SYNOPSIS
The ABCDEF bundle represents an evidence-based guide for clinicians to approach the organizational changes needed for optimizing ICU patient recovery and outcomes. The ABCDEF bundle includes: Assess, Prevent, and Manage Pain, Both Spontaneous Awakening Trials (SAT) and Spontaneous Breathing Trials (SBT), Choice of analgesia and sedation, Delirium: Assess, Prevent, and Manage, Early mobility and Exercise, and Family engagement and empowerment. In this chapter, we will review the core evidence and features behind the ABCDEF bundle. The bundle has individual components that are clearly defined, flexible to implement, and help empower multidisciplinary clinicians and families in the shared care of the critically ill. The ABCDEF bundle helps guide well-rounded patient care and optimal resource utilization resulting in more interactive ICU patients with better controlled pain, who can safely participate in higher-order physical and cognitive activities at the earliest point in their critical illness.
Keywords: Pain, Spontaneous Awakening Trials (SAT), Spontaneous Breathing Trials (SBT), Sedation, Analgesia, Delirium, Early Mobility, Family Engagement, Intensive Care Unit
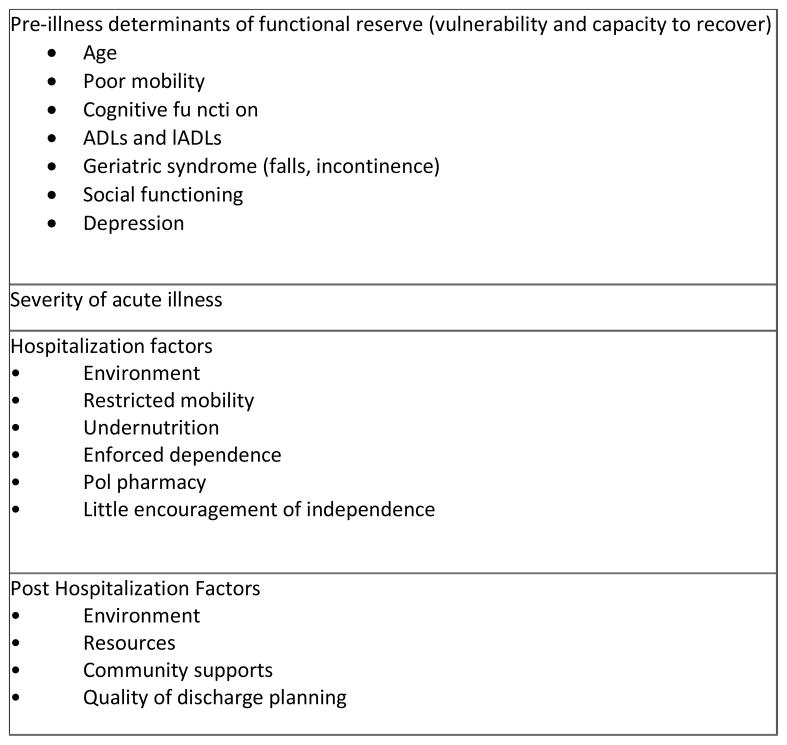
With more than 4 million ICU admissions per year in the US, there is increasing recognition of the long-term consequences of ICU care on the physical and mental health function of our patients. An acute care hospitalization and critical illness has tangible consequences of cognitive decline,1 post-traumatic stress disorder,2 and depression.3 In a multicenter cohort of 821 critically ill patients, with respiratory failure or shock, our group demonstrated that one of four ICU patients had cognitive impairment after 12 months after critical illness that was similar in severity to that of patients with mild Alzheimer’s disease and moderate traumatic brain injury.4 The largest risk factor for this ICU-related cognitive impairment was delirium. Disability associated with ICU care and hospitalization is an unfortunately common occurrence in older adults with significant consequences for patients and caregivers (Figure 1).5
Figure 1.
