Abstract
OBJECTIVES
To test a new cognitive behavioral therapy for insomnia (CBT-I) program designed for use by nonclinicians.
DESIGN
Randomized controlled trial.
SETTING
Department of Veterans Affairs healthcare system.
PARTICIPANTS
Community-dwelling veterans aged 60 and older who met diagnostic criteria for insomnia of 3 months duration or longer (N = 159).
INTERVENTION
Nonclinician “sleep coaches” delivered a five-session manual-based CBT-I program including stimulus control, sleep restriction, sleep hygiene, and cognitive therapy (individually or in small groups), with weekly telephone behavioral sleep medicine supervision. Controls received five sessions of general sleep education.
MEASUREMENTS
Primary outcomes, including self-reported (7-day sleep diary) sleep onset latency (SOL-D), wake after sleep onset (WASO-D), total wake time (TWT-D), and sleep efficiency (SE-D); Pittsburgh Sleep Quality Index (PSQI); and objective sleep efficiency (7-day wrist actigraphy, SE-A) were measured at baseline, at the posttreatment assessment, and at 6- and 12-month follow-up. Additional measures included the Insomnia Severity Index (ISI), depressive symptoms (Patient Health Questionnaire-9 (PHQ-9)), and quality of life (Medical Outcomes Study 12-item Short-form Survey version 2 (SF-12v2)).
RESULTS
Intervention subjects had greater improvement than controls between the baseline and posttreatment assessments, the baseline and 6-month assessments, and the baseline and 12-month assessments in SOL-D (−23.4, −15.8, and −17.3 minutes, respectively), TWT-D (−68.4, −37.0, and −30.9 minutes, respectively), SE-D (10.5%, 6.7%, and 5.4%, respectively), PSQI (−3.4, −2.4, and −2.1 in total score, respectively), and ISI (−4.5, −3.9, and −2.8 in total score, respectively) (all P < .05). There were no significant differences in SE-A, PHQ-9, or SF-12v2.
CONCLUSION
Manual-based CBT-I delivered by nonclinician sleep coaches improves sleep in older adults with chronic insomnia.
Keywords: insomnia, sleep, cognitive behavioral therapy, aged, randomized controlled trial
Insomnia is the most common sleep problem in adults, with 30% to 50% reporting symptoms.1 Insomnia is particularly problematic in older adults, in whom it is often chronic and comorbid with other health conditions. 2,3 Insomnia and sleep disturbance are associated with poor health,4 including greater risk of depression,5 falls,6 stroke,7 cognitive decline,8 and impaired functional status.9 Older adults are more likely than younger adults to take sedative–hypnotics,10 despite evidence that these agents increase falls,11,12 fractures,13 healthcare costs,12 and mortality.14
Behavioral sleep interventions, such as cognitive behavioral therapy for insomnia (CBT-I), are highly effective in treating chronic insomnia in older adults,15,16 including individuals with selected comorbid medical and psychiatric conditions.17 CBT-I typically combines stimulus control (associating the bed and bedroom with sleep), sleep restriction (increasing the homeostatic drive to sleep and reducing time in bed) and cognitive therapy (addressing maladaptive beliefs about sleep and reducing anxiety about sleep and the consequences of not sleeping).15 Sleep hygiene (establishing behavioral routines to promote restorative sleep) and other components may be included. Improvements in sleep with CBT-I are equal or greater in magnitude and more durable than improvements seen with sedative–hypnotics.18
Treatment guidelines universally recommend behavioral therapies as first-line treatment for insomnia in older adults,3,19 but these treatments have not been widely implemented. Many primary care providers do not use behavioral therapies because of time constraints, cost, and perceived difficulty motivating people to see a therapist.20 Limited access to behavioral sleep medicine (BSM) specialists is also a hurdle to widespread implementation. Alternative approaches have been developed, such as brief treatment programs,21 self-help manuals,22 video educational programs,23 and online approaches,24 but uptake of these approaches in primary care has also been limited, particularly in older adults and those with comorbidities. A new, manual-based CBT-I program designed to be delivered by nonclinicians with BSM telephone supervision was developed and tested to address this. This model of CBT-I was tested in older veterans with chronic insomnia disorder based on established diagnostic criteria.25 It was hypothesized that sleep outcomes would improve more at 6-month follow-up in the intervention group than in controls and that these improvements would be maintained for up to 12 months.
METHODS
Trial Design
This study was a randomized controlled trial comparing CBT-I provided by nonclinicians in a small-group or individual (one-on-one) format, with weekly telephone supervision by a BSM specialist, with a general sleep education control condition delivered in a group format by a similar nonclinician health educator. Recognizing that the intervention might be implemented in the future in group or individual settings, both formats were tested. Eligible subjects were community-dwelling veterans aged 60 and older with chronic (≥3 months’ duration) insomnia disorder (based on International Classification of Sleep Disorders, Second Edition (ICSD-2) criteria).25 Eligible subjects were randomized 1:1:1 to group CBT-I, individual CBT-I, or the control condition. Group and individual CBT-I were pooled to form the intervention group for analyses. Objective and subjective measures of sleep were collected at baseline, the posttreatment assessment, and 6- and 12-month follow-up. Subject recruitment and randomization ran from June 2010 to January 2012; follow-up testing ran from September 2010 to March 2013. The institutional review board of the Veterans Affairs (VA) Greater Los Angeles Healthcare System approved the study procedures, and written informed consent was obtained from all subjects (ClinicalTrials.gov Identifier: NCT00781963).
