Abstract
Background
The development of effective nutritional strategies in support of muscle growth for patients with chronic obstructive pulmonary disease (COPD) remains challenging. Dietary essential amino acids (EAAs) are the main driver of postprandial net protein anabolism. In agreement, EAA supplements in healthy older adults are more effective than supplements with the composition of complete proteins. In patients with COPD it is still unknown whether complete protein supplements can be substituted with only EAAs, and whether they are as effective as in healthy older adults.
Methods
According to a double-blind randomized crossover design, we examined in 23 patients with moderate to very severe COPD (age: 65 ± 2 y, FEV1: 40 ± 2% of predicted) and 19 healthy age-matched subjects (age: 64 ± 2 y), whether a free EAA mixture with a high proportion (40%) of leucine (EAA mixture) stimulated whole body net protein gain more than a similar mixture of balanced free EAAs and non-EAAs as present in whey protein (TAA mixture). Whole body net protein gain and splanchnic extraction of phenylalanine (PHE) were assessed by continuous IV infusion of L-[ring-2H5]-PHE and L-[ring-2H2]-tyrosine, and enteral intake of L-[15N]-PHE (added to the mixtures).
Results
Besides an excellent positive linear relationship between PHE intake and net protein gain in both groups (r=0.84–0.91, P<0.001), net protein gain was 42% higher in healthy controls and 49% higher in COPD patients after intake of the EAA mixture compared to the TAA mixture (P<0.0001). These findings could not be attributed to the high LEU content, as in both groups net protein gain per gram EAA intake was lower for the EAA mixture (P<0.0001). Net protein gain was higher in COPD patients for both mixtures due to a 40% lower splanchnic extraction (P<0.0001), but was similarly related to dietary PHE (i.e. EAA) plasma appearance.
Conclusions
In COPD patients, similarly to healthy older adults, free EAA supplements stimulate whole body protein anabolism more than free amino acid supplements with the composition of complete proteins. Therefore, free EAA supplements may aid in the prevention and treatment of muscle wasting in this patient population.
Trial registry
ClinicalTrials.gov; Nos.: NCT01173354 and NCT01172314; URL: www.clinicaltrials.gov
Keywords: essential amino acid, protein turnover, COPD, leucine
1. Introduction
It has been well established that weight and muscle loss in patients with chronic obstructive pulmonary disease (COPD) are independent disease related factors compromising health status. A BMI of < 25 kg/m2 is already associated with an increased overall risk of mortality in these patients [1], and severe muscle wasting (indicated by a fat-free mass index below the lowest 10th percentile of the general population) is in 26% of cases masked by a normal BMI [2]. As weight gain [1] and higher physical activity level [3] both reduce the overall risk of mortality in these patients, it remains important to develop more effective nutritional strategies that support muscle growth and improved function. Long-term intervention with essential amino acids (EAAs) may have the potential to do both [4].
Our recent data in COPD patients with muscle wasting [5] showed that the milk proteins whey and casein, both high in EAAs, are very effective at stimulating whole body protein anabolism, similar to our findings in normal-weight COPD patients [6, 7]. Importantly, we found an excellent positive linear relationship between EAA intake per kg fat-free mass and whole body protein anabolism in that study [5]. These results are similar to our findings in healthy older adults and in other disease states [8, 9]. It is however still unknown whether the efficacy of dietary EAAs in relation to protein anabolism is similar between patients with COPD and healthy older adults. Previous research in healthy older adults has shown that EAAs are primarily responsible for the stimulation of net muscle protein gain [10, 11], which strongly suggests that the use of only free EAAs instead amino acid mixtures with the composition of complete proteins might be more effective at preventing muscle loss in COPD patients.
The EAA leucine (LEU) has received particular interest because of its unique role in the stimulation of protein translation [12]. In healthy older adults, a higher proportion of LEU as part of a free EAA mixture was required for optimal stimulation of muscle protein synthesis, positively affecting net muscle protein balance [13]. In COPD patients, we found that a low quality soy protein mixture with added free branched-chain amino acids (leucine, isoleucine and valine) improved whole body protein synthesis [14]. On the contrary, free LEU added to hydrolyzed milk protein mixtures neither enhanced whole body protein synthesis, nor improved net protein balance [5]. From the latter study, it remains unclear whether the already high biological value of milk proteins is reason to believe that additional LEU was unnecessary, or whether the presence of non-EAAs in these proteins negated the positive effect of adding LEU.
Therefore, we tested in stable COPD patients whether (1) a supplement with only EAAs stimulated whole body net protein gain more than a supplement with both EAAs and non-EAAs, (2) whether this response was different from healthy older adults, and (3) whether a high proportion of LEU in a supplement with only EAAs is favorable.
