Abstract
Background:
We conducted a randomized trial to evaluate the efficacy and safety of dexmedetomidine for prophylactic analgesia and sedation in patients with delayed extubation after craniotomy.
Methods:
From June 2012 to July 2014, 150 patients with delayed extubation after craniotomy were randomized 1:1 and were assigned to the dexmedetomidine group that received a continuous infusion of 0.6 μg/kg/h (10 μg/mL) or the control group that received a maintenance infusion of 0.9% sodium chloride for injection. The mean percentage of time under optimal sedation (SAS3-4), the percentage of patients who required rescue with propofol/fentanyl, and the total dose of propofol/fentanyl required throughout the course of drug infusion, as well as VAS, HR, MAP, and SpO2 were recorded.
Results:
The percentage of time under optimal sedation was significantly higher in the dexmedetomidine group than in the control group (98.4%±6.7% vs. 93.0%±16.2%, P=0.008). The VAS was significantly lower in the dexmedetomidine group than in the control group (1.0 vs. 4.0, P=0.000). The HR and mean BP were significantly lower in the dexmedetomidine group than in the control group at all 3 time points (before endotracheal suctioning, immediately after extubation, and 30 min after extubation). No significant difference in SpO2 was observed between the 2 groups. For hemodynamic adverse events, patients in the dexmedetomidine group were more likely to develop bradycardia (5.3% vs. 0%, P=0.043) but had a lower likelihood of tachycardia (2.7% vs. 18.7%, P=0.002).
Conclusions:
Dexmedetomidine may be an effective prophylactic agent to induce sedation and analgesia in patients with delayed extubation after craniotomy. The use of dexmedetomidine (0.6 μg/kg/h) infusion does not produce respiratory depression, but may increase the incidence of bradycardia.
Key Words: dexmedetomidine, analgesia, sedation, ICU, craniotomy, stress, delayed extubation, efficacy, safety
Acute pain and emergence agitation are common in patients after craniotomy during the early postoperative period, particularly in those patients with delayed extubation.1–5 Undertreated pain and agitation in patients can result in serious consequences after intracranial operations.6 Physiological changes during pain and agitation may cause intracranial hemorrhage and brain edema. Elevated oxygen consumption may disturb the balance of the supply and demand of brain oxygen, ultimately resulting in ischemia.7,8 However, several surveys revealed that physicians were reluctant to use opioids and sedatives in the early postoperative period after craniotomy.9–12 The major concern was the side effects of these drugs, which primarily included the influence of consciousness and respiratory depression.1,2
Dexmedetomidine, a potent and highly selective α-2-adrenoceptor agonist, provides dose-dependent sedation, anxiolysis, and analgesia without respiratory depression.13 In addition to its sedative effects, dexmedetomidine has significant analgesic qualities and may significantly reduce concomitant opioid use.14 Because of its centrally mediated sympatholytic effect, it confers good hemodynamic control after intracranial operations.15,16 Recently, a large body of research in experimental models and clinical settings has demonstrated the neuroprotective properties of dexmedetomidine.17 These characteristics make dexmedetomidine a potential agent for the management of pain and agitation in neurosurgical patients.18,19
We performed this trial to investigate the efficacy and safety of dexmedetomidine for prophylactic analgesia and sedation in patients with delayed extubation after craniotomy.
MATERIALS AND METHODS
Study Design and Ethical Consideration
The study was a single-center, randomized, double-blind, placebo-controlled trial. The study protocol was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University (KY2012-006-02). Written informed consent was obtained from patients’ relatives upon admission to the intensive care unit (ICU). This study was registered with the Chinese Clinical Trial Registry (ChiCTR-PRC-12002903). The study protocol was published in 2013.20
Setting and Participants
This study was performed in a 20-bed neurosurgical ICU at Beijing Tiantan Hospital (1000 beds), Capital Medical University, Beijing, China, between June 2012 and July 2014. All adult patients admitted to the neurosurgical ICU after intracranial surgery with delayed extubation were screened for study eligibility. Delayed extubation was defined as the patient not extubated in the operating room at completion of the surgery The exclusion criteria included: age under 18 years; pregnancy or lactation; emergency operation; reoperation within 72 hours; operations related to the medulla oblongata; preoperative consciousness disorders (GCS≤14), epilepsy, dysphagia, or cough reflex impairment; unrecovered consciousness or spontaneous breathing within 2 hours after end of surgery; concomitant cardiovascular problems, including second-degree or third-degree atrioventricular block; heart rate (HR) <50 beats per minute; systolic blood pressure (BP) <90 mm Hg or the need for continuous infusions of a vasopressor; refusal to participate; and enrollment in other trials. Patients were enrolled only once unless they were discharged from the hospital and were readmitted at least 180 days after the first enrollment.
Randomization and Masking
Enrolled patients were randomly assigned at a 1:1 ratio to receive intravenous infusion of dexmedetomidine (the intervention group) or normal saline (the control group). The study secretary (M.X.) generated randomization with a computerized random digits table, placed the treatment assignments into numbered opaque envelopes, and sealed them.
Dexmedetomidine (10 μg/mL) or normal saline with the same characteristics was prepared by the clinical pharmacist (J.-J.H.). Patients and all study personnel except for the investigative pharmacist were blind to the treatment assignments.
