate that RAL-1 acts at multiple steps of exosome biogenesis, specifically ILV formation and MVB fusion (Fig. 1C-D). Thus, studies using the formation of alae in the model organism C. elegans helped to identify new regulators of exosome biogenesis and can be a valuable tool for further studies.
In contrast to RAL-1, the role of the exocyst complex (SEC-3, SEC-5, SEC-8, SEC-10, SEC-15, EXOC-7, EXOC-8) in alae formation still needs to be elucidated. Unlike RAL-1, the exocyst is unlikely to play a role in MVB biogenesis, because the number of ILVs and MVBs was normal in exocyst deletion mutants. In contrast, sec-8 depletion resulted in a higher accumulation of MVBs,45 suggesting the exocyst could have a role in MVB secretion. However, it is unclear why sec-8 knockdown showed increased MVB number while the sec-8 knockout had a normal number of MVBs. Given that both sec-8 RNAi and sec-8 deletion result in alae defects, the role of the exocyst is likely to be independent of MVB formation. Tethering of MVBs also appeared normal in exocyst deletion mutants and there was no evidence for MVB fusion or tethering defects in sec-8 RNAi. In summary, whether the exocyst affects exosome secretion or has another role in alae formation independent of exosomes remains to be determined.
Other proteins of interest
Two signaling pathways are also implicated in EV release that do not belong to known membrane-sculpting or trafficking pathways, including the MAP kinase PMK-1 and the morphogen Bmp. Stress-activated p38 mitogen-activated MAPK (PMK-1) was shown to be required for EV release from the cilia base to the sheath cell lumen.75 Fewer EVs are also released to the environment in pmk-1 mutants. PMK-1 was found to act independently of the innate immune MAPK cascade, making PMK-1 a novel regulator for EV biogenesis that acts through an unknown pathway. The morphogen Bmp is also implicated in EV release through 2 members of its signaling pathway. Overexpression of the Bmp antagonist Dad in Drosophila accessory glands blocks Bmp signaling and inhibits exosome release.42 Overexpressing Dad or the Bmp receptor Thickveins also disrupts MVB content or morphology, respectively. These data implicate the canonical Bmp signaling pathway in MVB biology. Further studies will be required to define how Bmp signaling proteins control exosome release.
Lipids and EV formation
The formation of membrane-wrapped vesicles requires membrane curvature either at the plasma membrane for microvesicle formation, or on the endosomal limiting membranes of MVBs for ILV formation. While many proteins are known to regulate membrane curvature, the lipid composition of the membrane also alters membrane curvature.76,77 MVB fusion with the plasma membrane also requires the regulation of lipids to fuse membranes and release ILVs as exosomes.78 Distinct lipids have different shapes as well as different protein binding partners. Phospholipids like phosphatidylserine (PS), phosphatidylethanolamine (PE), and phosphoinositides (PI) are restricted to the cytoplasmic leaflet of the plasma membrane. Phospholipids like phosphatidylcholine (PC), sphingomyelin (SM), and glycolipids are sequestered to the extracellular face of the plasma membrane, while cholesterol is symmetrically distributed.79 Microvesicles have altered lipid asymmetry, where PS and PE are often externalized and found on the outer surface of microvesicles (Fig. 1B).80,81 This observation suggested a role for lipid asymmetry in microvesicle release. Some exosomes also externalize PS,82,83 so it is possible lipid asymmetry is also regulated on the MVB during ILV formation.
Lipid asymmetry is first established in the ER and Golgi, but is also regulated directly in the plasma membrane.84 Translocating the hydrophilic head of lipids across the hydrophobic core of membrane bilayers is energetically unfavorable and requires the assistance of proteins. Three broad classes of proteins act as lipid translocators to regulate lipid asymmetry: flippases, floppases, and scramblases.85 Flippases translocate phospholipids from the extracellular face of the plasma membrane to the cytoplasmic face. A single family of transmembrane proteins has been identified as phospholipid flippases, the P4-ATPases.86 Floppases perform the backward translocation of phospholipids from the cytoplasmic face to the lumenal or extracellular face of a bilayer.87 Scramblases translocate phospholipids in both directions, destroying membrane asymmetry.85 Several families of transmembrane proteins have been proposed to be the scramblase responsible for externalizing phospholipids, including ABC transporters, SCRM proteins, and the Anoctamin TMEM16F. Provocatively, Scott syndrome patients have problems with platelet coagulation, PS & PE externalization, and EV release due to mutations in TMEM16F.88,89 However, whether TMEM16F is a Ca2+ transporter required for scramblase activity or the scramblase itself is still under debate. Regardless, the observation that Scott patients have defects in both lipid asymmetry and EV release support the idea that phospholipid asymmetry could have a role in EV release. Here we discuss the role of distinct lipid classes in EV formation, especially focusing on phospholipids.
Phosphatidylserine (PS)
The anionic phospholipid PS has a cylindrical shape and is found in all eukaryotic membranes.77,90 PS exposure is used as a marker for processes like apoptosis and platelet activation for coagulation.91,92 PS externalization also plays a role in fertilization, thus disruption of PS asymmetry is associated with both signaling and membrane fusion.93 Flipping PS to the cytoplasmic bilayer induces negative microdomains that can be recognized by trafficking proteins with positively-charged domains.94 In C. elegans, the P4-ATPase responsible for flipping PS in the plasma membrane and endolysosomal membranes is TAT-1, the human homolog of ATP8A1/2.95,96 TAT-1 is required for endocytosis and endolysosomal trafficking. TAT-1 mutants display severe MVB accumulation, suggesting they could have defects in MVB fusion. However, alae formation, which requires exosome release from MVBs, occurs normally in tat-1 mutants.96 Thus, MVB biogenesis and MVB fusion for exosome secretion are not likely to be affected in tat-1 mutants, suggesting a role for TAT-1 instead in lysosome biogenesis. Furthermore, tat-1 mutants have no increase in microvesicle release,15 suggesting that PS exposure does not induce microvesicle budding. However, PS exposure is an important signal for phagocytosis or membrane fusion,93,97 suggesting that PS exposure on EVs could be important for EV uptake or EV signaling, rather than EV formation.