Subjects
The VA national data warehouse was used to identify veterans with at least one outpatient visit at one urban VA healthcare system in the prior 18 months and lived within 30 miles of the facility. A 25-item postal survey that operationalized ICSD-2 criteria for insomnia disorder was mailed. Surveys were mailed over a 20-month enrollment period. A second postal survey was mailed to nonresponders. Of 9,080 veterans mailed a survey, 4,717 (51.9%) returned a completed survey (mean respondent age 74.1, range 60–100, 98.2% male, 78.6% non-Hispanic white), 2,461 (52.2%) of whom provided responses that met ICSD-2 diagnostic criteria for insomnia disorder with symptoms present for 3 months or longer. Of these, 1,947 (79.1%) indicated on their returned survey that they were willing to have the research team contact them, and 1,663 of these were assessed for eligibility for the study in a screening telephone call (221 could not be reached, 33 responded after study enrollment had ended, and 30 refused).
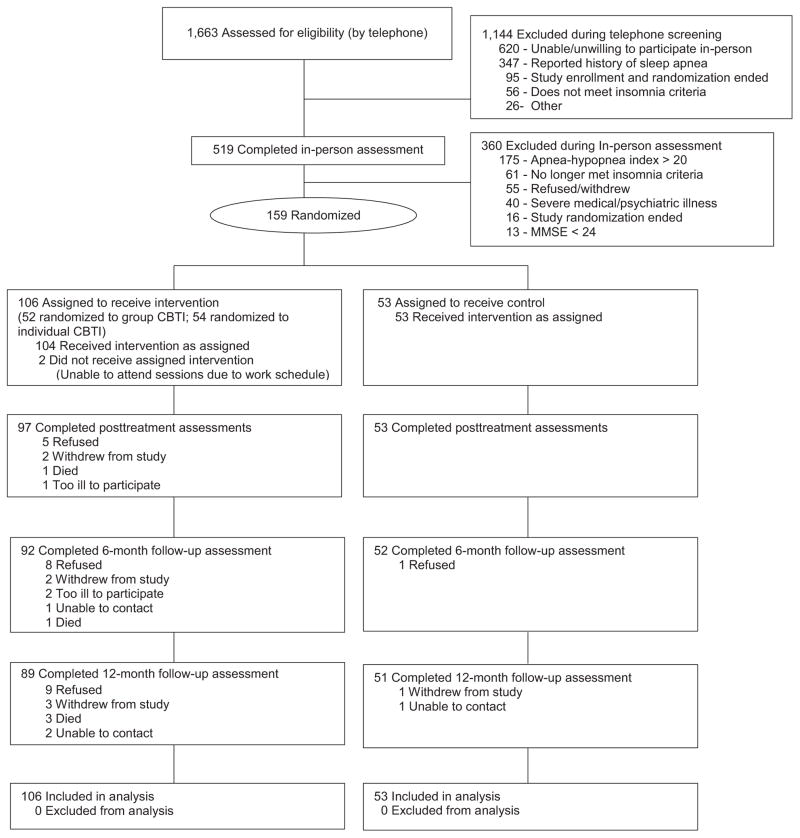
The figure shows the Consolidated Standards of Reporting Trials26 flow diagram of the 1,663 individuals assessed for eligibility using a multistep process over the telephone followed by in-person assessment. Subjects were excluded if their Mini-mental State Examination (MMSE)27 score was less than 24, they reported a history of sleep apnea or in-home portable sleep monitoring (using WatchPAT 200, Itamar Medical, Ltd, Caesarea, Israel) performed during assessment estimated an apnea-hypopnea index (AHI) greater than 20 (because the intervention did not address sleep apnea), or they had a severe unstable medical disorder (e.g., <6-month life expectancy) or active severe mental disorder (e.g., current active substance abuse, psychiatric hospitalization within the past 90 days, documented bipolar disorder). Trained research staff at a VA healthcare system collected all data.
Randomization
Subjects meeting eligibility criteria were randomized (using random allocation concealment) to one of three treatment groups (1:1:1 allocation to group CBT-I, individual CBT-I, or control), so two-thirds of subjects were randomized to intervention and one-third to control. Before the study began, a statistician (MM) created a randomization sequence using Stata version 13.1 (Stata Corp., College Station, TX), stratified according to nighttime sleep efficiency (<80%, ≥80%) estimated from the wrist actigraph in the WatchPAT device. A separate senior research staff member (KR) not involved in subject enrollment, assessment, or intervention prepared the opaque sequentially numbered envelopes and implemented the random allocation sequence. Subjects and assessment research staff were blinded to group assignment.
Intervention and Control Conditions
The CBT-I intervention was structured and manual based, with hard-copy materials provided to subjects, and delivered individually or in small groups of three to five subjects. Content included training in aspects of stimulus control, sleep restriction, cognitive therapy, and sleep hygiene, as defined above. The intervention was provided in five 1-hour sessions over 6 weeks (with a brief telephone check-in during Week 5). Specific content included (Session 1) sleep restriction and stimulus control, (Session 2) sleep hygiene and adjustment of sleep restriction parameters, (Session 3) cognitive therapy and adjustment of sleep restriction parameters, (Session 4) review of prior material and adjustment of sleep restriction parameters, and (Session 5) relapse prevention.