Intervention
Two hours after end of surgery, the intervention group received a continuous intravenous infusion of dexmedetomidine (Ai Bei Ning; Jiang Su Heng Rui Medicine Co. Ltd, Jiangsu Province, China) at a dose of 0.6 μg/kg/h. The control group received a maintenance infusion of normal saline at a volume and rate equal to that of the intervention group. The allocated intervention was terminated at 30 minutes after extubation or a maximum of 24 hours on the study infusion protocol.
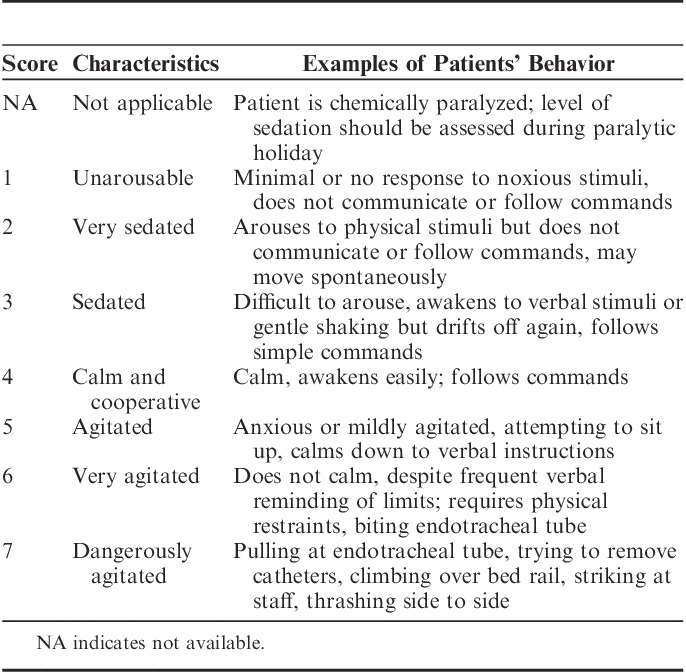
The level of sedation was assessed by bedside nurses using the Sedation Agitation Scale (SAS),21 which ranged from 1 (unarousable) to 7 (dangerous agitation) (Table 1). Routine SAS evaluation was incorporated into clinical practice in our ICU for >1 year before the start of this study.5 The assessment of SAS was performed every hour or as needed after the infusion of experimental agents. The target level of sedation was set at a SAS score of 3 to 4. If the patient exhibited agitation (SAS score of 5 to 7), an ICU physician was consulted, and the causes of agitation were investigated (eg, pain, hypoxia, hypotension, or endotracheal tube obstruction). A rescue bolus of propofol (0.5 mg/kg) was given as needed. If agitation was not eliminated, then a continuous infusion of propofol (20 mg/mL) was started and titrated to reach an SAS of 3 to 4. Pain was treated using bolus of fentanyl in 0.05 mg increments on an as-needed basis. For cases in which the SAS score was ≤2 (arouses to physical stimuli but does not communicate or follow commands), the trial intervention was stopped urgently. Other criteria for urgent stop included bradycardia (HR<50 beats/min after a 0.25 mg bolus of atropine), hypotension (systolic BP<90 mm Hg after infusion of 250 mL hydroxyethyl starch for 30 min), hypoxemia (pulse oxygen saturation [SpO2]<90%), or neurological complications (cerebral hemorrhage, infarction or brain edema diagnosed by computed tomography).
TABLE 1.
Sedation Agitation Scale
Weaning from mechanical ventilation and endotracheal extubation was performed according to our local extubation protocol.22 An ICU physician evaluated the patient using an extubation screening checklist, which included assessments of consciousness, respiration, and circulation status, muscle strength recovery, gag reflex, and swallowing function. If the patient passed the evaluation, then the endotracheal tube was extubated by a registered ICU nurse. Patients were discharged from the ICU the morning after their physiological status had been stabilized, usually with normal neurological, respiratory, and hemodynamic status. Patients with endotracheal intubation were not discharged.22 Patients with a tracheotomy were discharged after successful weaning from mechanical ventilation.
Study Endpoints
The primary endpoint was the percentage of time within the target sedation range (SAS of 3 to 4) during the infusion of experimental agents. Other efficacy endpoints included the following: (1) the percentage of patients exhibiting agitation (SAS≥5); (2) the percentage of patients requiring rescue propofol to achieve/maintain the targeted sedation range and the total dose of propofol required per patient throughout the infusion of the study drug; and (3) the percentage of patients requiring fentanyl for additional rescue of analgesia.
The safety endpoints included the following: (1) the incidence of an urgent stop of the study intervention; (2) the percentage of patients who remained intubated beyond 24 hours after operation; and (3) hemodynamic adverse events during the drug infusion, which included hypotension (systolic BP<90 mm Hg), hypertension (systolic BP>180 mm Hg), bradycardia (HR<50/min), and tachycardia (HR>120/min).
For patients who were extubated within 24 hours after operation, HR, mean BP, and SpO2 were recorded before endotracheal suctioning, immediately after extubation, and at 30 minutes after extubation. The visual analog scale (VAS) (VAS=0, not bad at all and well tolerable;VAS=10, very painful and beyond endurance) was evaluated immediately before extubation and 30 minutes after extubation.2,23 These parameters were measured and documented by the chief nurses (Y.Y. and W.C.).