Phosphatidylethanolamine (PE)
Although less studied than PS, the abundant phospholipid PE is also externalized on EVs.81 PE microdomains are thought to physically bend membranes because of their small headgroup and conical shape (Fig. 1).77 PE is exposed on the plasma membrane during cytokinesis and must be flipped back to the cytoplasmic leaflet for the plasma membrane to fuse during abscission.98 Given PE's shape, PE microdomains in the extracellular leaflet would cause budding away from the cytoplasm (Fig. 1B), while PE microdomains in the cytoplasmic leaflet would drive budding toward the cytoplasm.99 In addition to a direct role for externalized PE in driving membrane curvature, there are two hypotheses how PE externalization at the microvesicle bud neck could be sensed by proteins. First, PE microdomains at the extracellular leaflet would result in steric pressure that causes negative membrane curvature.77 Negative membrane curvature is necessary for ESCRT-III assembly at the bud neck.100 Indeed, the ESCRT-III subunit VPS-32 is recruited to the plasma membrane and required for microvesicle release in C. elegans embryos.15 Second, the movement of uncharged PE would result in a higher relative density of anionic lipids like PS and PI, resulting in more negatively charged microdomains at the cytoplasmic face of the plasma membrane. Mammalian Hrs, an ESCRT-0 component, binds anionic lipids like PI3P via its FYVE domain.44 Consistently, Drosophila Hrs and C. elegans HGRS-1 are required for microvesicle release.15,32 PE microdomains have also been linked to viral budding.101 Thus, PE is associated with membrane budding and scission events and is likely to play a role in EV release.
Strong evidence for a role for PE in EV release comes from studies in C. elegans on the P4-ATPase TAT-5,15 the homolog of ATP9A/B in mammals. TAT-5 flips PE from the extracellular face of the plasma membrane to the cytoplasmic face of the plasma membrane in order to maintain PE asymmetry. Loss of TAT-5 leads to PE exposure,15 probably because its activity is required to counteract an as yet unidentified PE scramblase or floppase. Loss of TAT-5 flippase activity results in a dramatic increase in microvesicle budding, shown by electron tomography. In fact, so many microvesicles are released that EVs become visible by light microscopy using fluorescent plasma membrane reporters. How cells regulate the quantity of microvesicle release is unclear, because no regulators of TAT-5 activity are yet reported. Thus, TAT-5 provides good evidence that PE plays a role in microvesicle budding.
A role for PE in exosome formation is unclear. In C. elegans, TAT-5 localizes to an array of endosomes and tat-5 mutant embryos have enlarged LMP-1-positive lysosomes. However, MVBs appear normal in tat-5 mutant embryos and the MVBs do not have an increased number of ILVs.15 There are 6 P4-ATPases in C. elegans, so another could maintain PE asymmetry in the endosomal membrane of MVBs. Also, the EVs observed in wild-type embryos or tat-5 mutants were larger than 90 nm in diameter, suggesting that significant amounts of exosome release may not occur in early C. elegans embryos. Therefore, the examination of another tissue such as seam cells may be necessary to find a role for PE in exosome release. Knocking down the Drosophila homolog of TAT-5 in wing discs results in a decrease in Hh-carrying EVs and reduced Hh signaling.32 However, it is not clear whether this is due to a loss in exosome or microvesicle release since the authors observed Hh in vesicles between 30–200 nm in diameter in wild-type wing discs. Thus, ultrastructural characterization of the Drosophila mutant is required to resolve this discrepancy with the C. elegans mutant. It is also unclear whether TAT-5 plays a role in intracellular vesicle trafficking.15 It is tempting to speculate that flipping PE to the cytoplasmic bilayer would curve membranes toward the cytoplasm and thereby drive endocytosis or tubulation on endosomes. However, given the absence of known PE-binding domains, analyzing the in vivo role of PE in intracellular trafficking remains a challenge.
Phosphatidylinositol (PI)
Phosphoinositides (PI) are a minor lipid species with major roles in intracellular signaling and vesicular trafficking.102 Phosphatidylinositol can be phosphorylated on three different positions, leading to an array of different binding partners.103 Specific kinases and phosphatases alter the phosphorylation of PI species and thereby help mature endosomes.104 The localization of different PI species specifies the identity of organelles, helping to recruit the right proteins to the right organelle at the right time. PI species play important roles in both exosome and microvesicle formation, regulating the recruitment of membrane-sculpting, tethering, and fusion proteins.
In order to form EVs, proteins such as the ESCRT complex depend on PI species to localize to the correct membrane (Fig. 1B). Phosphatidylinositol-3-phosphate (PI3P) localizes on the limiting membrane of the MVB and recruits proteins that promote ILV budding. The FYVE domain of Hrs recognizes PI3P and recruits ESCRT-0 and ESCRT-I to endosomal membranes for ILV formation.105 As discussed before, ESCRT-0 and ESCRT-I proteins are also required for microvesicle budding in C. elegans.15 Which PI species recruits ESCRT proteins to the plasma membrane in C. elegans is unclear, but in mammalian cells Nabhan et al. showed that the arrestin domain-containing protein ARRDC1 is required to recruit TSG101 (ESCRT-I) to the plasma membrane for microvesicle release.47 How the arrestin domain recruits ARRDC1 to the plasma membrane is also unclear, but arrestins have been shown to bind plasma membrane-localized PIs like PIP3.106 Thus, it is likely that PI species recruit ESCRT proteins to both endosomes and the plasma membrane. Interestingly, PIP3 also recruits Syx1A to the synapse in the Drosophila NMJ,107 but whether PI species recruit other proteins that lead to EV formation remains to be tested.