One of three nonclinicians (sleep coaches) with a master’s degree (public health, social work, or communication) delivered the intervention. Training of the sleep coaches included attendance at a 2-day educational workshop on CBT-I or completion of a six-session webinar published by the American Academy of Sleep Medicine.28 A clinical sleep psychologist investigator (JM) provided additional training on the study intervention materials and observed each sleep coach provide the intervention in a small number of pilot subjects. During the intervention period, a psychologist with BSM expertise (LF or JM) supervised the sleep coach weekly in a telephone consultation (one telephone call lasting 60–90 minutes) to discuss all currently active intervention subjects, review subject progress, and help solve problems with adherence. Fidelity of the intervention was monitored during these telephone consultations, and session content checklists (that the sleep coach completed during each intervention session) were reviewed to verify that topics had been covered as outlined in the treatment manual. Subjects in the group and individual active intervention conditions received identical intervention materials.
The control condition was a structured, manual-based, general sleep education program delivered in group format at the same frequency and intervals as the intervention condition to account for social attention and to encourage subject retention. A separate master’s-level nonclinician (with a master’s degree in public health) without CBT-I training delivered the control condition.
Subject Characteristics
Information on descriptive characteristics (e.g., age, sex, race and ethnicity, educational level, marital status, employment status) was collected at baseline. Self-reported comorbidity was recorded as number of health conditions endorsed (from a list of 36 common medical and psychiatric disorders).29 The seven-item pain intensity subscale of the Geriatric Pain Measure (GPM) was also administered. 30 All medication use (prescription and over the counter) was recorded for 1 week at each timepoint, and the total number of medications taken (excluding vitamins and other supplements) was calculated. Whether the subject took prescription medications commonly used for insomnia or other sedative medications, based on previously published definitions,10 was also recorded. As mentioned above, research staff also administered the MMSE and WatchPAT was used to estimate AHI.
Outcome Measures
Research staff blinded to group assignment, study research questions, and content of the intervention and control conditions collected information on outcome measures at baseline, within 1 week posttreatment, and at 6- and 12-month follow-up (from the date of randomization).
Primary sleep outcomes were measured using a sleep diary, wrist actigraphy, and sleep questionnaire. Subjects completed a 7-day sleep diary including bedtime, nighttime awakenings, rise time, and other items. Primary sleep diary variables included sleep onset latency (SOL-D (amount of time it takes to fall asleep)), wake after sleep onset (WASO-D), total wake time (TWT-D), and sleep efficiency (SE-D, time asleep divided by time in bed). Wrist actigraphy (Actiwatch Spectrum, Respironics) was performed during the same week as the sleep diary to estimate nighttime sleep efficiency (SE-A).31 The primary sleep questionnaire measure of subjective sleep quality was the Pittsburgh Sleep Quality Index (PSQI, total score 0–21; higher scores indicate worse sleep quality).32 The Insomnia Severity Index (ISI, total score 0–28; higher scores indicate worse insomnia severity) was included as a secondary measure of insomnia symptoms.33
Additional measures included the Patient Health Questionnaire-9 (PHQ-9, a 9-item scale, total score 0–27; higher scores indicate more-severe depressive symptoms)34 and the Medical Outcomes Study 12-item Short-Form Health Survey version 2 (SF-12v2)35 Mental Component Summary (MCS) and Physical Component Summary (PCS) scales (total score 0–100; higher scores indicate better functioning).
At the end of treatment, intervention and control subjects rated the program they received on four credibility items (total score for each item 0–6, higher scores indicate greater credibility).36 The items assessed how logical, successful, and acceptable they found the program and how confident they were that they would recommend the program to someone else.
Statistical Analysis
Baseline differences between the treatment and control groups were assessed using two-sample t-tests for continuous variables and chi-square tests for categorical variables.
The primary hypothesis was that sleep outcomes would show greater improvement from baseline to 6-month follow-up for the intervention group than for controls. The a priori primary sleep outcomes of interest were sleep diary SOL-D, WASO-D, and SE-D; SE-A; and PSQI total score. Secondary hypotheses were that sleep outcomes would show greater improvement in the intervention group than in controls from baseline to the posttreatment assessment and from baseline to the 12-month follow-up. The primary and secondary hypotheses were also assessed for the secondary outcomes (ISI, PHQ9, MCS, PCS) at these time points.
Mixed-effects models were used to test the hypotheses regarding difference in change in sleep outcomes between the intervention and control groups. When applied to repeated-measures designs, mixed-effects models accommodate incomplete data across time points (such as occurs with dropout or data missing at a particular time point) and can permit specification of a wide variety of residual covariance structures.37 Each outcome was analyzed using a two by four factorial mixed-effects model with a fixed intercept in which treatment group was a two-level between-subjects factor (intervention vs control), and time was a four-level repeated-measures factor (baseline, posttreatment, 6 months, 12 months). For each outcome, the best-fitting (based on the Bayesian information criterion) residual covariance structure was specified (unstructured residual covariance matrix for SOL-D, WASO-D, TWT-D, SE-D; compound symmetry for SE-A, PSQI, ISI, PHQ9, MCS, PCS).