Factors related to Hospitalization-Associated disability
Data from Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "She was probably able to ambulate, but I'm not sure". JAMA. 2011 Oct 26;306(16):1782–93. doi: 10.1001/jama.2011.1556.
ICU survivorship has become a top concern and methods to optimize patient recovery and outcomes are important objectives for the health provider, families, and researchers. In 2013, the American College of Critical Care Medicine, in collaboration with the Society of Critical Care Medicine and American Society of Health-System Pharmacists, updated the Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit (ICU PAD Guidelines) to provide recommendations for clinicians to better manage critically ill patients.6 Many elements of the symptom-based ICU PAD guideline can be implemented using an interdependent, multicomponent, evidence-based guide for the coordination multidisciplinary ICU care - the ABCDEF bundle. The ABCDEF bundle includes: Assess, Prevent, and Manage Pain (A), Both Spontaneous Awakening Trials (SAT) and Spontaneous Breathing Trials (SBT) (B), Choice of analgesia and sedation (C), Delirium: Assess, Prevent, and Manage (D), Early mobility and Exercise (E), and Family engagement and empowerment (F).
A: Assess, Prevent, and Manage Pain
ICU patients commonly experience pain, with an incidence of up to 50% in surgical and medical patients. It is a major clinical symptom that requires systematic diagnosis and treatment.7,8 In a prospective, cross-sectional, multicenter, multinational study of pain intensity associated with 12 procedures, the Europain study, Puntillo et al. showed that common ICU procedures induced a significant increase in pain, although no procedure caused severe pain. For the three most painful procedures (i.e., chest tube removal, wound drain removal, and arterial line insertion) pain intensity more than doubled during the procedure compared with the pre-procedural levels.9
Assessment of pain is the first step before administering pain relief. Pain assessments are often only performed 35% of the time before ICU procedures.7 Patient's self-report of pain using a 1–10 numerical rating scale (NRS) is considered the gold standard and is highly recommended by many critical care societies.6,8 Because of the high interrelation between delirium and pain,8 assessing and treating pain could be important in the prevention and/or management of delirium.
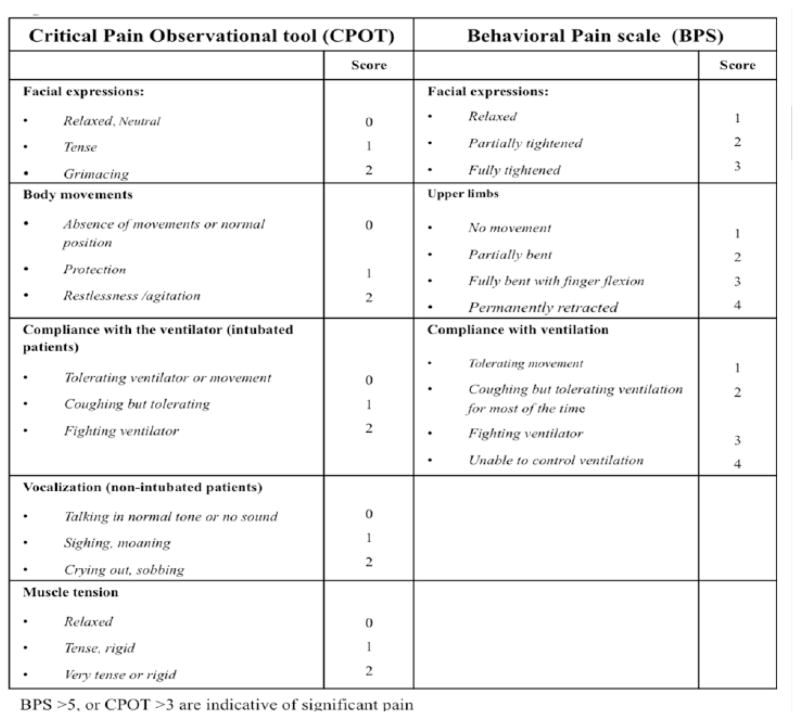
In the absence of a patient’s self-report, observable behavioral and physiological indicators become important indices for the assessment of pain.10 The Behavioral Pain Scale (BPS) and the Critical-Care Pain Observation Tool (CPOT) are the most valid and reliable behavioral pain scales for ICU patients unable to communicate (Figure 2). The BPS is composed of 3 subscales: facial expression, movement of the upper limbs, and compliance with mechanical ventilation (MV). Each subscale is scored from 1 (no response) to 4 (full response). A BPS score of 5 or higher is considered to reflect unacceptable pain. The CPOT has 4 components: facial expression, body movements, muscle tension, and compliance with the ventilator for intubated patients or vocalization for extubated patients. Each component is scored from 0 to 2 with a possible total score ranging from 0 to 8. A CPOT ≥ 3 is indicative of significant pain. Both the BPS and the CPOT provide guidance for the selection of pharmacological interventions for pain and in the evaluation of their effectiveness.11,12
Figure 2.