2. Materials and methods
2.1. Subject inclusion
Twenty-three patients with moderate to very severe airflow obstruction (GOLD stage II-IV) [15], and 19 healthy older adults from the Little Rock, AR area were included between 2009 and 2012 (Supplemental Figure 1). Recruitment took place through pulmonologist referral and local advertising efforts. Medical history and medication use were assessed as part of the screening process. All patients had a clinical diagnosis of COPD and did not experience a respiratory tract infection or exacerbation at least 4 weeks prior to the study. All received bronchodilator treatment, except for two patients who did not receive any maintenance therapy. Furthermore, 18 patients were taking inhalation corticosteroids and nine were on long-term oxygen therapy. Use of systemic corticosteroids one month prior to the study was an exclusion criterion, as well as malignancy, recent surgery and severe unstable endocrine, hepatic or renal disorders. Written informed consent was obtained from all participants, and the Institutional Review Board of the University of Arkansas for Medical Sciences approved the study (UAMS, IRB no. 105558).
2.2. Lung function and anthropometric data
Height and weight were measured using standard procedures. Body composition was assessed using Dual-Energy X-ray Absorptiometry (Hologic QDR 4500). Values were standardized for height. Forced expiratory volume in 1 second (FEV1) was assessed with the highest value from ≥ 3 technically acceptable maneuvers being used [16].
2.3. Study design
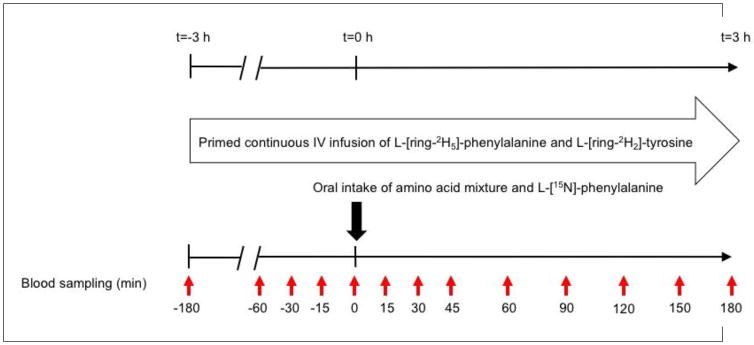
Participants were studied on two days (within one week, but ≥ one day apart) of six hours each, after an overnight fast (Figure 1). After insertion of a catheter into an antecubital vein, a blood sample was taken to measure natural stable isotope enrichment, glucose and insulin levels (t= −180 min). For the measurement of whole body protein kinetics, we subsequently started a primed, constant intravenous infusion of L-[ring-2H5]-phenylalanine (L-[ring-2H5]-PHE) (prime: 3.6 μmol/kg; IV infusion: 3.6 μmol/kg/h) and L-[ring-2H2]-tyrosine (prime: 1.14 μmol/kg; IV infusion: 1.14 μmol/kg/h), and L-[ring-2H4]-tyrosine was primed orally (0.31 μmol/kg). L-[15N]-PHE was given orally as well, mixed with each of the free amino acid mixtures (the amount in both mixtures was similar, and equal to 20% of the PHE content in the EAA mixture) for the measurement of splanchnic PHE extraction. A second catheter for arterialized venous blood sampling was placed in a superficial dorsal vein of the contralateral hand or lower arm, and the hand was placed in a thermostatically controlled heated box to mimic direct arterial sampling [17]. Whole body protein breakdown, synthesis, net protein gain (protein synthesis - protein breakdown), and splanchnic PHE extraction were calculated from the plasma isotope enrichment during either postabsorptive steady state or postprandial non-steady state conditions, using previously described equations [5] available in the online supplemental material. Blood was processed and analyzed in batch by LC-MS/MS using routine laboratory procedures [5].
Figure 1.
Study design. All participants were studied twice within one week (≥ one day apart) during a 6-hour infusion protocol, and received two different free amino acid mixtures according to a randomized crossover design.
2.4. Composition of the free amino acid mixtures
Participants were given two free amino acid mixtures (Table 1) as a single bolus, according to a double-blind randomized crossover design (randomizer.org). The EAA mixture contained a balanced mixture of solely EAAs similar to the composition of whey protein, enriched with additional leucine (40% w/w EAA content) [9]. The total amino acid (TAA) mixture contained a balanced mixture of EAAs and non-EAAs also similar to the composition of whey protein (24% leucine w/w EAA content). As shown in Table 1 the EAA profile (i.e. distribution) of both mixtures was identical, with the exception of LEU (24 vs. 40%). Healthy controls were also in part recruited for another clinical trial [9], and as such received larger mixtures, but with the same composition as the mixtures provided to the COPD group. As we express the data per kg fat-free mass, the net protein gain response of the control group falls mostly within the range of the COPD group. Therefore, we consider it acceptable to compare the responses of healthy subjects who received more amino acids to those of COPD patients who received less amino acids. Maltodextrin was added to both mixtures to stimulate postprandial insulin secretion, and to thereby achieve a greater whole body net protein gain than attainable with amino acids only [18].
Table 1.