To date, there are no reports of PI externalization on EVs or the plasma membrane,108 but several observations from yeast could suggest a role for PI asymmetry in EV formation. The yeast homolog of the PE flippase TAT-5, called Neo1p, was originally identified due to neomycin sensitivity.109 Neomycin is an aminoglycoside antibiotic that can bind PI(4,5)P2.110 Yeast neo1 mutants are also sensitive to the PE-binding lantibiotic duramycin,111 demonstrating that PE is externalized in neo1 mutants. The increased neomycin sensitivity of yeast neo1 mutants could therefore suggest that PI(4,5)P2 is externalized in neo1 mutants. This would imply that Neo1p (as well as its homologs in other species) could also flip PI species. By electron microscopy, temperature-sensitive neo1 mutants accumulate unusual elongated tubules on endosomes in addition to increased MVBs.112 The increased MVB phenotype is reminiscent of the increased microvesicle phenotype of tat-5 mutants in C. elegans.15 Since Neo1p localizes to endosomes and TAT-5 to the plasma membrane,15,112 both flippases could flip PI and PE to prevent their respective membranes from budding away from the cytoplasm. In support of this idea, decreased PI levels suppress the abnormal vesicle phenotypes in yeast flippase mutants, including neo1 mutants.113 Thus, Neo1p could flip PE and PI to the cytofacial layer on endosomal membranes that later fuse with the plasma membrane to establish PE and PI asymmetry there. Therefore, it is important to determine whether PI externalization actually occurs and whether PI externalization is required for EV formation.
Other lipids
Not only phospholipids have been implicated in EV biogenesis pathways.5,46,114 For example, the sphingolipid ceramide can induce membrane invagination on MVBs and is required for ILV budding independent of the ESCRT complex. Thus, the enzyme neutral sphingomyelinase nSMase that hydrolyzes sphingomyelin into ceramide is required for ILV formation.115 Knocking down αSMase in Drosophila wing discs decreased EV release and Hh signaling in vivo,32 suggesting that ceramide plays a role in exosome or microvesicle budding. In contrast, in cultured Drosophila S2 cells, knockdown of nSMase did not disrupt EV biogenesis.29 Together, these data suggest that different cells may also use ceramide-dependent pathways to release exosomes and microvesicles.
Conclusion
From studies in genetic model organism, we have gained our first insights into the in vivo functions of EVs. In Drosophila and C. elegans, EVs carry lipidated morphogens important for the patterning of tissues ranging from epithelia to neurons (Fig. 2B, D-E). In both worms and flies, adult males release EVs important for inducing mating-specific behaviors in other males or females (Fig. 2C, F). Thus, EVs have conserved roles during development as well as in adult behavior. In addition to their role in signaling, EVs have other notable functions. For example, microvesicles are thought to sculpt membrane during development, such as removing extra membrane from the intercellular bridge during cytokinesis.53 EVs can also serve as a membrane repair mechanism. ESCRT-dependent membrane budding seals holes in the plasma membrane by releasing EVs after injury.116 Interestingly, in C. elegans embryos with reduced TAT-5 levels, microvesicles first appear after fertilization.15 Fertilization is a time of massive endocytosis and exocytosis,117 potentially damaging the plasma membrane and thereby inducing microvesicle budding. EVs can also be a way to deal with toxic compounds. For example, bacteria release outer membrane vesicles containing misfolded or overexpressed proteins.3 Heat-shock proteins are also commonly found in animal EVs, including Drosophila imaginal disc microvesicles.31 Additionally, polar bodies are large EVs released by oocytes that contain extra copies of maternal DNA that would be toxic to the developing embryo.118 Thus, understanding EV biology is likely to impact not only signal transduction cascades, but also a broad range of physiological functions.
Studies on the functions of purified EVs are likely complicated by the diversity of EVs produced by cells. Most purification strategies focus on EVs smaller than 100 nm in diameter. However, in vivo studies in C. elegans and Drosophila have attributed functions to EVs from 30–500 nm in diameter. Studies on Drosophila morphogens have also shown that the same cells release the same signaling molecules on both exosomes and microvesicles.28,29,31,32 These data suggest that focusing on exosome-sized vesicles may not reveal the diverse roles of EVs in vivo. In addition, some cells predominantly release microvesicle-sized vesicles, like C. elegans early embryos. Thus, it is important to characterize a cell type's native EV complement before designing appropriate EV purification methods to analyze the protein, lipid, or nucleic acid content of EVs.
The studies described here give us exciting glimpses into the signaling roles of EVs, but our limited understanding of the mechanisms of EV release restricts our studies of EV function. Work in C. elegans, Drosophila, and mammalian cells has identified a number of interesting starting points for determining the conserved mechanisms of EV release, but the field is still in its early days. For example, the role of small GTPases (Rab and Ral) provide entry points to define the effector proteins specific for MVB transport, docking, tethering, and fusion. Notably however, many of the proteins originally identified for their role in exosome biogenesis are also required for microvesicle budding. This finding suggests that cells are likely to regulate both secretion pathways simultaneously. In many cases, a role for a protein in microvesicle budding has not been tested, so it is unclear which mechanisms are actually specific for exosome biogenesis. Similarly, the role of lipids in exosome biogenesis is insufficiently studied. To date, studies have used candidate screens based on known membrane sculpting proteins or vesicle trafficking regulators to identify proteins regulating EV release. However, the proteins that will allow us to cleanly dissect EV functions are proteins that do not act in other endosomal trafficking pathways.