Clinical Pain Observational Tool (CPOT) and Behavioral Pain Scale (BPS)
Adapted from Payen JF, Bru O, Bosson JL, et al Assessing pain in critically ill sedated patients by using a behavioral pain scale Crticial Care Med. 2001 Dec;29(12):2258–63; with permission.
According to ICU PAD Guidelines, pain medications should be routinely administered in the presence of significant pain (i.e., NRS >4, BPS >5, or CPOT >3) and prior to performing painful invasive procedures. Parenteral opioids are first-line pharmacologic agents for treating non-neuropathic pain in critically ill patients. All opioids have the potential to induce tolerance over time, resulting in the need for escalating doses to achieve the same analgesic effect. For the treatment of neuropathic pain in ICU patient gabapentin or carbamazepine should be administered enterally, in addition to opioids. Non-opioid analgesics, such as acetaminophen, nonsteroidal anti-inflammatory drugs, or ketamine, should be used as adjunctive pain medications to reduce opioid requirements and opioid-related side effects ill. Use of regional analgesia in ICU patients is limited to the use of epidural analgesia in specific subpopulations of surgical patients, and in patients with traumatic rib fractures.6 In managing pain in the ICU, non-pharmacological methods are often effective and safe (e.g., injury stabilization, patient repositioning, use of heat/cold).13
B: Both Spontaneous Awakening Trials (SAT) and Spontaneous Breathing Trials (SBT)
Daily SATs are the stopping of narcotics (as long as pain is controlled) and sedatives every day and, if needed, restarting either narcotics or sedatives at half the previous dose and titrating as need. Daily interruption of sedation shortens the duration of mechanical ventilation and the ICU length of stay. The 2013 ICU PAD Guidelines emphasize the importance of minimizing sedative use and maintaining a light level of sedation in patients, using either a daily sedative interruption strategy (i.e., SAT), or by continuously titrating sedatives to maintain a light level of sedation (i.e., targeted sedation strategy). Kress et al. conducted a randomized, controlled trial involving 128 adult patients who were receiving mechanical ventilation and continuous infusions of sedative drugs in a medical ICU (MICU). In the intervention group, the sedative infusions were interrupted daily until the patients were awake; in the control group, the infusions were interrupted only at the discretion of the clinicians. In this study, daily interruption of the infusion of sedative drugs shortened the duration of mechanical ventilation by more than 2 days and the length of stay in the intensive care unit by 3.5 days.14 These data suggest that daily SAT uses less analgosedation while improving ICU outcomes.14
There is a consistent relationship between deeper sedation and worse ICU outcomes. Deep sedation in the first 48 hours of an ICU stay has been associated with delayed time to extubation, higher need for tracheostomy, increased risk of hospital and long term death.15–17 Shehabi et al. examined the relationships between early sedation and time to extubation, delirium, hospital and 180-day mortality among ventilated critically ill patients in the intensive care unit. Every additional Richmond Agitation-Sedation Score (RASS) assessment in the deep sedation range in the first 48 hours was associated with delayed time to extubation of 12.3 hours, a 10% increased risk of hospital death, and an 8% increased risk of death at 6 months.15 Balzer et al. examined short and long-term survival after deep sedation during the first 48 hours after ICU admission. In this study, 1,884 patients receiving mechanical ventilation were grouped as either lightly or deeply sedated (light sedation: RASS -2 to 0; deep: RASS -3 or below). Deep sedation (27.2%, n=513) was associated with an in-hospital mortality hazard ratio of 1.661 (95% CI: 1.074 to 2.567; P = 0.022) and a two-year hazard ratio of 1.866 (95% CI: 1.351 to 2.576; P <0.001). In summary, deeply sedated patients had longer ventilation times, increased length of stay and higher rates of mortality.17 These studies show that early deep sedation is a modifiable risk factor and that the implementation of sedation protocols to achieve light sedation is feasible and reproducible in the early phase of ICU treatment.