Composition of the TAA and EAA mixture per study group1
| Healthy | COPD | |||
|---|---|---|---|---|
|
| ||||
| TAA mixture2 | EAA mixture3 | TAA mixture | EAA mixture | |
| Histidine | 0.256 (4) | 0.386 (4) | 0.128 (4) | 0.193 (4) |
| Isoleucine | 0.714(11) | 1.252 (11) | 0.357 (11) | 0.626 (11) |
| Leucine | 1.562 (24) | 2.690 (24) | 0.781 (24) | 1.345 (24) |
| Lysine | 1.582 (25) | 2.674 (24) | 0.791 (25) | 1.337 (24) |
| Methionine | 0.358 (6) | 0.654 (6) | 0.179 (6) | 0.327 (6) |
| Phenylalanine | 0.458 (7) | 0.794 (7) | 0.229 (7) | 0.397 (7) |
| Threonine | 0.830 (13) | 1.428 (13) | 0.415 (13) | 0.714 (13) |
| Valine | 0.652 (10) | 1.140 (10) | 0.326 (10) | 0.570 (10) |
| Sum balanced EAA | 6.412 (100) | 11.018 (100) | 3.206 (100) | 5.509 (100) |
| Extra leucine | 0.000 | 2.886 | 0.000 | 1.443 |
| Sum leucine | 1.562 | 5.576 | 0.781 | 2.788 |
| Sum BCAA4 | 2.928 | 7.968 | 1.464 | 3.989 |
| Sum EAA | 6.412 | 13.904 | 3.206 | 6.952 |
| Sum NEAA5 | 7.000 | 0.000 | 3.487 | 0.000 |
| Sum amino acids | 13.412 | 13.904 | 6.693 | 6.952 |
| Maltodextrin | 30.000 | 30.000 | 15.000 | 15.000 |
Values are in gram (% of balanced EAA content).
TAA: total amino acid;
EAA: essential amino acid;
BCAA: branched-chain amino acid (includes isoleucine, leucine and valine);
NEAA: non-essential amino acid (includes alanine, arginine, aspartic acid, glutamic acid, glycine, proline, serine, and tyrosine). The amino acid mixtures were prepared, packaged, coded and analyzed by Ajinomoto Co., Inc.
2.5. Statistical analysis
Results were expressed as mean ± standard error. Population characteristics and baseline measurements were compared using either the unpaired Student’s t-test or Mann-Whitney test, depending on the distribution of the data. Calculations for the postabsorptive phase of the study were done using the median value for measurements taken at t= −30, −15 and 0 min prior to mixture intake. Postprandial phenylalanine kinetics were expressed as the 3 h area under the curve, derived from all postprandial measurements. For the within group comparison of postprandial phenylalanine kinetics we used either the paired Student’s t-test or Wilcoxon matched-pairs signed rank test. Computation of Pearson’s correlation coefficient and linear regression analysis was used for the comparison of PHE intake, dietary PHE plasma appearance, and change in PHE concentration with net protein gain. Two-way (Repeated) Measures Analysis Of Variance (two-way RM ANOVA) with “group” and “mixture”, or ”time” and “mixture” as factors were used to compare differences in plasma amino acid and insulin concentrations, and net protein gain between mixtures and/or groups and/or over time (during the 3 h postprandial period). We applied Bonferroni post hoc testing to evaluate within-time differences between mixtures. The level of significance was set at P<0.05, and Graphpad Prism (version 6.07) was used for data analysis.
3. Results
COPD patients had a significantly lower pulmonary function (FEV1 % of predicted) than healthy older adults (40±2 vs. 95±4, P<0.0001), and included n=3 GOLD II patients (albeit FEV1 < 60% of predicted), n=15 GOLD III patients, and n=5 GOLD IV patients. Diffusion capacity (DLCO) from the medical record was available for n=15 COPD patients, of which n=9 had a moderate to severe reduction in diffusing capacity (DLCO < 60% of predicted). As previously published [19], gender division, age (64±2 vs. 65±2 y), BMI (27.8±1.1 vs. 26.3±1.0 kg/m2), and fat-free mass index (17.7±0.6 vs. 17.0±0.5 kg/m2) were all similar between the controls and COPD patients. Furthermore, COPD patients varied in comorbidities and exacerbation frequency [19]. Fasting glucose (5.7±0.2 vs. 5.2±0.1 mmol/L), insulin (8.0± 1.2 vs. 8.7±1.1 μIU/mL), and HOMA-IR (1.16±0.16 vs. 1.22±0.15) were not different between the groups.
3.1. Plasma amino acid and insulin kinetics
Postabsorptive plasma concentration of the three branched-chain amino acids was significantly lower in COPD patients compared to controls (leucine: 71±2 vs. 95±3 μM, isoleucine: 33±1 vs. 44±2 μM, and valine: 141±5 vs. 192±8 μM, P<0.0001). As previously described [19], this contributed to an overall lower plasma EAA concentration in COPD patients.