In the future, model organisms are likely to contribute significantly to identifying more proteins involved in EV release. The establishment of genetic models for EV release allows unbiased genetic screens to identify novel genes in the future. This is especially promising in tissues where one type of EV release is predominant, like exosomes in C. elegans seam cells and Drosophila secretory glands or like microvesicles in C. elegans early embryos. Studies on the functional roles of EVs have also revealed that proteins involved in morphogen secretion as well as ciliary proteins are interesting candidates for playing a role in EV release. Consequently, identifying new regulators involved in EV release will help us determine their functions and new functions will help us identify new regulators. These studies in genetic model organisms will also identify new EV biomarkers for use in studies from the laboratory to the clinic.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Tamas Matusek for his suggestions on the manuscript.
References
- [1].Tannetta D, Dragovic R, Alyahyaei Z, Southcombe J. Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell Mol Immunol 2014; 11:548-63; PMID:24954226; http://dx.doi.org/ 10.1038/cmi.2014.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun 2012; 80:1948-57; PMID:22409932; http://dx.doi.org/ 10.1128/IAI.06014-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 2015; 13:605-19; PMID:26373371; http://dx.doi.org/ 10.1038/nrmicro3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 2016; 17:160-72; PMID:26891626; http://dx.doi.org/ 10.1038/nrn.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30:255-89; PMID:25288114; http://dx.doi.org/ 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- [6].Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 2015; 25:364-72; PMID:25683921; http://dx.doi.org/ 10.1016/j.tcb.2015.01.004 [DOI] [PubMed] [Google Scholar]
- [7].McGough IJ, Vincent JP. Exosomes in developmental signalling. Dev 2016; 143:2482-93; PMID:27436038; http://dx.doi.org/ 10.1242/dev.126516 [DOI] [PubMed] [Google Scholar]
- [8].Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14:195-208; PMID:24566916; http://dx.doi.org/ 10.1038/nri3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Desrochers LM, Antonyak MA, Cerione RA. Extracellular vesicles: Satellites of information transfer in cancer and stem cell biology. Dev Cell 2016; 37:301-9; PMID:27219060; http://dx.doi.org/ 10.1016/j.devcel.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Iraci N, Leonardi T, Gessler F, Vega B, Pluchino S. Focus on extracellular vesicles: Physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci 2016; 17:171; PMID:26861302; http://dx.doi.org/ 10.3390/ijms17020171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Altan-Bonnet N. Extracellular vesicles are the Trojan horses of viral infection. Curr Opin Microbiol 2016; 32:77-81; PMID:27232382; http://dx.doi.org/ 10.1016/j.mib.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gong J, Jaiswal R, Dalla P, Luk F, Bebawy M. Microparticles in cancer: A review of recent developments and the potential for clinical application. Semin Cell Dev Biol 2015; 40:35-40; PMID:25843775; http://dx.doi.org/ 10.1016/j.semcdb.2015.03.009 [DOI] [PubMed] [Google Scholar]
- [13].Torrano V, Royo F, Peinado H, Loizaga-Iriarte A, Unda M, Falcón-Pérez JM, Carracedo A. Vesicle-MaNiA: extracellular vesicles in liquid biopsy and cancer. Curr Opin Pharmacol 2016; 29:47-53; PMID:27366992; http://dx.doi.org/ 10.1016/j.coph.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].De Palma G, Sallustio F, Schena FP. Clinical application of human urinary extracellular vesicles in kidney and urologic diseases. Int J Mol Sci 2016; 17:1043; PMID:27376269; http://dx.doi.org/ 10.3390/ijms17071043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wehman AM, Poggioli C, Schweinsberg P, Grant BD, Nance J. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr Biol 2011; 21:1951-9; PMID:22100064; http://dx.doi.org/ 10.1016/j.cub.2011.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol 2013; 14:141-52; PMID:23429793; http://dx.doi.org/ 10.1038/nrm3531 [DOI] [PubMed] [Google Scholar]
- [17].Dionne LK, Wang X-J, Prekeris R. Midbody: from cellular junk to regulator of cell polarity and cell fate. Curr Opin Cell Biol 2015; 35:51-8; PMID:25950842; http://dx.doi.org/ 10.1016/j.ceb.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kalra H, Drummen GPC, Mathivanan S. Focus on extracellular vesicles: Introducing the next small big thing. Int J Mol Sci 2016; 17:170; PMID:26861301; http://dx.doi.org/ 10.3390/ijms17020170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, et al.. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 2012; 10:e1001450; PMID:23271954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al.. Tumour exosome integrins determine organotropic metastasis. Nature 2015; 527:329-35; PMID:26524530; http://dx.doi.org/ 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Desrochers LM, Bordeleau F, Reinhart-King CA, Cerione RA, Antonyak MA. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat Commun 2016; 7:11958; PMID:27302045; http://dx.doi.org/ 10.1038/ncomms11958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287:795-801; PMID:6776413; http://dx.doi.org/ 10.1038/287795a0 [DOI] [PubMed] [Google Scholar]