Daily SBT has been proven to be effective and superior to other techniques to ventilator weaning. Numerous randomized trials support the use of ventilator weaning protocols that include daily SBTs as their centerpiece.18,19 About two-thirds of the time on mechanical ventilation is spent during weaning, so anything that reduced this period would have a very high likelihood of improving outcomes. Girard et al. undertook the Awakening and Breathing Controlled (ABC) trial, a multicenter, randomized controlled trial to assess the efficacy and safety of a protocol of daily SATs paired with SBTs (intervention group, n=168) versus a standard SBT protocol in patients receiving patient-targeted sedation as part of usual care (control group, n=168).20 Patients in the intervention group (both SAT and SBT) spent more days breathing without assistance during the 28-day study period (14.7 days versus 11.6 days; mean difference 3.1 days, 95% CI: 0.7–5.6, p=0.02) and were discharged earlier from the ICU (median time in ICU of 9.1 days versus 12.9 days, p=0.01) and earlier from the hospital (median hospital time 14.9 days versus 19.2 days, p=0.04).20 During the year after enrollment, patients receiving SATs with SBTs (intervention) were less likely to die than were patients receiving only SBTs (control) (hazard ratio=0.68, 95% CI: 0.50–0.92, p=0·01). For every seven patients treated with the intervention, one life was saved (number needed to treat was 7.4, 95% CI: 4.2–35.5).20 Conversely in the SLEAP trial (protocolized light sedation in combination with daily SAT versus protocolized light sedation alone), found no difference between the groups with regard to time to extubation, duration of ICU and hospital stays.21 One reason the SLEAP study might not have showed an effect is because both the treatment and control groups received high sedative doses that would result in moderate to deep levels, rather than light levels of sedation.22