The primary hypotheses were tested using an interaction contrast that compared change in outcome from baseline to 6 months in the treatment group with change in the control group. The intervention effect was estimated as the change in the intervention group from baseline to 6 months minus the change in the control group from baseline to 6 months, and a 95% confidence interval for this effect was computed. The intervention effect was also estimated using a variation of Cohen’s d formulated for a pretest-posttest-control (PPC) design that forms a standardized measure of the difference in the change (from baseline to follow-up) between the intervention group and the control group.38 The secondary hypotheses were tested in a similar manner, but focusing on the change from baseline to posttreatment and from baseline to 12 months.
Sample size calculations performed before the start of the study established that a total sample size of 150 subjects (using α = 0.05 and power = 80%) would be adequate to test for differences between groups (intervention vs control) in outcome variables. The study was not designed to compare individual with group CBT-I, and because there were not significant differences between individual and group CBT-I in primary outcomes, subjects who received individual and group CBT-I were pooled to form the intervention group.
All outcomes were assessed using intention-to-treat analyses. Systematic attrition was tested for using two-sample t-tests to compare the baseline characteristics for each outcome variable of those who did and did not have complete data at each follow-up time point. No such tests were significant (P > .05), indicating no evidence of systematic attrition.
Data preparation and data analyses were performed using Stata version 13.1. The linear mixed models were fit using the Stata mixed command. All significance tests were two-tailed and tested using α = 0.05. No adjustments were made for multiple tests, so the tests with regard to the secondary hypotheses and secondary outcomes should be considered exploratory.
RESULTS
Of 1,663 postal survey respondents screened for study eligibility over the telephone, 519 (31.9%) completed in-person eligibility screening, and 159 were randomized to intervention (n = 106; n = 52 group CBT-I, n = 54 individual CBT-I) or control (n = 53, Figure 1).
Figure 1.
Participant flow in the study.
Table 1 provides information on baseline demographic characteristics, sleep measures, and other characteristics of randomized subjects. Subjects were predominantly non-Hispanic white men with a mean age of 72.2 (range 60–91) and reported an average of six comorbidities. More than 90% of subjects reported that their sleep problems had been present for longer than 12 months. There were no significant differences in these characteristics between the intervention and control groups at baseline.
Table 1.
Baseline Demographic, Sleep, and Other Characteristics of Randomized Subjects
| Variable | Overall, N = 159 | Intervention, n = 106 | Control, n = 53 |
|---|---|---|---|
| Demographic | |||
| Age, mean±SD | 72.2 ± 7.7 | 72.1 ± 7.9 | 72.4 ± 7.3 |
| Male, n (%) | 154 (96.9) | 102 (96.2) | 52 (98.1) |
| Race and ethnicity, n (%)a | |||
| Hispanic | 10 (6.3) | 7 (6.6) | 3 (5.7) |
| Black | 7 (4.4) | 6 (5.7) | 1 (1.9) |
| White | 125 (78.6) | 83 (78.3) | 42 (79.2) |
| Other | 12 (7.6) | 7 (6.6) | 5 (9.4) |
| No response | 5 (3.1) | 3 (2.8) | 2 (3.8) |
| Education | |||
| <High school | 6 (3.8) | 6 (5.7) | 0 (0.0) |
| High school graduate | 25 (15.7) | 18 (17.0) | 7 (13.2) |
| Some college | 70 (44.0) | 44 (41.5) | 26 (49.1) |
| College graduate | 30 (18.9) | 18 (17.0) | 12 (22.6) |
| Postbaccalaureate | 28 (17.6) | 20 (18.9) | 8 (15.1) |
| Marital status | |||
| Married | 66 (41.5) | 43 (40.6) | 23 (43.4) |
| Living as married | 11 (6.9) | 8 (7.5) | 3 (5.7) |
| Divorced, separated | 48 (30.2) | 34 (32.1) | 14 (26.4) |
| Widowed | 14 (8.8) | 10 (9.4) | 4 (7.5) |
| Single, never married | 20 (12.6) | 11 (10.4) | 9 (17.0) |
| Employment | |||
| Not working | 121 (76.1) | 81 (76.4) | 40 (75.5) |
| Working part time | 31 (19.5) | 19 (17.9) | 12 (22.6) |
| Working full time | 7 (4.4) | 6 (5.7) | 1 (1.9) |
| Sleep measures | |||
| From sleep diary, mean ± SD | |||
| Sleep onset latency, minutes | 41.4 ± 42.4 | 43.3 ± 47.9 | 37.5 ± 28.5 |
| Wake after sleep onset, minutes | 56.6 ± 49.0 | 55.8 ± 40.3 | 58.1 ± 63.3 |
| Total wake time, minutes | 143.2 ± 88.9 | 144.0 ± 89.5 | 141.6 ± 88.5 |
| Sleep efficiency, % | 72.3 ± 15.4 | 72.0 ± 14.8 | 72.8 ± 16.8 |
| Sleep efficiency from actigraphy, %, mean±SD | 83.4 ± 6.3 | 83.7 ± 6.1 | 82.8 ± 6.9 |
| Pittsburgh Sleep Quality Index, total score, mean±SD | 9.1 ± 3.4 | 9.4 ± 3.5 | 8.3 ± 3.2 |
| Duration of sleep problems > 12 months, n (%) | 144 (90.6) | 96 (90.6) | 48 (90.6) |
| Insomnia Severity Index, total score, mean±SD | 11.1 ± 5.3 | 11.7 ± 5.3 | 10.1 ± 5.3 |
| Apnea–hypopnea index, mean±SD | 9.4 ± 5.3 | 9.9 ± 5.4 | 8.3 ± 5.1 |
| Other measures, mean±SD | |||
| Comorbidity indexb | 6.6 ± 3.4 | 7.0 ± 3.5 | 6.0 ± 3.0 |
| Geriatric Pain Measure 7-item pain intensity subscale | 14.8 ± 11.5 | 15.0 ± 11.6 | 14.3 ± 11.3 |
| Patient Health Questionnaire-9 score | 4.8 ± 4.3 | 5.1 ± 4.3 | 4.4 ± 4.3 |
| Medical Outcomes Study 12-item Short-form Survey v2 | |||
| Mental Component Summary | 52.8 ± 9.5 | 52.9 ± 10.0 | 52.6 ± 8.3 |
| Physical Component Summary | 45.3 ± 10.7 | 44.2 ± 10.9 | 47.6 ± 10.3 |
SD = standard deviation.