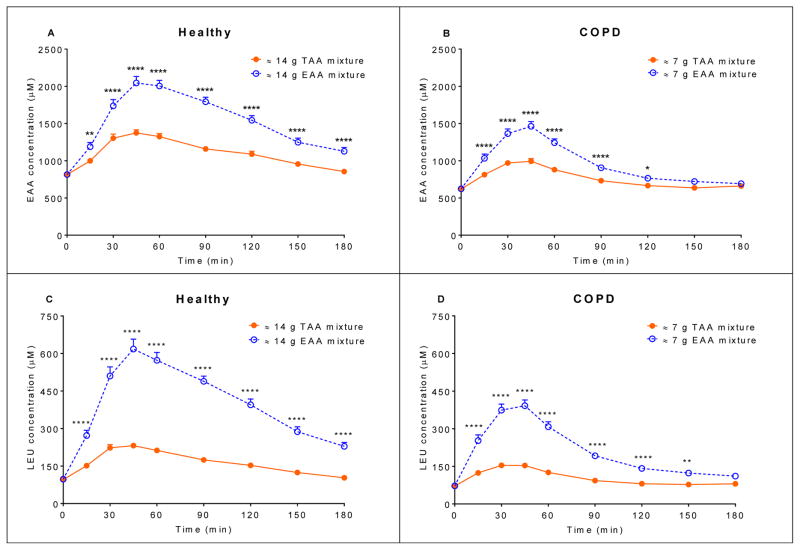
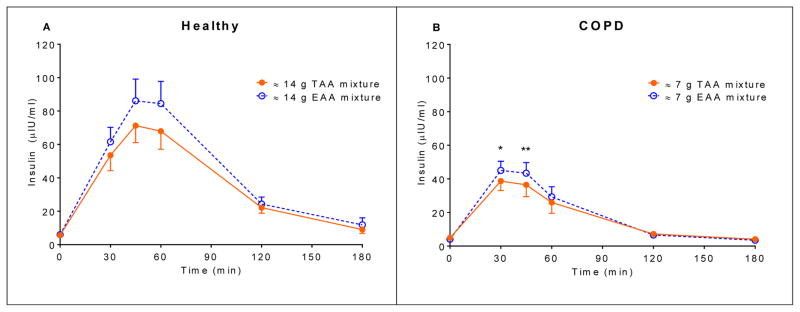
In both groups, postprandial plasma EAA and LEU concentrations (Figure 2) were significantly higher (P<0.0001) for the EAA mixture compared to the TAA mixture. Also, the insulin response for the EAA mixture (Figure 3) was significantly higher, albeit small, in both groups (healthy controls: P=0.0382, COPD patients: P=0.0285).
Figure 2.
Mean (± SE) (A) plasma EAA concentration after intake of the 14 g TAA and EAA mixtures in healthy controls (n=19) (B) plasma EAA concentration after intake of the 7 g TAA and EAA mixtures in COPD patients (n=23) (C) plasma LEU concentration after intake of the 14 g TAA and EAA mixtures in healthy controls (n=19) (D) plasma LEU concentration after intake of the 7 g TAA and EAA mixtures in COPD patients (n=23). Statistics were done using two-way repeated measures analysis of variance with “time” and “mixture” as factors used to compare differences between mixtures and over time. In both groups, and for the EAA as well as the LEU concentration: time effect, P<0.0001, mixture effect, P<0.0001, and time x mixture interaction, P<0.0001. EAA mixture significantly different from TAA mixture, ****P<0.0001, **P<0.01, *P<0.05. TAA mixture: total amino acid mixture (balanced mixture of EAA and non-EAA). EAA mixture: essential amino acid mixture (balanced mixture of EAA and additional LEU). EAA: essential amino acid. LEU: leucine.
Figure 3.
Mean (± SE) (A) plasma insulin concentration after intake of the 14 g TAA and EAA mixtures in healthy controls (n=19) (B) plasma insulin concentration after intake of the 7 g TAA and EAA mixtures in COPD patients (n=23). Statistics were done using two-way repeated measures analysis of variance with “time” and “mixture” as factors used to compare differences between mixtures and over time. Healthy controls: time effect, P<0.0001, mixture effect, P=0.0382, and no time x mixture interaction. COPD patients: time effect, P<0.0001, mixture effect, P=0.0290, and time x mixture interaction, P=0.0167. EAA mixture significantly different from TAA mixture, **P<0.01, *P<0.05. TAA mixture: total amino acid mixture (balanced mixture of EAA and non-EAA). EAA mixture: essential amino acid mixture (balanced mixture of EAA and additional LEU).
3.2. Postprandial whole body protein kinetics
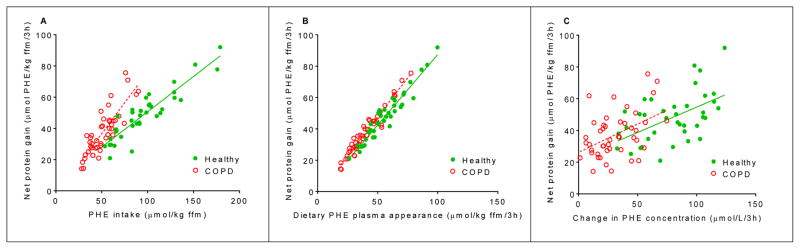
Data regarding plasma isotope enrichments can be found in Supplemental Figures 2 and 3. Postabsorptive values for whole body protein turnover did not differ between controls and COPD patients [19]. Figure 4A and Supplemental Figure 4 illustrate that in both groups there was an excellent positive linear relationship between the amount of PHE (r=0.84–0.91, P<0.001) and total EAAs (r=0.73–0.81, P<0.0001) consumed and net protein gain. Although the relationship between PHE intake and net protein gain differed between the groups, the relationship between the amounts of PHE that appeared from the mixture (dietary PHE plasma appearance) and net protein gain were similar (Figure 4B).