Patients were followed up until hospital discharge, death, or 60 days after the trial intervention on a first-come, first-serve basis. Data regarding clinical outcomes were collected: reintubation and reoperation within 72 hours after the trial intervention, the 5-category Glasgow Outcome Scale, and ICU stay and overall hospital length of stay (LOS).
Statistical Analysis
All analyses were conducted according to the intention-to-treat principle, that is, all randomized patients were analyzed in the groups to which they were originally allocated and were blinded to the treatment assignments. Baseline characteristics were summarized by performing univariate analyses. Categorical variables were presented as numbers and percentages and analyzed by the χ2 test. Continuous variables were checked for normal distribution and presented as the mean and SD or median and interquartile range as appropriate. Comparisons of continuous variables were performed using the Student t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. HR, mean BP, and SpO2 during extubation were analyzed by repeated measures analysis of variance (ANOVA). All tests of significance were 2-sided and were considered significant at the 5% level. Analyses were conducted using SPSS 17.0.
Sample Size
In our previous study, we found that the frequency of agitation in delayed extubation patients after craniotomy was 30%5 and that the mean percentage of time in optimal sedation was 88% (unpublished data). We hypothesized that the mean percentage of time in optimal sedation would increase to 95% after the use of dexmedetomidine. Using the Power and Sample Size Calculation program, we determined that we needed to enroll 72 patients in each arm to be able to reject the null hypothesis that the population means of the experimental and control groups were equal with a probability (power) of 0.8. The type I error probability of testing this null hypothesis was 0.05.
RESULTS
From June 2012 to July 2014, 328 patients with delayed extubation after craniotomy were assessed for eligibility, and 150 of them were enrolled in the study (75 patients in each arm). The reasons for exclusion are shown in the study flow chart (Fig. 1).
FIGURE 1.
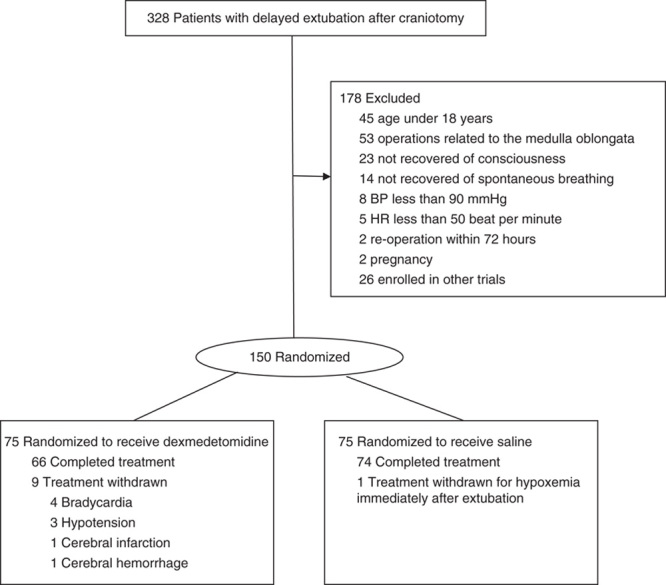
Flow diagrams for the dexmedetomidine versus saline trial.
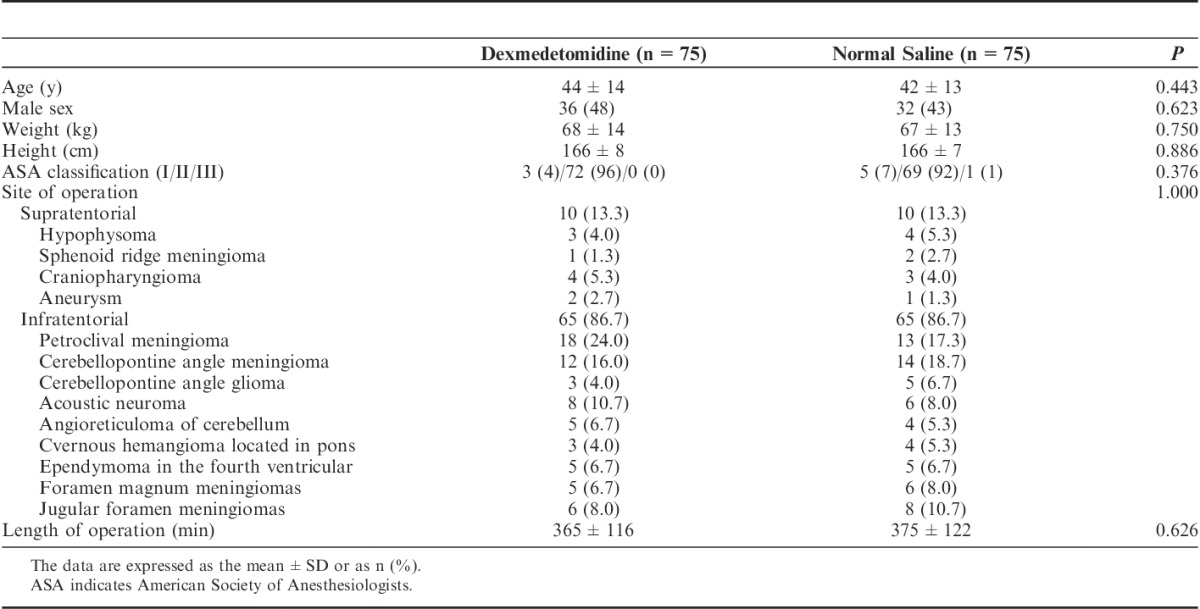
Demographic and clinical characteristics at baseline were comparable between the 2 arms (Table 2). There were 11 hypertensive patients per group included in the study. There was no difference between the 2 groups. The durations of the infusion of the study agent were 12.7±4.4 and 13.4±3.1 hours in the dexmedetomidine group and the control group, respectively (P=0.258).