Participants were asked to select all that applied.
Total number of self-reported health conditions endorsed.
Attendance and Attrition
Attendance at the five intervention or control sessions was 100% for individual CBT-I subjects, 85% for group CBT-I subjects, and 100% for control subjects. At the conclusion of treatment, the range of mean responses to the credibility items of intervention and control subjects was 4.6–5.8 (on a 6-point scale, with higher scores indicating better credibility). Control subjects reported lower program credibility than intervention subjects (all P < .05), although the largest difference in mean credibility between the intervention and control groups on an item was only 0.8. No harms or unintended effects were identified in either group.
Outcomes
Table 2 presents the mean values and 95% confidence intervals for sleep and other measures at each time point (baseline, posttreatment, 6- and 12-month follow-up) for the intervention and control groups. Table 3 presents the results of the repeated-measures analysis comparing change in sleep outcomes in the intervention and control groups from baseline to posttreatment, from baseline to 6 months, and from baseline to 12 months. For example, decrease in time to fall asleep from baseline to the posttreatment assessment was 23.4 minutes greater for the intervention than the control group (SOL-D, P < .001), decrease in time awake once they fell asleep was 17.7 minutes greater (WASO-D, P = .01), decrease in time awake throughout the night was 68.4 minutes greater (TWT-D, P < .001), and sleep efficiency improved 10.5% more (SE-D, P < .001). At 6 months (the primary outcome time point), the intervention group showed significantly greater improvements in SOL-D (P = .02), TWT-D (P = .004), and SE-D (P = .005) than controls but not in WASO-D (P = .21). At 12 months, treatment effects remained significant for SOL-D, TWT-D, and SE-D (all P < .05). The effect sizes for the significant treatment effects in sleep diary measures ranged from 0.34 to 0.76. In contrast, there was no significant treatment effect for SE-A at any time point.
Table 2.
Sleep and Other Outcome Measures at Each Time Point According to Treatment Group (n = 106 Intervention, n = 53 Control)
| Outcome | Mean (95% Confidence Interval) | |||
|---|---|---|---|---|
| Baseline Assessment | Posttreatment Assessment | 6-Month Assessment | 12-Month Assessment | |
| According to sleep diary | ||||
| Sleep onset latency, minutes | ||||
| Intervention | 43.3 (35.3–51.3) | 18.7 (14.7–22.7) | 21.3 (16.8–25.8) | 24.0 (18.3–29.7) |
| Control | 37.5 (26.2–48.9) | 36.3 (31.0–41.7) | 31.3 (25.4–37.2) | 35.5 (28.2–42.9) |
| Wake after sleep onset, minutes | ||||
| Intervention | 55.8 (46.5–65.1) | 24.7 (19.1–30.3) | 30.3 (22.9–37.7) | 34.5 (27.7–41.2) |
| Control | 58.1 (45.0–71.3) | 44.7 (37.0–52.4) | 42.8 (33.0–52.7) | 38.9 (30.3–47.4) |
| Total wake time at night, minutes | ||||
| Intervention | 144.0 (127.1–160.9) | 59.1 (48.9–69.3) | 73.4 (61.4–85.4) | 85.9 (72.8–99.0) |
| Control | 141.6 (117.7–165.5) | 125.1 (111.2–138.9) | 108.0 (91.8–124.1) | 114.4 (97.3–131.5) |
| Sleep efficiency, % | ||||
| Intervention | 72.0 (69.0–74.9) | 85.9 (83.8–88.0) | 84.8 (82.6–87.1) | 82.5 (80.1–84.8) |
| Control | 72.8 (68.7–76.9) | 76.2 (73.4–79.1) | 79.0 (76.0–82.0) | 77.8 (74.7–80.9) |
| Sleep efficiency according to actigraphy, % | ||||
| Intervention | 83.7 (82.4–85.1) | 84.7 (83.4–86.1) | 82.7 (81.3–84.1) | 82.4 (81.0–83.8) |
| Control | 82.8 (80.9–84.7) | 82.5 (80.6–84.4) | 83.1 (81.2–85.0) | 82.6 (80.7–84.5) |