No sedation has also been applied as a strategy in ICU patients. Strøm et al. enrolled 140 critically ill adult patients who were undergoing mechanical ventilation and were expected to need ventilation for more than one day. Patients were randomly assigned in a 1:1 ratio (unblinded) to receive no sedation (n=70 patients) or sedation (n=70, control group). Patients receiving no sedation had significantly more days without ventilation (mean 13.8 days, SD 11.0 vs mean 9.6 days, SD 10.0; mean difference 4.2 days, 95%: CI 0.3–8·1. p=0.0191) in a 28-day period, and reduced stays in the ICU and hospital. This study did find increased hyperactive delirium in the group receiving no sedation.23
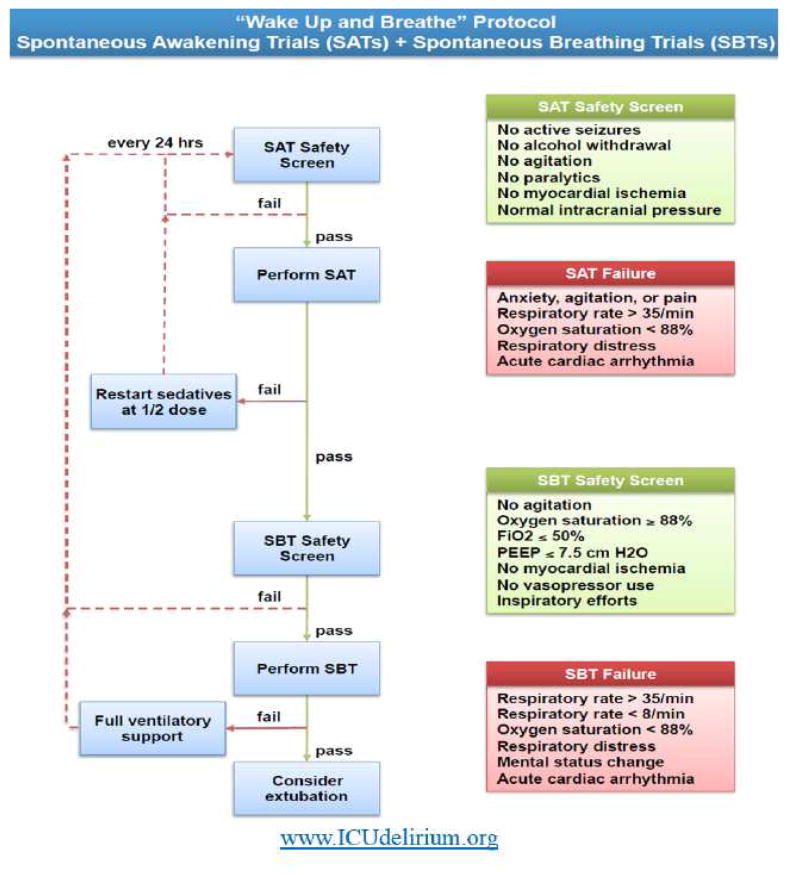
Ultimately, the core features of the ABCDEF bundle involve coordination of SATs and SBTs emphasizing narcotic and sedation titration resulting in earlier liberation from mechanical ventilation, ICU, and hospitalization (Figure 3).
Figure 3.
“Wake up and Breath” Protocol: Spontaneous Awakening Trials (SATs) with Spontaneous Breathing Trials
© 2008 Vanderbilt University. All rights reserved
C: Choice of analgesia and sedation
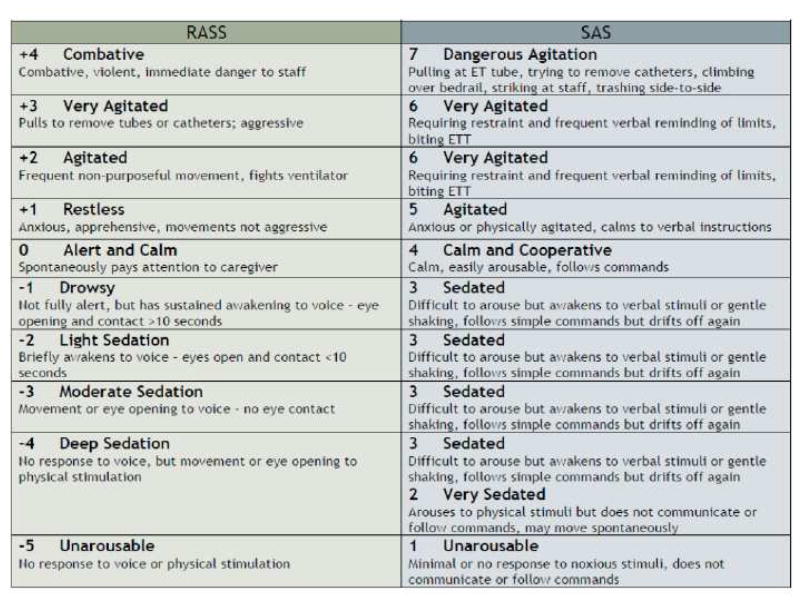
Although, we have discussed pain assessment and management earlier, the 2013 PAD guidelines emphasize the need for goal-directed delivery of psychoactive medications to avoid over-sedation, to promote earlier extubation, and to help the medical team agree on a target sedation level by using sedation scales. Of the available reliable and valid sedation scales, the PAD guidelines recommend the use of the Richmond Agitation-Sedation Scale (RASS) and the Riker Sedation-Agitation Scale (SAS). Figure 4 shows the psychometric properties of both the RASS and SAS. The SAS has 7 individual tiers ranging from “1” (unarousable) to “7” (dangerous agitation).24 RASS is a 10-point scale, with four levels of escalating agitation (RASS +1 to +4), one level denoting a calm and alert state (RASS 0), three levels of sedation (RASS -1 to –3), and two levels of coma (RASS -4 to -5). A unique feature of RASS is that it relies on the duration of eye contact following verbal stimulation. The RASS takes less than 20 seconds to perform with minimal training, and has been shown highly reliability among multiple types of healthcare providers and an excellent interrater reliability in a broad range of adult medical and surgical ICU patients.25
Figure 4.