TABLE 2.
Baseline Characteristics of the Participants
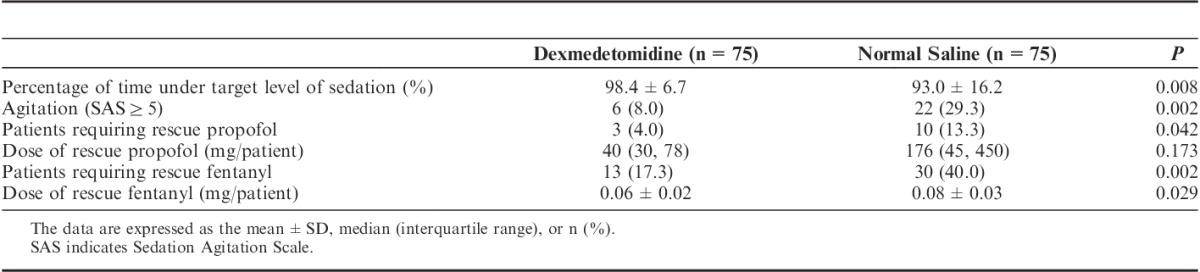
Efficacy
The results of efficacy endpoints are shown in Table 3. The percentage of time within the target sedation range was significantly higher in the dexmedetomidine group than in the control group. The incidence of agitation was significantly lower in the dexmedetomidine group (8.0%) than in the control group (29.3%). Three (4.0%) and 10 (13.3%) patients required rescue propofol in the dexmedetomidine group and the control group, respectively. No patient required continuous infusion of propofol in the dexmedetomidine group, whereas 5 patients in the control group received continuous infusion of propofol during the experimental period. No significant difference in the dose of rescue propofol per patient was observed between the 2 groups (P=0.173). A significantly lower percentage of patients was found to have self-reported pain in the dexmedetomidine group (17.3%) relative to the control group (40.0%). Patients in the dexmedetomidine group received less rescue fentanyl than in the control group (P=0.029).
TABLE 3.
Efficacy of Sedation and Analgesia
Safety
The results of the safety endpoints are shown in Table 4.
TABLE 4.
Safety Endpoints
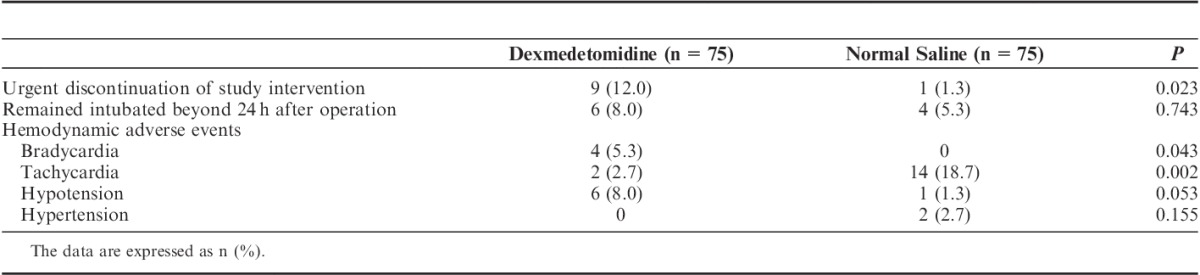
Urgent discontinuation of the study intervention was required in 9 (12.0%) patients in the dexmedetomidine group and in 1 (1.3%) patient in the control group (P=0.023, Table 4, and Fig. 1). The reasons for urgent discontinuation in the dexmedetomidine group were as follows: bradycardia in 4 patients (at 2 to 10 h after the start of agent infusion), hypotension in 3 patients (at 2 to 10 h after the start of agent infusion), cerebral infarction in 1 patient (unilateral dilation of the pupil and lack of response to stimulation were found at 3 h after agent infusion, and cerebral infarction was diagnosed by computed tomography; decompressive craniectomy was performed at 64 h after the operation); and cerebral hemorrhage in 1 patient (diagnosed by routine postoperative computed tomography at 4 h after agent infusion). In the control group, the study intervention was stopped in 1 patient due to hypoxemia immediately after extubation, and the patient was reintubated.
No significant difference in the percentage of patients who remained intubated beyond 24 hours after operation was observed between the 2 groups (Table 4). In the dexmedetomidine group, 5 patients remained intubated for 43 to 132 hours, and 1 patient received tracheostomy at 131 hours after the operation. In the control group, 3 patients remained intubated for 25 to 84 hours, and 1 patient received tracheostomy at 38 hours after operation. For hemodynamic adverse events, patients in the dexmedetomidine group were more likely to develop bradycardia (5.3% vs. 0%, P=0.043) but had a lower likelihood of tachycardia (2.7% vs. 18.7%, P=0.002) (Table 4).