Figure 4.
Correlation after intake of the TAA and EAA mixtures between whole body net protein gain and (A) PHE intake, in healthy controls (n=19) and COPD patients (n=23). Healthy: net protein gain = 0.45 × + 5.42, r=0.91, P<0.0001. COPD: net protein gain = 0.79 × EAA intake – 3.1, r=0.85, P<0.0001. Slopes are significantly different between groups, P<0.0001. (B) Dietary PHE plasma appearance after splanchnic extraction, in healthy controls (n=19) and COPD patients (n=23). Healthy: net protein gain = 0.90 × EAA intake – 2.58, r=0.96, P<0.0001. COPD: net protein gain = 0.99 × EAA intake – 1.81, r=0.97, P<0.0001. Slopes are similar between groups. (C) Change in PHE concentration, in controls (n=19) and COPD patients (n=23). Healthy: net protein gain = 0.32 × EAA intake + 22.76, r=0.53, P=0.0006. COPD: net protein gain = 0.36 × EAA intake + 24.93, r=0.45, P=0.0007. Slopes are similar between groups. Statistics were done using linear regression analysis and computation of Pearson’s correlation coefficient. Net protein gain, dietary PHE plasma appearance and change in PHE concentration are expressed as the 3h postprandial area under the curve. TAA mixture: total amino acid mixture (balanced mixture of EAA and non-EAA). EAA mixture: essential amino acid mixture (balanced mixture of EAA and additional LEU). PHE: phenylalanine. Net protein gain = protein synthesis – protein breakdown.
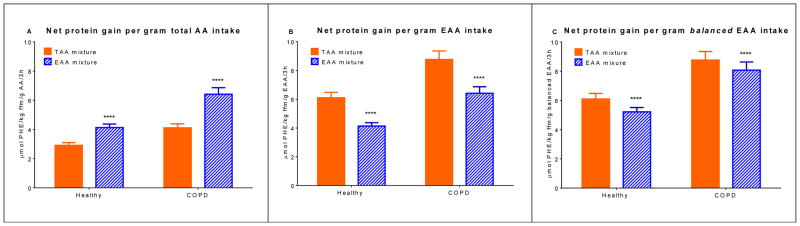
Taking into account the exact size of each mixture (Table 1), whole body net protein gain was 42% and 49% higher after intake of the EAA mixture compared to the TAA mixture in the control group and the COPD group, respectively (P<0.0001) (Figure 5A). Per gram of total amino acid intake, COPD patients had a higher net protein gain (i.e. efficiency) than controls for both mixtures (P<0.0001). Splanchnic extraction was about 40% lower in the COPD group for both mixtures (Table 2 and 3).
Figure 5.
Mean (± SE) whole body net protein gain after intake of the TAA and EAA mixtures in healthy controls (n=19) and COPD patients (n=23) (A) per gram total AA intake (B) per gram EAA intake (C) per gram balanced EAA intake (=EAA intake minus additional LEU). Statistics were done using two-way measures analysis of variance with “group” and “mixture” as factors used to compare differences between groups and mixtures. Net protein gain per gram total AA intake: group effect, P<0.0001, mixture effect, P<0.0001, and no group x mixture interaction. Net protein gain per gram EAA intake: group effect, P=0.0005, mixture effect, P<0.0001, and no group x mixture interaction. Net protein gain per gram balanced EAA intake: group effect, P=0.0004, mixture effect, P<0.0001, and no group x mixture interaction. Net protein gain is expressed as the 3h postprandial area under the curve. EAA mixture significantly different from TAA mixture, ****P<0.0001. TAA mixture: total amino acid mixture (balanced mixture of EAA and non-EAA). EAA mixture: essential amino acid mixture (balanced mixture of EAA and additional LEU). AA: amino acid. EAA: essential amino acid. PHE: phenylalanine. Net protein gain = protein synthesis – protein breakdown.
Table 2.
Whole body postprandial phenylalanine kinetics in healthy older adults1
| TAA mixture2 | EAA mixture3 | |
|---|---|---|
| Protein breakdown | 154 ± 5 | 158 ± 5 |
| Protein synthesis | 193 ± 6 | 215 ± 6**** |
| Hydroxylation | 19 ± 1 | 22 ± 1*** |
| Net protein gain | 39 ± 2 | 57 ± 3**** |
| Splanchnic extraction | 39 ± 2 | 41 ± 2 |
Values are mean ± SE. Values are expressed as the 3h postprandial area under the curve in μmol PHE/kg fat-free mass, except for splanchnic extraction which is expressed as a percentage.
TAA mixture: total amino acid mixture (balanced mixture of EAA and non-EAA);
EAA mixture: essential amino acid mixture (balanced mixture of EAA and additional LEU). Protein breakdown = endogenous phenylalanine rate of appearance. Protein synthesis = phenylalanine rate of appearance – phenylalanine hydroxylation. Net protein gain = protein synthesis – protein breakdown. Statistics were performed using either the paired Student’s t-test (if data were distributed normally) or Wilcoxon matched-pairs signed rank test. Significant difference between TAA and EAA mixture,
P<0.001,
P<0.0001.
Table 3.