| Pittsburgh Sleep Quality Index, total score | ||||
| Intervention | 9.4 (8.8–10.1) | 5.5 (4.8–6.2) | 6.0 (5.3–6.7) | 6.5 (5.8–7.2) |
| Control | 8.3 (7.3–9.2) | 7.7 (6.7–8.6) | 7.2 (6.2–8.2) | 7.5 (6.5–8.5) |
| Insomnia Severity Index, total score | ||||
| Intervention | 11.7 (10.7–12.7) | 6.0 (5.0–7.0) | 5.5 (4.4–6.5) | 6.5 (5.4–7.5) |
| Control | 10.1 (8.7–11.5) | 8.9 (7.5–10.3) | 7.8 (6.4–9.2) | 7.7 (6.3–9.1) |
| Patient Health Questionnaire-9, total score | ||||
| Intervention | 5.1 (4.3–5.8) | 3.1 (2.3–3.9) | 3.0 (2.2–3.8) | 3.4 (2.6–4.2) |
| Control | 4.4 (3.3–5.4) | 2.9 (1.9–4.0) | 3.3 (2.2–4.4) | 2.6 (1.5–3.7) |
| Mental Outcomes Study 12-item Short-Form Study | ||||
| Mental Component Summary | ||||
| Intervention | 52.9 (51.1–54.7) | 52.6 (50.7–54.4) | 53.3 (51.5–55.2) | 52.5 (50.6–54.4) |
| Control | 52.6 (50.0–55.1) | 54.7 (52.2–57.3) | 54.4 (51.8–56.9) | 53.0 (50.4–55.6) |
| Physical Component Summary | ||||
| Intervention | 44.2 (42.2–46.1) | 45.9 (43.9–47.9) | 44.8 (42.8–46.9) | 43.7 (41.7–45.8) |
| Control | 47.6 (44.8–50.4) | 49.8 (47.0–52.6) | 46.7 (43.9–49.5) | 48.4 (45.5–51.2) |
Table 3.
Results of Repeated-Measures Analysis Comparing Difference in Change in Sleep Outcomes from Baseline to Each Follow-Up Time Point Between the Intervention (n = 106) and Control (n = 53) Groups
| Outcome | Difference Between Groups in Change from Baseline (95% Confidence Interval) | P-Value | Effect Size |
|---|---|---|---|
| According to sleep diary | |||
| Sleep onset latency, minutes | |||
| Posttreatment assessment | −23.4 (−37.2 to −9.7) | <.001 | −0.55 |
| 6 months | −15.8 (−28.7 to −3.0) | .02 | −0.37 |
| 12 months | −17.3 (−30.2 to −4.4) | .009 | −0.41 |
| Wake after sleep onset, minutes | |||
| Posttreatment assessment | −17.7 (−31.5 to −3.8) | .01 | −0.36 |
| 6 months | −10.2 (−26.3–5.9) | .21 | −0.21 |
| 12 months | −2.1 (−19.2–15.1) | .81 | −0.04 |
| Total wake time at night, minutes | |||
| Posttreatment assessment | −68.4 (−96.4 to −40.4) | <.001 | −0.76 |
| 6 months | −37.0 (−62.2 to −11.7) | .004 | −0.41 |
| 12 months | −30.9 (−57.8 to −4.1) | .02 | −0.34 |
| Sleep efficiency, % | |||
| Posttreatment assessment | 10.5 (5.5–15.5) | <.001 | 0.68 |
| 6 months | 6.7 (2.0–11.3) | .005 | 0.43 |
| 12 months | 5.4 (0.5–10.4) | .03 | 0.35 |
| Sleep efficiency according to actigraphy, % | |||
| Posttreatment assessment | 1.3 (−0.6–3.2) | .17 | 0.20 |
| 6 months | −1.4 (−3.3–0.5) | .15 | −0.22 |
| 12 months | −1.1 (−3.0–0.9) | .27 | −0.17 |
| Pittsburgh Sleep Quality Index, total score | |||
| Posttreatment assessment | −3.4 (−4.4 to −2.3) | <.001 | −0.98 |
| 6 months | −2.4 (−3.5 to −1.3) | <.001 | −0.69 |
| 12 months | −2.1 (−3.2 to −1.0) | <.001 | −0.63 |
| Insomnia Severity Index, total score, total score | |||
| Posttreatment assessment | −4.5 (−6.2 to −2.8) | <.001 | −0.85 |
| 6 months | −3.9 (−5.7 to −2.1) | <.001 | −0.74 |
| 12 months | −2.8 (−4.6 to −1.1) | .002 | −0.54 |
Significant treatment effects were also observed for self-reported sleep quality. Intervention subjects had decreases (improvement) in PSQI total score from baseline that were 3.4 points better than those of controls at the posttreatment assessment, 2.4 points better at 6 months, and 2.1 points better at 12 months (all P < .05). Effect sizes for improvement in PSQI ranged from 0.63 to 0.98. Although not a primary outcome measure, the ISI also showed significantly greater improvement from baseline in intervention subjects than controls at each follow-up time point, with effect sizes that ranged from 0.54 to 0.85.