Richmond Agitation-Sedation Scale (RASS) and Riker Sedation-Agitation Scale (SAS)
From ICU Delirium, Vanderbilt University. Available at www.ICUdelerium.org. Adapted from Riker RR, Picard JT, Fraser GL. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med 1999;27(7):1327, and Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation–Sedation Scale. Am J Resp Crit Care Med 166:1339
To maximize patient outcomes, it is essential to carefully choose sedatives and analgesic medications, as well as consider medication doses, titration, and discontinuation.25 For example, there is a clear association between decreased exposure to sedatives, particularly benzodiazepines, and improved patient outcomes.15,17,26,27 Pandharipande et al. evaluated 198 mechanically ventilated patients to determine the probability of daily transition to delirium, as a function of sedative and analgesic dose administration during the previous 24-hour period. They found that every unit dose of lorazepam was associated with a higher risk for daily transition to delirium (odds ratio=1.2, 95% CI: 1.1–1.4, p=0.003).28 Similarly Seymour et al. confirmed that benzodiazepines are an independent risk factor for development of delirium during critical illness even when given more than 8 hours before a delirium assessment.29 These results expand and support the recommendation made in the 2013 ICU PAD guidelines that non-benzodiazepine sedative options may be preferred over benzodiazepine-based sedative regimens.6
Two major studies evaluated benzodiazepines against a novel alpha-2-agonist sedative, dexmedetomidine. The SEDCOM trial (Safety and Efficacy of Dexmedetomidine Compared with Midazolam) showed a reduction in the prevalence of delirium and in the duration of mechanical ventilation in patients sedated with dexmedetomidine compared with midazolam30 The MENDS study (Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction) evaluated the role of changing sedation paradigms on acute brain dysfunction, comparing dexmedetomidine with lorazepam.31 The dexmedetomidine sedative strategy resulted in more days alive without delirium or coma, but without differences in mortality or ventilator-free days. Notably, the subgroup of septic patients sedated with dexmedetomidine in the MENDS study had shorter durations of delirium and coma, lower daily probability of delirium, shorter time on the ventilator, and improved 28-day survival.32 There is an ongoing trial (MENDS II study) to determine the best sedative medication to reduce delirium and improve survival and long-term brain function in the ventilated septic patient (ClinicalTrials.gov Identifier: NCT01739933).
D: Delirium: Assess, Prevent, and Manage
An important third element in the PAD guidelines is monitoring and management of delirium. Delirium is a disturbance in attention and awareness that develops over a short period of time, hours to days, and fluctuates over time.33 Over 80% of patients developed delirium during their hospital stay, with the majority of cases occurring in the ICU with an average time of onset between the second and the third day.