Whole body postprandial phenylalanine kinetics in COPD patients1
| TAA mixture2 | EAA mixture3 | |
|---|---|---|
| Protein breakdown | 171 ± 6 | 164 ± 5** |
| Protein synthesis | 200 ± 7 | 209 ± 7** |
| Hydroxylation | 16 ± 1 | 17 ± 1** |
| Net protein gain | 29 ± 2 | 45 ± 3**** |
| Splanchnic extraction | 23 ± 3 | 22 ± 3 |
Values are mean ± SE. Values are expressed as the 3h postprandial area under the curve in μmol PHE/kg fat-free mass, except for splanchnic extraction which is expressed as a percentage.
TAA mixture: total amino acid mixture (balanced mixture of EAA and non-EAA);
EAA mixture: essential amino acid mixture (balanced mixture of EAA and additional LEU). Protein breakdown = endogenous phenylalanine rate of appearance. Protein synthesis = phenylalanine rate of appearance – phenylalanine hydroxylation. Net protein gain = protein synthesis – protein breakdown; Statistics were performed using either the paired Student’s t-test (if data were distributed normally) or Wilcoxon matched-pairs signed rank test. Significant difference between TAA and EAA mixture,
P<0.01,
P<0.0001.
In control subjects, the higher net protein gain after intake of the EAA mixture was caused by a greater increase in whole body protein synthesis (P<0.0001) (Table 2). In COPD patients this was explained by a greater increase in protein synthesis (P=0.0027), as well as a larger reduction in protein breakdown (P=0.0043) (Table 3).
Per gram EAA intake, the TAA mixture stimulated net protein gain to a greater extent than the EAA mixture (P<0.0001) (Figure 5B). Per gram balanced EAA intake, this difference was smaller, but still significantly lower for the EAA mixture (P<0.0001) (Figure 5C).
4. Discussion
In both healthy older adults and COPD patients, we found that a mixture with only EAAs and additional LEU caused a greater whole body net protein gain than a similar mixture with the amino acid composition of complete proteins. The favorable response to a mixture with EAAs only was supported by an excellent positive linear relationship between PHE (i.e. EAA) intake per kg fat-free mass and net protein gain, but could not be attributed to the high LEU content. COPD patients had an overall higher net protein gain per gram amino acids, associated with a reduced splanchnic extraction. However, based on the dietary PHE plasma appearance net protein gain was comparable to the healthy control group.
4.1. Whole body net protein gain
EAAs are primarily responsible for the stimulation of net muscle protein gain [11]. Although we did not measure specifically muscle protein turnover rates, a greater whole body net protein gain after intake of the EAA mixture is likely to result in a greater net muscle protein gain as well. Whether the extent of net muscle gain differs between healthy older adults and COPD patients due to potential disease related factors remains to be studied. The crucial role of dietary EAAs is further emphasized by the excellent positive linear relationship between EAA intake and whole body net protein gain, in agreement with our previous findings in COPD [5], Cystic Fibrosis [8], and lung cancer patients [9]. Although we used relatively small mixtures in our study, with an EAA content comparable to 7 and 15 g of whey protein, a recent study in healthy older adults showed the same linear relationship for EAA intake levels up to ~90 g [20]. It is yet to be determined whether specifically muscle protein follows the same pattern. However, that protein intake beyond the upper limit for muscle protein synthesis [20–22] in healthy older adults does not limit whole body net protein gain [20], suggests that net muscle protein anabolism above a certain protein intake level is driven by a reduction in muscle protein breakdown. Assuming a 4% contribution of PHE to protein [23], we can convert net protein gain from μmol PHE/kg fat-free mass to g protein/kg fat-free mass. With that, the intake of 1 g of the EAA mixture in comparison to the TAA mixture resulted in approx. 0.25 g greater net protein gain in the control group (0.6 g vs. 0.85 g protein/3h), and 0.43 g greater net protein gain in the COPD group (0.9 g vs.1.3 g protein/3h), for an average person with 50 kg fat-free mass. The higher efficiency in COPD patients as shown in Figure 4A was related to a lower splanchnic extraction.
4.2. Role of leucine
In the current study, the specific anabolic properties ascribed to LEU [12] did not translate into an improved whole body net protein gain in either healthy older adults or COPD patients. One could even argue that the added LEU was slightly counterproductive. Also, in our previous study in COPD [5] we observed that additional LEU when given in combination with hydrolyzed milk proteins did not change whole body protein turnover. From that study we can now conclude that it was not the presence of non-EAA that masked a potentially beneficial effect of LEU. Also, the small differences in digestion and/or absorption kinetics of the hydrolyzed proteins and free LEU were apparently not important. On the contrary, in another group of healthy older adults, it was the additional LEU that was needed in combination with an EAA mixture similar to ours to optimally stimulate skeletal muscle protein anabolism [13]. Thus, LEU may exert its effects specifically on skeletal muscle protein.
This being said, we are skeptical towards the additional use of LEU as a single free amino acid instead of the combined use of all EAAs to stimulate skeletal muscle protein anabolism, especially at greater intake levels. In support, a 21-day study in neonatal pigs showed that the use of more EAAs in the form of milk protein was better than the use of more LEU to achieve an increase in lean mass [24]. Furthermore, the available long-term intervention studies in humans that used LEU supplementation have not shown an increase in muscle mass [25, 26].