- [23].Wang X, Yang C. Programmed cell death and clearance of cell corpses in Caenorhabditis elegans. Cell Mol Life Sci 2016; 73:2221-36; PMID:27048817; http://dx.doi.org/ 10.1007/s00018-016-2196-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Panáková D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 2005; 435:58-65; PMID:Can't; http://dx.doi.org/ 10.1038/nature03504 [DOI] [PubMed] [Google Scholar]
- [25].Zugasti O, Rajan J, Kuwabara PE. The function and expansion of the Patched- and Hedgehog-related homologs in C. elegans. Genome Res 2005; 15:1402-10; PMID:16204193; http://dx.doi.org/ 10.1101/gr.3935405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kolotuev I, Apaydin A, Labouesse M. Secretion of Hedgehog-related peptides and WNT during Caenorhabditis elegans development. Traffic 2009; 10:803-10; PMID:19210682; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00871.x [DOI] [PubMed] [Google Scholar]
- [27].Liégeois S, Benedetto A, Garnier J-M, Schwab Y, Labouesse M. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. JCB 2006; 173:949-61; PMID:16785323; http://dx.doi.org/ 10.1083/jcb.200511072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 2009; 139:393-404; PMID:19837038; http://dx.doi.org/ 10.1016/j.cell.2009.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. JBC 2012; 287:16820-34; PMID:22437826; http://dx.doi.org/ 10.1074/jbc.M112.342667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol 2012; 14:1036-45; PMID:22983114; http://dx.doi.org/ 10.1038/ncb2574 [DOI] [PubMed] [Google Scholar]
- [31].Matusek T, Wendler F, Polès S, Pizette S, D'Angelo G, Fürthauer M, Thérond PP. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature 2014; 516:99-103; PMID:25471885; http://dx.doi.org/ 10.1038/nature13847 [DOI] [PubMed] [Google Scholar]
- [32].Gradilla A-C, González E, Seijo I, Andrés G, Bischoff M, González-Mendez L, Sánchez V, Callejo A, Ibáñez C, Guerra M, et al.. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun 2014; 5:5649; PMID:25472772; http://dx.doi.org/ 10.1038/ncomms6649 [DOI] [PubMed] [Google Scholar]
- [33].Cadigan KM. Regulating morphogen gradients in the Drosophila wing. Semin Cell Dev Biol 2002; 13:83-90; PMID:12127140; http://dx.doi.org/ 10.1016/S1084-9521(02)00014-9 [DOI] [PubMed] [Google Scholar]
- [34].Ramírez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 1999; 97:599-607; PMID:Can't; http://dx.doi.org/ 10.1016/S0092-8674(00)80771-0 [DOI] [PubMed] [Google Scholar]
- [35].Danilchik M, Williams M, Brown E. Blastocoel-spanning filopodia in cleavage-stage Xenopus laevis: Potential roles in morphogen distribution and detection. Dev Biol 2013; 382:70-81; PMID:23916849; http://dx.doi.org/ 10.1016/j.ydbio.2013.07.024 [DOI] [PubMed] [Google Scholar]
- [36].Wood CR, Rosenbaum JL. Ciliary ectosomes: transmissions from the cell's antenna. Trends Cell Biol 2015; 25:276-85; PMID:25618328; http://dx.doi.org/ 10.1016/j.tcb.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cao M, Ning J, Hernandez-Lara CI, Belzile O, Wang Q, Dutcher SK, Liu Y, Snell WJ. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. Elife 2015; 4:183; PMID:25688564; http://dx.doi.org/ 10.7554/eLife.05242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang J, Silva M, Haas LA, Morsci NS, Nguyen KCQ, Hall DH, Barr MM. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol 2014; 24:519-25; PMID:24530063; http://dx.doi.org/ 10.1016/j.cub.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maguire JE, Silva M, Nguyen KCQ, Hellen E, Kern AD, Hall DH, Barr MM. Myristoylated CIL-7 regulates ciliary extracellular vesicle biogenesis. Mol Biol Cell 2015; 26:2823-32; PMID:26041936; http://dx.doi.org/ 10.1091/mbc.E15-01-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 1999; 401:386-9; PMID:10517638 [DOI] [PubMed] [Google Scholar]
- [41].Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol 2001; 11:1341-6; PMID:11553327; http://dx.doi.org/ 10.1016/S0960-9822(01)00423-7 [DOI] [PubMed] [Google Scholar]
- [42].Corrigan L, Redhai S, Leiblich A, Fan S-J, Perera SMW, Patel R, Gandy C, Wainwright SM, Morris JF, Hamdy F, et al.. BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior. JCB 2014; 206:671-88; PMID:25154396; http://dx.doi.org/ 10.1083/jcb.201401072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hurley JH. ESCRTs are everywhere. EMBO J 2015; 34:2398-407; PMID:26311197; http://dx.doi.org/ 10.15252/embj.201592484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. CSH Perspect Biol 2013; 5(9); pii: a016766; PMID:24003212; http://dx.doi.org/26459596 10.1101/cshperspect.a016766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, Tak S, Lefebvre O, Schwab Y, Goetz JG, et al.. RAL-1 controls multivesicular body biogenesis and exosome secretion. JCB 2015; 211:27-37; PMID:26459596; http://dx.doi.org/ 10.1083/jcb.201504136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Abels ER, Breakefield XO. Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 2016; 36:301-12; PMID:27053351; http://dx.doi.org/ 10.1007/s10571-016-0366-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. PNAS 2012; 109:4146-51; PMID:22315426; http://dx.doi.org/ 10.1073/pnas.1200448109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gradilla A-C, Guerrero I. Hedgehog on the move: a precise spatial control of Hedgehog dispersion shapes the gradient. Curr Opin Genet Dev 2013; 23:363-73; PMID:23747033; http://dx.doi.org/ 10.1016/j.gde.2013.04.011 [DOI] [PubMed] [Google Scholar]