Clinical Outcomes and Costs
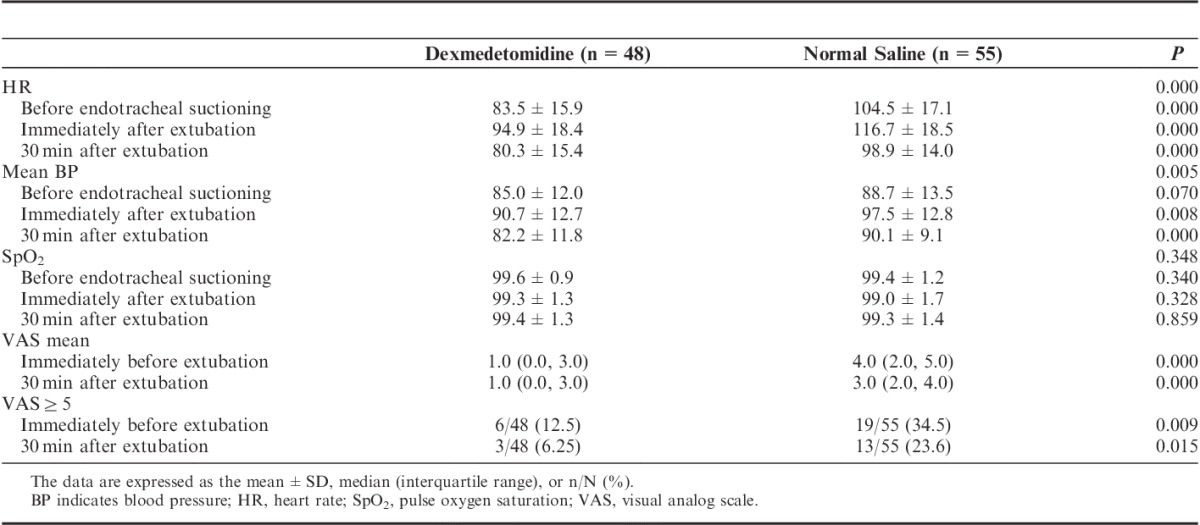
The clinical outcomes and costs are shown in Table 5. No significant differences in the percentages of reintubation, reoperation, Glasgow Outcome Scale, ICU LOS, hospital LOS, and cost were observed between the 2 groups.
TABLE 5.
Clinical Outcomes and Cost
Parameters During Extubation
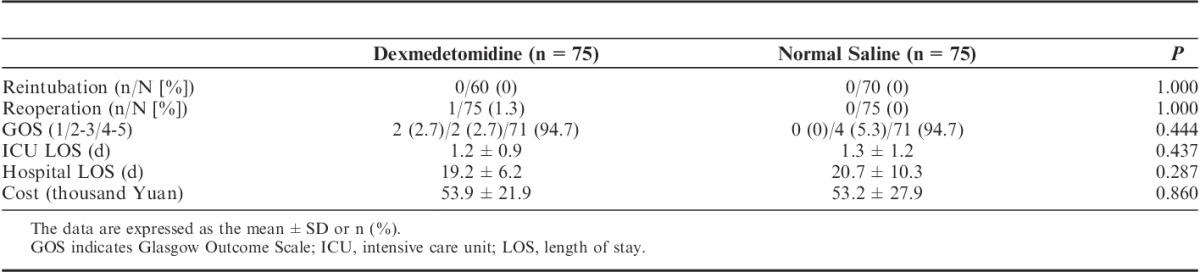
Of the 150 enrolled patients, 130 patients were extubated within 24 hours after operation and received dexmedetomidine until 30 minutes after extubation. HR, mean BP, SpO2, and VAS parameters were documented in 103 patients (48 in the dexmedetomidine group and 55 in the control group). No relative data for the remaining 27 patients were obtained due to protocol violation.
Significant differences in the HR and mean BP during extubation were observed between the 2 groups (Table 6). The HR and mean BP were significantly lower in the dexmedetomidine group than in the control group at all 3 time points (before endotracheal suctioning, immediately after extubation, and 30 min after extubation). The within-group comparison showed that the HR and mean BP increased significantly during extubation and then resumed to preextubation levels at 30 minutes after extubation. No significant difference in SpO2 during extubation was observed between the 2 groups. The VAS was significantly lower in the dexmedetomidine group than in the control group (1.0 vs. 4.0, P=0.000), and severe pain (VAS≥5) was less likely to occur in the dexmedetomidine group (12.5% vs. 34.5%, P=0.009).
TABLE 6.