There were no significant treatment effects for the mental and physical components of the SF-12 or for the PHQ-9 between baseline and any follow-up time point. In secondary analyses, there were no significant differences in treatment effect between subjects who did and did not receive medications commonly used for insomnia or other sedative medications. In the intervention group, there were also no significant differences between intervention subjects receiving individual and group CBT-I on any outcome at any time point (data not shown). In particular, there were no significant differences for the primary sleep outcomes between individual and group CBT-I in change from baseline to 6 months (all P > .12).
DISCUSSION
A manual-based behavioral insomnia treatment program delivered by nonclinician sleep coaches with weekly telephone supervision by a psychologist with expertise in BSM improved subjective sleep measures and self-reported sleep quality in older adults with chronic insomnia. Overall effectiveness of the intervention on sleep outcomes was similar to findings from reviews of insomnia treatment studies in older adults involving CBT-I or sedative–hypnotics, 16,39 and most improvements were maintained for 12 months.
This intervention provides a promising option for increasing access to behavioral treatment for insomnia in older adults, in keeping with treatment guidelines that recommend behavioral therapy for older adults.3,19 Several approaches to provide behavioral treatment for insomnia have been developed, such as brief treatment programs with a sleep psychologist,21 self-help manuals,22 video educational programs,23 and online approaches,24 but use of these approaches in clinical settings has been limited, and many people do not receive behavioral treatment for their insomnia.
The intervention tested in this trial addresses several barriers to implementation of behavioral therapies for insomnia. First, use of a nonclinician, non-mental health provider to interact directly with individuals may increase access to treatment and address concerns of people who do not want to see a mental health specialist for treatment of their insomnia. Second, this program provides for supervision by BSM specialists (who are limited in number), which may increase treatment fidelity and effectiveness while efficiently using this scarce resource. Finally, the program offers in-person support from the sleep coach, which may be helpful for older adults with comorbidities.
There were also several strengths of the study design, particularly the randomized controlled methodology with long-term follow-up. It is likely that the clinical population–based screening with a postal survey provided a study sample that was more representative of the general clinical population than recruitment from sleep clinics or self-referral through advertisements. In addition, the high adherence to the intervention suggests good feasibility of providing the intervention in clinical care. Finally, the active control condition delivered by an individual with a comparable education level using similarly formatted program materials provided a strong comparison group to address potential nonspecific placebo effects. The high adherence to control sessions and the high program credibility ratings of control subjects suggests they remained blinded to group assignment.
A potential limitation of the study was the predominantly male veteran population; findings may not be generalizable to older women and nonveterans. In addition, although home sleep apnea testing was used to exclude those with severe sleep-disordered breathing, polysomnography was not performed, and SE-A did not show a treatment effect. Nevertheless, diagnostic criteria for insomnia are based on self-report, and objective sleep measurement is not required unless another sleep disorder (e.g., sleep apnea) is suspected.25,40 As such, subjective improvement in sleep is generally the critical measure of effectiveness in treatment of insomnia. There is often a mismatch between subjective and objective measures of sleep,41,42 and prior studies of sedative–hypnotics43 and CBT-I44 have found greater subjective than objective improvements in sleep. As a final limitation, diagnostic criteria for insomnia in use at study commencement (ICSD-2)25 were recently updated (ICSD-3).39 In addition to other changes, ICSD-3 criteria require symptoms to occur at least three times per week, which was not a diagnostic criterion used to identify participants for the study.
In summary, a structured, manual-based CBT-I program delivered by nonclinician sleep coaches significantly improved subjective sleep measures and self-reported sleep quality in older adults with chronic insomnia, and these improvements in sleep were maintained for up to 12 months. This intervention provides a promising option for increasing access to CBT-I in older adults, including those with significant comorbidity, which is in keeping with treatment guidelines that recommend behavioral therapies as first-line management of insomnia in older adults.
Acknowledgments
We wish to thank Sergio Martinez, Anna Gregorian, Jessica Bautista, Jaime Hughes, Therese Fernandez, Rebecca Saia, Lilia Lukowsky, Jessica Kim, and Rebecca Vivrette for their excellent work on this project. This manuscript is written in loving memory of Terry Vandenburg, MA, sleep coach extraordinaire.
Funded by VA Health Services Research and Development (Alessi IIR 08–295) and the VA Greater Los Angeles Geriatric Research, Education and Clinical Center.
Conflict of Interest: None.
Author Contributions: Alessi, Martin, Fiorentino, Josephson, Mitchell: study concept and design. Alessi, Martin, Fiorentino, Josephson: acquisition of subjects and/or data. Alessi, Martin, Fung, Dzierzewski, Rodriguez Tapia, Song, Josephson, Jouldjian, Mitchell: analysis and interpretation of data. Alessi, Martin, Fung, Fiorentino, Dzierzewski, Rodriguez Tapia, Song, Josephson, Jouldjian, Mitchell: preparation of manuscript.
Sponsor’s Role: The sponsor had no role in the design, methods, subject recruitment, data collections, analysis and preparation of the paper.