- [49].Beckett K, Monier S, Palmer L, Alexandre C, Green H, Bonneil E, Raposo G, Thibault P, Le Borgne R, Vincent JP. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic 2013; 14:82-96; PMID:23035643; http://dx.doi.org/ 10.1111/tra.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Utley TJ, Ducharme NA, Varthakavi V, Shepherd BE, Santangelo PJ, Lindquist ME, Goldenring JR, Crowe JE. Respiratory syncytial virus uses a Vps4-independent budding mechanism controlled by Rab11-FIP2. PNAS 2008; 105:10209-14; PMID:18621683; http://dx.doi.org/ 10.1073/pnas.0712144105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Campsteijn C, Vietri M, Stenmark H. Novel ESCRT functions in cell biology: spiraling out of control? Curr Opin Cell Biol 2016; 41:1-8; PMID:27031044; http://dx.doi.org/ 10.1016/j.ceb.2016.03.008 [DOI] [PubMed] [Google Scholar]
- [52].Crowell EF, Gaffuri A-L, Gayraud-Morel B, Tajbakhsh S, Echard A. Engulfment of the midbody remnant after cytokinesis in mammalian cells. JCS 2014; 127:3840-51; PMID:25002399; http://dx.doi.org/ 10.1242/jcs.154732 [DOI] [PubMed] [Google Scholar]
- [53].Sherman S, Kirchenbuechler D, Nachmias D, Tamir A, Werner S, Elbaum M, Elia N. Resolving new ultrastructural features of cytokinetic abscission with soft-X-ray cryo-tomography. Sci Rep 2016; 6:27629; PMID:27282220; http://dx.doi.org/ 10.1038/srep27629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; http://dx.doi.org/ 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- [55].Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al.. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12:19-30–suppp1–13; PMID:19966785; http://dx.doi.org/ 10.1038/ncb2000 [DOI] [PubMed] [Google Scholar]
- [56].Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol 2007; 19:394-401; PMID:17628466; http://dx.doi.org/ 10.1016/j.ceb.2007.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gerber PP, Cabrini M, Jancic C, Paoletti L, Banchio C, Bilderling C, Sigaut L, Pietrasanta LI, Duette G, Freed EO, et al.. Rab27a controls HIV-1 assembly by regulating plasma membrane levels of phosphatidylinositol 4,5-bisphosphate. JCB 2015; 209:435-52; PMID:25940347; http://dx.doi.org/ 10.1083/jcb.201409082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang M, Chen L, Wang S, Wang T. Rab7: roles in membrane trafficking and disease. Biosci Rep 2009; 29:193-209; PMID:19392663; http://dx.doi.org/ 10.1042/BSR20090032 [DOI] [PubMed] [Google Scholar]
- [59].Jaé N, McEwan DG, Manavski Y, Boon RA, Dimmeler S. Rab7a and Rab27b control secretion of endothelial microRNA through extracellular vesicles. FEBS Lett 2015; 589:3182-8; PMID:Can't; http://dx.doi.org/ 10.1016/j.febslet.2015.08.040 [DOI] [PubMed] [Google Scholar]
- [60].Sato M, Grant BD, Harada A, Sato K. Rab11 is required for synchronous secretion of chondroitin proteoglycans after fertilization in Caenorhabditis elegans. JCS 2008; 121:3177-86; PMID:18765566; http://dx.doi.org/ 10.1242/jcs.034678 [DOI] [PubMed] [Google Scholar]
- [61].Takahashi S, Kubo K, Waguri S, Yabashi A, Shin H-W, Katoh Y, Nakayama K. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. JCS 2012; 125:4049-57; PMID:22685325; http://dx.doi.org/ 10.1242/jcs.102913 [DOI] [PubMed] [Google Scholar]
- [62].Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. JCS 2002; 115:2505-15; PMID:12045221 [DOI] [PubMed] [Google Scholar]
- [63].Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 2005; 6:131-43; PMID:15634213; http://dx.doi.org/ 10.1111/j.1600-0854.2004.00257.x [DOI] [PubMed] [Google Scholar]
- [64].Klinkert K, Echard A. Rab35 GTPase: A Central Regulator of Phosphoinositides and F-actin in Endocytic Recycling and Beyond. Traffic 2016; 17(10):1063-77; PMID:27329675; http://dx.doi.org/20404108 10.1111/tra.12422 [DOI] [PubMed] [Google Scholar]
- [65].Hsu C, Morohashi Y, Yoshimura S-I, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, et al.. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. JCB 2010; 189:223-32; PMID:20404108; http://dx.doi.org/ 10.1083/jcb.200911018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bombardier JP, Munson M. Three steps forward, two steps back: mechanistic insights into the assembly and disassembly of the SNARE complex. Curr Opin Chem Biol 2015; 29:66-71; PMID:26498108; http://dx.doi.org/ 10.1016/j.cbpa.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fader CM, Sánchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta 2009; 1793:1901-16; PMID:19781582; http://dx.doi.org/ 10.1016/j.bbamcr.2009.09.011 [DOI] [PubMed] [Google Scholar]
- [68].Jahn R, Lang T, Südhof TC. Membrane fusion. Cell 2003; 112:519-33; PMID:12600315; http://dx.doi.org/ 10.1016/S0092-8674(03)00112-0 [DOI] [PubMed] [Google Scholar]
- [69].Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell 1995; 80:311-20; PMID:7834751; http://dx.doi.org/ 10.1016/0092-8674(95)90414-X [DOI] [PubMed] [Google Scholar]
- [70].Marshansky V, Rubinstein JL, Grüber G. Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta 2014; 1837:857-79; PMID:24508215; http://dx.doi.org/ 10.1016/j.bbabio.2014.01.018 [DOI] [PubMed] [Google Scholar]
- [71].Maxson ME, Grinstein S. The vacuolar-type H+-ATPase at a glance - more than a proton pump. JCS 2014; 127:4987-93; PMID:25453113; http://dx.doi.org/ 10.1242/jcs.158550 [DOI] [PubMed] [Google Scholar]