Parameters During Extubation
DISCUSSION
Emergence agitation is a significant clinical issue during recovery from general anesthesia.24 Observational and controlled studies demonstrated that the incidence of emergence agitation in patients after craniotomy was higher than that after other types of operations, such as ear, nose, and throat surgeries or ophthalmologic, abdominal, urologic, or vascular surgeries.5,25–27 In our pilot study,5 we observed that emergence agitation was common in patients after elective craniotomy for brain tumors performed under general anesthesia. Of the total included patients, 29% suffered at least 1 episode of agitation during the first 12 hours after surgery. After intracranial operations, patients are more vulnerable to the stress that results from emergence agitation during the recovery from general anesthesia.6,28 Agitation can cause tachycardia, hypertension, increased catecholamine production, increased oxygen consumption, and immunosuppression.29,30 Increased catecholamine levels have been shown to contribute to myocardial ischemia, disturbed sleep, and catabolism.31 Hypertension can cause an increase in brain edema or hemorrhage, which may cause brain herniation.32
Acute pain is common after craniotomy.33 Prospective observational studies showed that a substantial number of patients (approximately 50%) suffered from moderate to severe pain during the first postoperative day after craniotomy.34–36 In a recent study by Mordhorst et al,34 55% of patients had moderate or severe postoperative pain in the first 24 hours following craniotomy. This study had a result similar to the pilot study by De Benedittis et al,37 in which 60% of patients experienced postoperative pain. In our previous study,5 51% of patients complained of pain after elective craniotomy for brain tumors.
However, despite a greater awareness of pain and agitation after craniotomy, clinicians remain reluctant to administer analgesics and sedatives in patients following craniotomy due to the drugs’ side effects, primarily reducing the clinician’s ability to monitor the level of consciousness and that inducing respiratory depression in patients.38
Dexmedetomidine, a potent and highly selective α-2-adrenoceptor agonist, provides dose-dependent sedation, anxiolysis, and analgesia (involving spinal and supraspinal sites) without respiratory depression39 by acting on α-2 receptors in the LC.40–42 Dexmedetomidine may induce a sedative state similar to physiological sleep, and patients may be aroused easily with stimulation and are cooperative once aroused. In addition to its sedative effects, dexmedetomidine has significant analgesic qualities and may significantly reduce concomitant opioid use. Analgesia with dexmedetomidine is mediated primarily through an interaction at α-2a within the spinal cord, where drug activity attenuates nociceptive signal transduction. The actual mechanism of action appears to involve an interaction with opioid receptors, and although dexmedetomidine alone has been documented to reduce pain, the effect when given jointly with opioids may be additive or synergistic.43 All these advantages allow dexmedetomidine to be used after craniotomy.
Data reflecting the present study at our institution revealed that emergence agitation is common for the first day after major elective intracranial surgery, with approximately 29.3% of patients in the control group exhibiting agitation at some point during their hospitalization. Approximately 40.0% of patients exhibited self-reported pain in the control group. In the dexmedetomidine group, the incidences of agitation and pain decreased to 8.0% and 17.3%, respectively. The VAS was significantly lower in the dexmedetomidine group than in the control group. Patients in the dexmedetomidine group received less rescue fentanyl than in the control group.
All sedatives have cardiovascular adverse effects. Bradycardia and hypotension were most common with dexmedetomidine, found in up to 10% of cases.44,45 The hemodynamic effects of dexmedetomidine may result from both peripheral and central mechanisms. Dexmedetomidine is the pharmacologically active dextroisomer of medetomidine.46 The stimulation of α-2 adrenoceptors by dexmedetomidine in the pontine LC results in decreased firing of LC neurons secondary to their hyperpolarization. This drug also has a sympatholytic effect by decreasing the concentration of norepinephrine, which subsequently decreases the BP and the HR.47–50 Bradycardia and hypotension were shown to be dose dependent, with no remarkable detrimental effect on cardiac function.51 However, the risk of bradycardia was significantly higher in studies that used both a loading dose and high maintenance doses (>0.7 μg/kg/h) than in studies that did not use both.52 To avoid cardiovascular adverse effects, we performed continuous infusion (0.6 μg/kg/h) without the use of a loading dose. In the present study, bradycardia was reported in 4 of 75 dexmedetomidine patients (5.3%) versus 0 control patients (0%); hypotension was observed in 6 dexmedetomidine patients (8%) and in 1 control patient (1.3%).
Dexmedetomidine also can prevent hyperdynamic responses during extubation without causing significant respiratory depression, allowing comfortable and high-quality recovery after intracranial neurosurgery.48,53 Dexmedetomidine attenuated the increases in HR and BP after extubation and improved extubation conditions but did not prolong recovery in patients presenting for craniotomy. In the present study, the hemodynamic parameters in the dexmedetomidine group were significantly stable during extubation compared with the control group. No significant difference in SpO2 during extubation was observed between the 2 groups.
The present study has some limitations that should be considered when interpreting these results. First, we selected a constant dexmedetomidine infusion rate of 0.6 μg/kg/h, without adjusting the dose according to the individual’s ability to metabolize this drug. Therefore, the incidence of side effects increased. With a study design in which the caregiver was permitted to titrate the dexmedetomidine dose, further improvements in hemodynamic stability might be observed. Second, another possible limitation of the present study was the relatively small number of patients analyzed. Regarding sample size, this study was powered to detect differences in sedation and, thus, could not definitively detect differences in safety. Third, we assessed sedation from a caregiver’s perspective. Future studies should also include the patient’s perspective concerning the quality of sedation. Fourth, the study’s findings cannot account for lesion location.