References
- 1.Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 2.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Bloom HG, Ahmed I, Alessi CA, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57:761–789. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivertsen B, Lallukka T, Sala P, et al. Insomnia as a risk factor for ill health: Results from the large population-based prospective HUNT study in Norway. J Sleep Res. 2014;23:124–132. doi: 10.1111/jsr.12102. [DOI] [PubMed] [Google Scholar]
- 5.Jaussent I, Bouyer J, Ancelin ML, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34:1103–10. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone KL, Blackwell TL, Ancoli-Israel S, et al. Sleep disturbances and increased risk of falls in older community-dwelling men: The Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. J Am Geriatr Soc. 2014;62:299–305. doi: 10.1111/jgs.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu MP, Lin HJ, Weng SF, et al. Insomnia subtypes and the subsequent risks of stroke: Report from a nationally representative sample. Stroke. 2014;45:1349–1354. doi: 10.1161/STROKEAHA.113.003675. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017–1028. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 9.Spira AP, Kaufmann CN, Kasper JD, et al. Association between insomnia symptoms and functional status in U.S. older adults. J Gerontol B Psychol Sci Soc Sci. 2014;69B(Suppl 1):S35–S41. doi: 10.1093/geronb/gbu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37:343–349. doi: 10.5665/sleep.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diem SJ, Ewing SK, Stone KL, et al. Use of non-benzodiazepine sedative hypnotics and risk of falls in older men. J Gerontol Geriatr Res. 2014;3:158. doi: 10.4172/2167-7182.1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tannenbaum C, Diaby V, Singh D, et al. Sedative-hypnotic medicines and falls in community-dwelling older adults: A cost-effectiveness (decision-tree) analysis from a US Medicare perspective. Drugs Aging. 2015;32:305–314. doi: 10.1007/s40266-015-0251-3. [DOI] [PubMed] [Google Scholar]
- 13.Bakken MS, Engeland A, Engesæter LB, et al. Risk of hip fracture among older people using anxiolytic and hypnotic drugs: A nationwide prospective cohort study. Eur J Clin Pharmacol. 2014;70:873–880. doi: 10.1007/s00228-014-1684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weich S, Pearce HL, Croft P, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: Retrospective cohort study. BMJ. 2014;348:1–12. doi: 10.1136/bmj.g1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 16.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 17.Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: A meta-analysis. Sleep Med Rev. 2014;23C:54–67. doi: 10.1016/j.smrv.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: A randomized controlled trial. JAMA. 2006;295:2851–2858. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 19.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–1419. [PubMed] [Google Scholar]
- 20.Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: Family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398–e406. [PMC free article] [PubMed] [Google Scholar]
- 21.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho FY, Chung KF, Yeung WF, et al. Self-help cognitive-behavioral therapy for insomnia: A meta-analysis of randomized controlled trials. Sleep Med Rev. 2015;19:17–28. doi: 10.1016/j.smrv.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Savard J, Ivers H, Savard MH, et al. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37:1305–1314. doi: 10.5665/sleep.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espie CA, Kyle SD, Williams C, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35:769–781. doi: 10.5665/sleep.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Classification of Sleep Disorders. Diagnostic and Coding Manual. 2. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 26.CONSORT. [Accessed May 18, 2016];2010 [on-line]. Available at http://www.consort-statement.org/consort-2010.
- 27.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’ A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Behavioral Sleep Medicine Series Webinar. Darien, IL: American Academy of Sleep Medicine; [Google Scholar]
- 29.Selim AJ, Fincke G, Ren XS, et al. Comorbidity assessments based on patient report: Results from the Veterans Health Study. J Ambul Care Manage. 2004;27:295. doi: 10.1097/00004479-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Ferrell BA, Stein WM, Beck JC. The geriatric pain measure: Validity, reliability and factor analysis. J Am Geriatr Soc. 2000;48:1669–1673. doi: 10.1111/j.1532-5415.2000.tb03881.x. [DOI] [PubMed] [Google Scholar]
- 31.Morgenthaler T, Alessi C, Friedman L, et al. Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007 Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9. Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware JE, Jr, Koskiski M, Turner-Bowker DM, et al. User’s Manual for the SF-12v2 Health Survey with a Supplement Documenting SF-12 Health Survey. Lincoln, RI: QualityMetric Inc; 2002. [Google Scholar]
- 36.Borkovec T, Nau S. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3:257–260. [Google Scholar]
- 37.Littell RC, Pendergast J, Natarajan R. Tutorial in biostatistics: Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19:1793–1819. doi: 10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 38.Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11:364–386. [Google Scholar]
- 39.Brasure M, MacDonald R, Fuchs E, et al. Management of Insomnia Disorder. Rockville, MD: Agency for Healthcare Research and Quality; 2015. Comparative Effectiveness Review No. 159 (AHRQ Publication No. 15-EHC027-EF) [PubMed] [Google Scholar]
- 40.International Classification of Sleep Disorders. Diagnostic and Coding Manual. 3. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 41.Bianchi MT, Williams KL, McKinney S, et al. The subjective-objective mismatch in sleep perception among those with insomnia and sleep apnea. J Sleep Res. 2013;22:557–568. doi: 10.1111/jsr.12046. [DOI] [PubMed] [Google Scholar]
- 42.Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychol Bull. 2012;138:77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. Can Med Assoc J. 2000;162:225–233. [PMC free article] [PubMed] [Google Scholar]
- 44.Epstein DR, Sidani S, Bootzin RR, et al. Dismantling multicomponent behavioral treatment for insomnia in older adults: A randomized controlled trial. Sleep. 2012;35:797–805. doi: 10.5665/sleep.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]