After we expressed net protein gain in this study per gram balanced EAAs (thereby correcting for the high LEU content), the EAA mixture still had a lower efficacy per gram EAAs. We first considered the possibility of a leucine-induced branched-chain amino acid antagonism where plasma valine and isoleucine concentrations fall below baseline values [27] and limit net protein gain. However, our data were unsupportive (Supplemental Figure 5), and thus the amounts of valine and isoleucine in the EAA mixture were sufficient to prevent a branched-chain amino acid antagonism. Possibly, there was a small anabolic effect of the non-EAAs that made up the difference in net protein gain per gram balanced EAAs between the EAA and TAA mixtures.
4.3. Splanchnic extraction of phenylalanine
We found a reduced splanchnic extraction of PHE in COPD patients. This is in line with our previous findings for PHE and other amino acids in this condition [6, 7], and similarly related to a higher net protein gain [6, 7]. The effect of the splanchnic area on protein turnover in COPD patients is intriguing, as weight loss and muscle atrophy are common phenomenon in COPD [2]. Possibly, there are more or higher catabolic events in between meals that negate the more positive protein balance following meals.
The lower splanchnic extraction of amino acids in COPD could be related to changes in the gut and/or liver. However, less extraction by the liver seems unlikely, as the inflammatory process in COPD stimulates the production of acute phase proteins [28, 29]. Use of inhaled corticosteroids, which was 78% in our COPD group, can significantly reduce the acute phase response [30], and may explain why we did not see an increase in the concentration of C-reactive protein in our study.
For several reasons we are inclined to think that the gut plays a crucial role in the altered splanchnic extraction in COPD patients. It was recently discovered that COPD patients are characterized by an increased permeability of the small intestine and colon [31], similar to heart failure patients [32]. Furthermore, dietary intake induced hypoxia [33] with episodes of intestinal oxygen deprivation, and “inflammatory organ cross-talk” [34] are potential mechanisms that could induce COPD related intestinal inflammation and damage. It is, however, still unknown whether this also indicates changes in amino acid absorption capacity. We could not find a difference in splanchnic extraction between COPD patients with or without supplemental oxygen (data not shown). Another factor that supports involvement of the gut, is the presence of metabolic acidosis in COPD due to the development of stable hypercapnia [35]. Metabolic acidosis is a process known to reduce protein turnover in the splanchnic area [36]. Finally, all COPD patients except for three in this study used inhalation beta-agonists as a maintenance medication. Use of beta-agonists is known to reduce gut mass in favor of increased muscle anabolism [37–39], and in that sense may even provide COPD patients some degree of protection from muscle atrophy. It is clear that more COPD research is needed aimed at the intrinsic factors that contribute to alterations in splanchnic amino acid extraction and an enhanced net anabolic response to feeding.
4.4. Study limitations
The measurement of blood gasses could potentially have given us more insight in the relation between hypoxia, metabolic acidosis and splanchnic extraction. Secondly, the comparison of data regarding whole body protein kinetics between the healthy controls and COPD patients would have been facilitated by the use of mixtures similar in size. The greater load of dietary amino acids in controls resulted in a less complete absorption of dietary PHE after 3 h (Supplemental Figure 2D and 3D), and therefore to some extent this contributed to a higher value for splanchnic extraction in this group. However, as our previous studies have also shown that splanchnic extraction of amino acids is lower in COPD patients [6, 7], we expect that our current findings are not primarily due to methodological issues. Based on our comparable studies in cystic fibrosis [8] and lung cancer [9], the number of subjects included in this study was greater than the number required. However, with a group of 23 COPD patients we were able to perform within group comparisons for other previously published primary outcomes [19].
In conclusion, our data indicate that a more optimal stimulation of whole body protein anabolism in COPD patients can be achieved by supplements that contain a balanced mixture of EAAs only, instead of both EAAs and non-EAAs. As supplements often become replacements due to their effect on satiety, supplements with only EAA may be a more energy efficient means to promote muscle gain in COPD patients.
Supplementary Material
Acknowledgments
Source of Support
The project described was supported by grants UL1RR029884, R01HL095903, and S10RR027047 through the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of abbreviations
- COPD
chronic obstructive pulmonary disease
- EAA
essential amino acid
- PHE
phenylalanine
- TAA
total amino acid
Footnotes
Author contributions: None of the authors had any financial or personal interest in any company or organization sponsoring the research, including advisory board affiliations. RJ, NEPD and MKPJE had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. NEPD and MPKJE designed the research and RJ, NEPD and MPKJE were involved in the conduct of the research, data analysis and writing of the manuscript. MLE and PJA were involved in the recruitment of study participants. Other contributions: We thank chief analytical chemist John Thaden, PhD, for sample analysis, and acknowledge Ajinomoto Co., Inc. for providing the amino acid mixtures.