In summary, as a new sedative and analgesic drug, dexmedetomidine may be an effective agent for prophylactic sedation and analgesia in patients with delayed extubation after craniotomy. Dexmedetomidine can increase the mean percentage of time under optimal sedation and reduce the frequency of agitation and the degree of pain in patients with delayed extubation after craniotomy surgery. The infusion of dexmedetomidine (0.6 μg/kg/h) does not produce respiratory depression, but may increase the incidence of bradycardia.
Footnotes
Supported by Beijing Health Bureau (No: 2009-3-28).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Nemergut EC, Durieux ME, Missaghi NB, et al. Pain management after craniotomy. Best Pract Res Clin Anaesthesiol. 2007;21:557–573. [DOI] [PubMed] [Google Scholar]
- 2.Nair S, Rajshekhar V. Evaluation of pain following supratentorial craniotomy. Br J Neurosurg. 2011;25:100–103. [DOI] [PubMed] [Google Scholar]
- 3.Lai LT, Ortiz-Cardona JR, Bendo AA. Perioperative pain management in the neurosurgical patient. Anesthesiol Clin. 2012;30:347–367. [DOI] [PubMed] [Google Scholar]
- 4.Bilotta F, Guerra C, Rosa G. Update on anesthesia for craniotomy. Curr Opin Anaesthesiol. 2013;26:517–522. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Xu M, Li GY, et al. Incidence, risk factors and consequences of emergence agitation in adult patients after elective craniotomy for brain tumor: a prospective cohort study. PLoS One. 2014;9:e114239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruder N, Ravussin P. Recovery from anesthesia and postoperative extubation of neurosurgical patients: a review. J Neurosurg Anesthesiol. 1999;11:282–293. [DOI] [PubMed] [Google Scholar]
- 7.Manninen PH, Raman SK, Boyle K, et al. Early postoperative complications following neurosurgical procedures. Can J Anaesth. 1999;46:7–14. [DOI] [PubMed] [Google Scholar]
- 8.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1055. discussion 1055-1056. [DOI] [PubMed] [Google Scholar]
- 9.Kotak D, Cheserem B, Solth A. A survey of post-craniotomy analgesia in British neurosurgical centres: time for perceptions and prescribing to change? Br J Neurosurg. 2009;23:538–542. [DOI] [PubMed] [Google Scholar]
- 10.Hassouneh B, Centofanti JE, Reddy K. Pain management in post-craniotomy patients: a survey of Canadian neurosurgeons. Can J Neurol Sci. 2011;38:456–460. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro Mdo C, Pereira CU, Sallum AM, et al. Knowledge of doctors and nurses on pain in patients undergoing craniotomy. Rev Lat Am Enfermagem. 2012;20:1057–1063. [DOI] [PubMed] [Google Scholar]
- 12.Massad IM, Mahafza TM, Abu-Halawah SA, et al. Postoperative pain is undertreated: results from a local survey at Jordan University Hospital. East Mediterr Health J. 2013;19:485–489. [PubMed] [Google Scholar]
- 13.Reardon DP, Anger KE, Adams CD, et al. Role of dexmedetomidine in adults in the intensive care unit: an update. Am J Health Syst Pharm. 2013;70:767–777. [DOI] [PubMed] [Google Scholar]
- 14.Weinbroum AA, Ben-Abraham R. Dextromethorphan and dexmedetomidine: new agents for the control of perioperative pain. Eur J Surg. 2001;167:563–569. [DOI] [PubMed] [Google Scholar]
- 15.Soliman RN, Hassan AR, Rashwan AM, et al. Prospective, randomized study to assess the role of dexmedetomidine in patients with supratentorial tumors undergoing craniotomy under general anaesthesia. Middle East J Anesthesiol. 2011;21:325–334. [PubMed] [Google Scholar]
- 16.Tanskanen P, Kytta J, Randell T, et al. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumor surgery: a double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97:658–665. [DOI] [PubMed] [Google Scholar]
- 17.Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28:3–6. [DOI] [PubMed] [Google Scholar]
- 18.Mirski MA, Lewin JJ, 3rd, Ledroux S, et al. Cognitive improvement during continuous sedation in critically ill, awake and responsive patients: the Acute Neurological ICU Sedation Trial (ANIST). Intensive Care Med. 2010;36:1505–1513. [DOI] [PubMed] [Google Scholar]
- 19.Grof TM, Bledsoe KA. Evaluating the use of dexmedetomidine in neurocritical care patients. Neurocrit Care. 2010;12:356–361. [DOI] [PubMed] [Google Scholar]
- 20.Zhao LH, Shi ZH, Yin NN, et al. Use of dexmedetomidine for prophylactic analgesia and sedation in delayed extubation patients after craniotomy: a study protocol and statistical analysis plan for a randomized controlled trial. Trials. 2013;14:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329. [DOI] [PubMed] [Google Scholar]
- 22.Cai YH, Zeng HY, Shi ZH, et al. Factors influencing delayed extubation after infratentorial craniotomy for tumour resection: a prospective cohort study of 800 patients in a Chinese neurosurgical centre. J Int Med Res. 2013;41:208–217. [DOI] [PubMed] [Google Scholar]