- [72].Wang D, Epstein D, Khalaf O, Srinivasan S, Williamson WR, Fayyazuddin A, Quiocho FA, Hiesinger PR. Ca2+-Calmodulin regulates SNARE assembly and spontaneous neurotransmitter release via v-ATPase subunit V0a1. JCB 2014; 205:21-31; PMID:24733584; http://dx.doi.org/ 10.1083/jcb.201312109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wu B, Guo W. The exocyst at a glance. JCS 2015; 128:2957-64; PMID:26240175; http://dx.doi.org/ 10.1242/jcs.156398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Armenti ST, Chan E, Nance J. Polarized exocyst-mediated vesicle fusion directs intracellular lumenogenesis within the C. elegans excretory cell. Dev Biol 2014; 394:110-21; PMID:25102190; http://dx.doi.org/ 10.1016/j.ydbio.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang J, Kaletsky R, Silva M, Williams A, Haas LA, Androwski RJ, Landis JN, Patrick C, Rashid A, Santiago-Martinez D, et al.. Cell-specific transcriptional profiling of ciliated sensory neurons reveals regulators of behavior and extracellular vesicle biogenesis. Curr Biol 2015; 25:3232-8; PMID:26687621; http://dx.doi.org/ 10.1016/j.cub.2015.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Graham TR, Kozlov MM. Interplay of proteins and lipids in generating membrane curvature. Curr Opin Cell Biol 2010; 22:430-6; PMID:20605711; http://dx.doi.org/ 10.1016/j.ceb.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].McMahon HT, Boucrot E. Membrane curvature at a glance. JCS 2015; 128:1065-70; PMID:25774051; http://dx.doi.org/ 10.1242/jcs.114454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem 2003; 72:175-207; PMID:14527322; http://dx.doi.org/ 10.1146/annurev.biochem.72.121801.161504 [DOI] [PubMed] [Google Scholar]
- [79].Murate M, Kobayashi T. Revisiting transbilayer distribution of lipids in the plasma membrane. Chem Phys Lipids 2016; 194:58-71; PMID:26319805; http://dx.doi.org/ 10.1016/j.chemphyslip.2015.08.009 [DOI] [PubMed] [Google Scholar]
- [80].Bassé F, Gaffet P, Rendu F, Bienvenue A. Translocation of spin-labeled phospholipids through plasma membrane during thrombin- and ionophore A23187-induced platelet activation. Biochemistry 1993; 32:2337-44; PMID:Can't; http://dx.doi.org/ 10.1021/bi00060a027 [DOI] [PubMed] [Google Scholar]
- [81].Larson MC, Woodliff JE, Hillery CA, Kearl TJ, Zhao M. Phosphatidylethanolamine is externalized at the surface of microparticles. Biochim Biophys Acta 2012; 1821:1501-7; PMID:22960380; http://dx.doi.org/ 10.1016/j.bbalip.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999; 94:3791-9; PMID:10572093 [PubMed] [Google Scholar]
- [83].Arraud N, Linares R, Tan S, Gounou C, Pasquet J-M, Mornet S, Brisson AR. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost 2014; 12:614-27; PMID:24618123; http://dx.doi.org/ 10.1111/jth.12554 [DOI] [PubMed] [Google Scholar]
- [84].van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008; 9:112-24; PMID:18216768; http://dx.doi.org/ 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Montigny C, Lyons J, Champeil P, Nissen P, Lenoir G. On the molecular mechanism of flippase- and scramblase-mediated phospholipid transport. Biochim Biophys Acta 2016; 1861:767-83; PMID:26747647; http://dx.doi.org/ 10.1016/j.bbalip.2015.12.020 [DOI] [PubMed] [Google Scholar]
- [86].Andersen JP, Vestergaard AL, Mikkelsen SA, Mogensen LS, Chalat M, Molday RS. P4-ATPases as Phospholipid Flippases-Structure, Function, and Enigmas. Front Physiol 2016; 7:275; PMID:27458383; http://dx.doi.org/ 10.3389/fphys.2016.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hankins HM, Baldridge RD, Xu P, Graham TR. Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic 2015; 16:35-47; PMID:25284293; http://dx.doi.org/ 10.1111/tra.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 2010; 468:834-8; PMID:21107324; http://dx.doi.org/ 10.1038/nature09583 [DOI] [PubMed] [Google Scholar]
- [89].Castoldi E, Collins PW, Williamson PL, Bevers EM. Compound heterozygosity for 2 novel TMEM16F mutations in a patient with Scott syndrome. Blood 2011; 117:4399-400; PMID:21511967; http://dx.doi.org/ 10.1182/blood-2011-01-332502 [DOI] [PubMed] [Google Scholar]
- [90].Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annual review of biophysics 2010; 39:407-27; PMID:20192774; http://dx.doi.org/ 10.1146/annurev.biophys.093008.131234 [DOI] [PubMed] [Google Scholar]
- [91].Williamson P, Schlegel RA. Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim Biophys Acta 2002; 1585:53-63; PMID:12531537; http://dx.doi.org/ 10.1016/S1388-1981(02)00324-4 [DOI] [PubMed] [Google Scholar]
- [92].Kay JG, Grinstein S. Phosphatidylserine-mediated cellular signaling. Adv Exp Med Biol 2013; 991:177-93; PMID:23775696; http://dx.doi.org/ 10.1007/978-94-007-6331-9_10 [DOI] [PubMed] [Google Scholar]
- [93].Zhou X, Platt JL. Molecular and cellular mechanisms of mammalian cell fusion. Adv Exp Med Biol 2011; 713:33-64; PMID:21432013; http://dx.doi.org/ 10.1007/978-94-007-0763-4_4 [DOI] [PubMed] [Google Scholar]
- [94].Xu P, Baldridge RD, Chi RJ, Burd CG, Graham TR. Phosphatidylserine flipping enhances membrane curvature and negative charge required for vesicular transport. JCB 2013; 202:875-86; PMID:24019533; http://dx.doi.org/ 10.1083/jcb.201305094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Darland-Ransom M, Wang X, Sun C-L, Mapes J, Gengyo-Ando K, Mitani S, Xue D. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science 2008; 320:528-31; PMID:18436785; http://dx.doi.org/ 10.1126/science.1155847 [DOI] [PubMed] [Google Scholar]