Conflict of interest
None of the authors had any financial or personal interest in any company or organization sponsoring the research, including advisory board affiliations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 1998;157:1791–7. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. American journal of respiratory and critical care medicine. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 3.Waschki B, Kirsten A, Holz O, Muller KC, Meyer T, Watz H, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331–42. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 4.Dal Negro RW, Testa A, Aquilani R, Tognella S, Pasini E, Barbieri A, et al. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace / Fondazione clinica del lavoro. Vol. 77. IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio, Universita di Napoli; Secondo ateneo: 2012. Essential amino acid supplementation in patients with severe COPD: a step towards home rehabilitation; pp. 67–75. [DOI] [PubMed] [Google Scholar]
- 5.Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin Nutr. 2014;33:211–20. doi: 10.1016/j.clnu.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelen MP, De Castro CL, Rutten EP, Wouters EF, Schols AM, Deutz NE. Enhanced anabolic response to milk protein sip feeding in elderly subjects with COPD is associated with a reduced splanchnic extraction of multiple amino acids. Clin Nutr. 2012;31:616–24. doi: 10.1016/j.clnu.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Altered interorgan response to feeding in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:366–72. doi: 10.1093/ajcn.82.2.366. [DOI] [PubMed] [Google Scholar]
- 8.Engelen MP, Com G, Wolfe RR, Deutz NE. Dietary essential amino acids are highly anabolic in pediatric patients with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2013;12:445–53. doi: 10.1016/j.jcf.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelen MP, Safar AM, Bartter T, Koeman F, Deutz NE. High anabolic potential of essential amino acid mixtures in advanced nonsmall cell lung cancer. Ann Oncol. 2015;26:1960–6. doi: 10.1093/annonc/mdv271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Experimental gerontology. 2006;41:215–9. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino acids. 2010;38:1533–9. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 13.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 14.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007;85:431–9. doi: 10.1093/ajcn/85.2.431. [DOI] [PubMed] [Google Scholar]
- 15.Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. 2016 Available from: http://www.goldcopd.org/
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 17.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30:936–40. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 18.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Alterations in whole-body arginine metabolism in chronic obstructive pulmonary disease. Am J Clin Nutr. 2016;103:1458–64. doi: 10.3945/ajcn.115.125187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, et al. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab. 2015;308:E21–8. doi: 10.1152/ajpendo.00382.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109:1582–6. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70:57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 23.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol. 1995;268:E75–84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 24.Columbus DA, Steinhoff-Wagner J, Suryawan A, Nguyen HV, Hernandez-Garcia A, Fiorotto ML, et al. Impact of prolonged leucine supplementation on protein synthesis and lean growth in neonatal pigs. Am J Physiol Endocrinol Metab. 2015;309:E601–10. doi: 10.1152/ajpendo.00089.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, et al. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr. 2011;141:1070–6. doi: 10.3945/jn.111.138495. [DOI] [PubMed] [Google Scholar]
- 26.Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–75. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- 27.Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. American Journal of Clinical Nutrition. 2014;99:276–86. doi: 10.3945/ajcn.113.068775. [DOI] [PubMed] [Google Scholar]
- 28.Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto-Plata VM, Mullerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61:23–8. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sin DD, Lacy P, York E, Man SFP. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2004;170:760–5. doi: 10.1164/rccm.200404-543OC. [DOI] [PubMed] [Google Scholar]
- 31.Rutten EPA, Lenaerts K, Buurman WA, Wouters EFM. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145:245–52. doi: 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- 32.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered Intestinal Function in Patients With Chronic Heart Failure. Journal of the American College of Cardiology. 2007;50:1561–9. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins S, Cecins N. Six-minute walk test: observed adverse events and oxygen desaturation in a large cohort of patients with chronic lung disease. Internal medicine journal. 2011;41:416–22. doi: 10.1111/j.1445-5994.2010.02169.x. [DOI] [PubMed] [Google Scholar]
- 34.Keely S, Hansbro PM. Lung-gut cross talk: a potential mechanism for intestinal dysfunction in patients with COPD. Chest. 2014;145:199–200. doi: 10.1378/chest.13-2077. [DOI] [PubMed] [Google Scholar]
- 35.Bruno CM, Valenti M. Acid-base disorders in patients with chronic obstructive pulmonary disease: a pathophysiological review. J Biomed Biotechnol. 2012;2012:915150. doi: 10.1155/2012/915150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tessari P, Sofia A, Saffioti S, Vettore M, Verzola D, Millioni R, et al. Effects of chronic metabolic acidosis on splanchnic protein turnover and oxygen consumption in human beings. Gastroenterology. 2010;138:1557–65. doi: 10.1053/j.gastro.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 37.MacRae JC, Lobley GE. Physiological and metabolic implications of conventional and novel methods for the manipulation of growth and production. Livestock Production Science. 1991;27:43–59. [Google Scholar]
- 38.Caine WR, Mathison GW. Influence of the β-adrenergic agonist cimaterol on body composition and whole body synthesis and degradation of protein in growing lambs. Canadian Journal of Animal Science. 1992;72:569–87. [Google Scholar]
- 39.Bracher-Jakob A, Blum JW. Effects of a β-andrenergic agonist on growth performance, body composition and nutrient retention in finishing pigs fed normal or low amounts of protein. Animal Production. 2010;51:601–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.