- 23.Yu A, Teitelbaum J, Scott J, et al. Evaluating pain, sedation, and delirium in the neurologically critically ill—feasibility and reliability of standardized tools: a multi-institutional study. Crit Care Med. 2013;41:2002–2007. [DOI] [PubMed] [Google Scholar]
- 24.Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiol. 2011;77:448–456. [PMC free article] [PubMed] [Google Scholar]
- 25.Todd MM, Warner DS, Sokoll MD, et al. A prospective, comparative trial of three anesthetics for elective supratentorial craniotomy. Propofol/fentanyl, isoflurane/nitrous oxide, and fentanyl/nitrous oxide. Anesthesiology. 1993;78:1005–1020. [DOI] [PubMed] [Google Scholar]
- 26.Kanaya N, Kuroda H, Nakayama M, et al. Does propofol anesthesia increase agitation in neurosurgical patients?—a pilot study. Can J Anaesth. 2002;49:638–639. [DOI] [PubMed] [Google Scholar]
- 27.Citerio G, Pesenti A, Latini R, et al. A multicentre, randomised, open-label, controlled trial evaluating equivalence of inhalational and intravenous anaesthesia during elective craniotomy. Eur J Anaesthesiol. 2012;29:371–379. [DOI] [PubMed] [Google Scholar]
- 28.Bruder NJ. Awakening management after neurosurgery for intracranial tumours. Curr Opin Anaesthesiol. 2002;15:477–482. [DOI] [PubMed] [Google Scholar]
- 29.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. [DOI] [PubMed] [Google Scholar]
- 30.Gehlbach BK, Kress JP. Sedation in the intensive care unit. Curr Opin Crit Care. 2002;8:290–298. [DOI] [PubMed] [Google Scholar]
- 31.Epstein J, Breslow MJ. The stress response of critical illness. Crit Care Clin. 1999;15:17–33. [DOI] [PubMed] [Google Scholar]
- 32.Basali A, Mascha EJ, Kalfas I, et al. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000;93:48–54. [DOI] [PubMed] [Google Scholar]
- 33.Flexman AM, Ng JL, Gelb AW. Acute and chronic pain following craniotomy. Curr Opin Anaesthesiol. 2010;23:551–557. [DOI] [PubMed] [Google Scholar]
- 34.Mordhorst C, Latz B, Kerz T, et al. Prospective assessment of postoperative pain after craniotomy. J Neurosurg Anesthesiol. 2010;22:202–206. [DOI] [PubMed] [Google Scholar]
- 35.Klimek M, Ubben JF, Ammann J, et al. Pain in neurosurgically treated patients: a prospective observational study. J Neurosurg. 2006;104:350–359. [DOI] [PubMed] [Google Scholar]
- 36.Gottschalk A, Berkow LC, Stevens RD, et al. Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J Neurosurg. 2007;106:210–216. [DOI] [PubMed] [Google Scholar]
- 37.De Benedittis G, Lorenzetti A, Migliore M, et al. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996;38:466–469. [DOI] [PubMed] [Google Scholar]
- 38.Beretta L, De Vitis A, Grandi E. Sedation in neurocritical patients: is it useful? Minerva Anestesiol. 2011;77:828–834. [PubMed] [Google Scholar]
- 39.Bekker A, Sturaitis MK. Dexmedetomidine for neurological surgery. Neurosurgery. 2005;57(suppl 1):1–10. [DOI] [PubMed] [Google Scholar]
- 40.Huupponen E, Maksimow A, Lapinlampi P, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–294. [DOI] [PubMed] [Google Scholar]
- 41.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venn RM, Bradshaw CJ, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–1142. [DOI] [PubMed] [Google Scholar]
- 43.Fairbanks CA, Stone LS, Kitto KF. Alpha-2c adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J Pharmacol Exp Ther. 2002;300:282–290. [DOI] [PubMed] [Google Scholar]
- 44.Schaller B. Trigemino-cardiac reflex during transsphenoidal surgery for pituitary adenomas. Clin Neurol Neurosurg. 2005;107:468–474. [DOI] [PubMed] [Google Scholar]
- 45.Schaller B, Cornelius JF, Prabhakar H, et al. The trigemino-cardiac reflex: an update of the current knowledge. J Neurosurg Anesthesiol. 2009;21:187–195. [DOI] [PubMed] [Google Scholar]
- 46.Nelson LE, Lu J, Guo T, et al. The alpha 2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. [DOI] [PubMed] [Google Scholar]
- 47.Ebert TJ, Hall JE, Barney JA, et al. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. [DOI] [PubMed] [Google Scholar]
- 48.Turan G, Ozgultekin A, Turan C, et al. Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816–820. [DOI] [PubMed] [Google Scholar]
- 49.Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol. 2008;4:619–627. [DOI] [PubMed] [Google Scholar]
- 50.Bekker AY, Basile J, Gold M, et al. Dexmedetomidine for awake carotid endarterectomy: efficacy, hemodynamic profile, and side effects. J Neurosurg Anesthesiol. 2004;16:126–135. [DOI] [PubMed] [Google Scholar]
- 51.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–268. [DOI] [PubMed] [Google Scholar]
- 52.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36:926–939. [DOI] [PubMed] [Google Scholar]
- 53.Gopalakrishna KN, Dash PK, Chatterjee N, et al. Dexmedetomidine as an anesthetic adjuvant in patients undergoing transsphenoidal resection of pituitary tumor. J Neurosurg Anesthesiol. 2015;27:209–215. [DOI] [PubMed] [Google Scholar]