- [96].Ruaud A-F, Nilsson L, Richard F, Larsen MK, Bessereau J-L, Tuck S. The C. elegans P4-ATPase TAT-1 regulates lysosome biogenesis and endocytosis. Traffic 2009; 10:88-100; PMID:18939953; http://dx.doi.org/ 10.1111/j.1600-0854.2008.00844.x [DOI] [PubMed] [Google Scholar]
- [97].Li Z, Venegas V, Nagaoka Y, Morino E, Raghavan P, Audhya A, Nakanishi Y, Zhou Z. Necrotic cells actively attract phagocytes through the collaborative action of two distinct ps-exposure mechanisms. PLoS Genet 2015; 11:e1005285; PMID:26061275; http://dx.doi.org/ 10.1371/journal.pgen.1005285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, Umeda M. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. PNAS 1996; 93:12867-72; PMID:8917511; http://dx.doi.org/ 10.1073/pnas.93.23.12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Morris RJ. Ionic control of the metastable inner leaflet of the plasma membrane: Fusions natural and artefactual. FEBS Lett 2010; 584:1665-9; PMID:19913542; http://dx.doi.org/ 10.1016/j.febslet.2009.11.017 [DOI] [PubMed] [Google Scholar]
- [100].Lee I-H, Kai H, Carlson L-A, Groves JT, Hurley JH. Negative membrane curvature catalyzes nucleation of endosomal sorting complex required for transport (ESCRT)-III assembly. PNAS 2015; 112:15892-7; PMID:26668364; http://dx.doi.org/ 10.1073/pnas.1518765113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Booth AM, Fang Y, Fallon JK, Yang J-M, Hildreth JEK, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. JCB 2006; 172:923-35; PMID:16533950; http://dx.doi.org/ 10.1083/jcb.200508014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Posor Y, Eichhorn-Grünig M, Haucke V. Phosphoinositides in endocytosis. Biochim Biophys Acta 2015; 1851:794-804; PMID:25264171; http://dx.doi.org/ 10.1016/j.bbalip.2014.09.014 [DOI] [PubMed] [Google Scholar]
- [103].Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 2008; 9:99-111; PMID:18216767; http://dx.doi.org/ 10.1038/nrm2328 [DOI] [PubMed] [Google Scholar]
- [104].Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev 2013; 93:1019-137; PMID:23899561; http://dx.doi.org/ 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat Rev Mol Cell Biol 2010; 11:556-66; PMID:20588296; http://dx.doi.org/ 10.1038/nrm2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J 1999; 18:871-81; PMID:10022830; http://dx.doi.org/ 10.1093/emboj/18.4.871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Khuong TM, Habets RLP, Kuenen S, Witkowska A, Kasprowicz J, Swerts J, Jahn R, van den Bogaart G, Verstreken P. Synaptic PI(3,4,5)P3 is required for Syntaxin1A clustering and neurotransmitter release. Neuron 2013; 77:1097-108; PMID:23522045; http://dx.doi.org/ 10.1016/j.neuron.2013.01.025 [DOI] [PubMed] [Google Scholar]
- [108].Murate M, Abe M, Kasahara K, Iwabuchi K, Umeda M, Kobayashi T. Transbilayer distribution of lipids at nano scale. JCS 2015; 128:1627-38; PMID:25673880; http://dx.doi.org/ 10.1242/jcs.163105 [DOI] [PubMed] [Google Scholar]
- [109].Prezant TR, Chaltraw WE, Fischel-Ghodsian N. Identification of an overexpressed yeast gene which prevents aminoglycoside toxicity. Microbiology (Reading, Engl) 1996; 142(Pt 12):3407-14; PMID:9004503; http://dx.doi.org/ 10.1099/13500872-142-12-3407 [DOI] [PubMed] [Google Scholar]
- [110].Arbuzova A, Martushova K, Hangyás-Mihályné G, Morris AJ, Ozaki S, Prestwich GD, McLaughlin S. Fluorescently labeled neomycin as a probe of phosphatidylinositol-4, 5-bisphosphate in membranes. Biochim Biophys Acta 2000; 1464:35-48; PMID:10704918; http://dx.doi.org/ 10.1016/S0005-2736(99)00243-6 [DOI] [PubMed] [Google Scholar]
- [111].Takar M, Wu Y, Graham TR. The essential neo1 protein from budding yeast plays a role in establishing aminophospholipid asymmetry of the plasma membrane. JBC 2016; 291:15727-39; PMID:27235400; http://dx.doi.org/ 10.1074/jbc.M115.686253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wicky S, Schwarz H, Singer-Krüger B. Molecular interactions of yeast Neo1p, an essential member of the Drs2 family of aminophospholipid translocases, and its role in membrane trafficking within the endomembrane system. Mol Cell Biol 2004; 24:7402-18; PMID:15314152; http://dx.doi.org/ 10.1128/MCB.24.17.7402-7418.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yamagami K, Yamamoto T, Sakai S, Mioka T, Sano T, Igarashi Y, Tanaka K. Inositol depletion restores vesicle transport in yeast phospholipid flippase mutants. PLoS One 2015; 10:e0120108; PMID:25781026; http://dx.doi.org/ 10.1371/journal.pone.0120108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 2014; 29:116-25; PMID:24959705; http://dx.doi.org/ 10.1016/j.ceb.2014.05.004 [DOI] [PubMed] [Google Scholar]
- [115].Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319:1244-7; PMID:18309083; http://dx.doi.org/ 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- [116].Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F. ESCRT machinery is required for plasma membrane repair. Science 2014; 343:1247136; PMID:24482116; http://dx.doi.org/ 10.1126/science.1247136 [DOI] [PubMed] [Google Scholar]
- [117].Sato K, Sato M, Audhya A, Oegema K, Schweinsberg PJ, Grant BD. Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol Biol Cell 2006; 17:3085-94; PMID:16672374; http://dx.doi.org/ 10.1091/mbc.E06-03-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Maddox AS, Azoury J, Dumont J. Polar body cytokinesis. Cytoskeleton (Hoboken) 2012; 69:855-68; PMID:22927361; http://dx.doi.org/ 10.1002/cm.21064 [DOI] [PubMed] [Google Scholar]