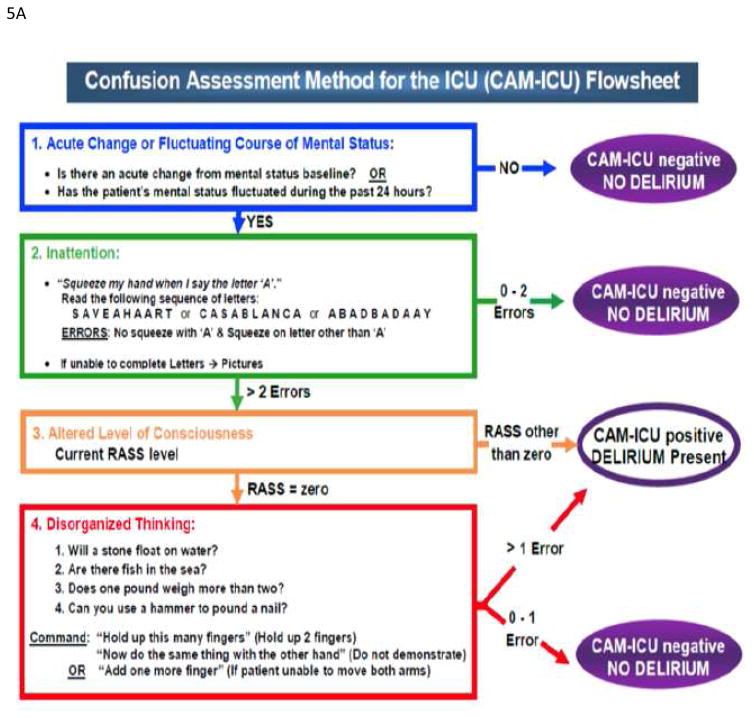
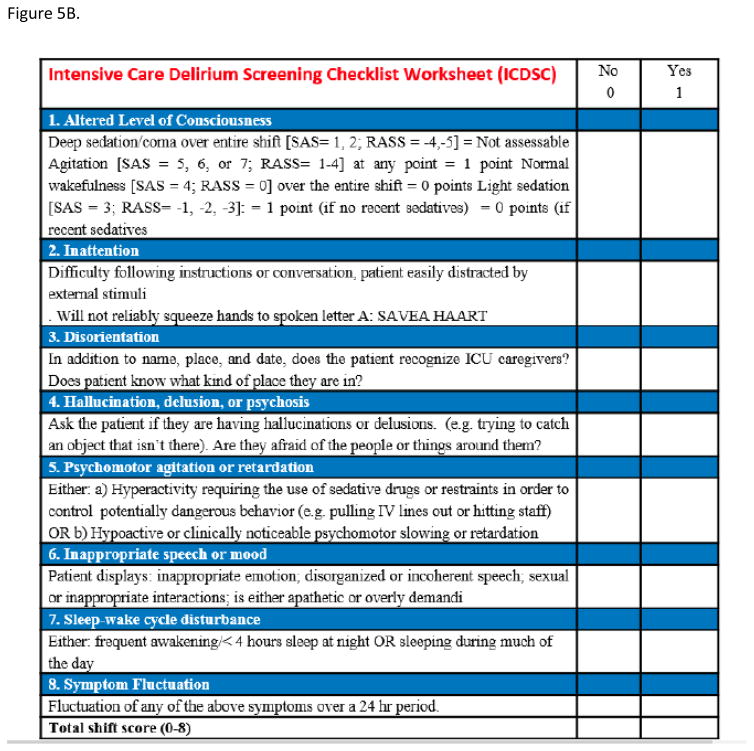
Several methods have been developed and validated to diagnose delirium in ICU patients but the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU, Figure 5A) and the Intensive Care Delirium Screening Checklist (ICDSC, Figure 5B) are the most frequently employed tools for this purpose.34 The ICDSC checklist is an eight-item screening tool (one point for each item) that is based on DSM criteria and applied to data that can be collected through medical records or to information obtained from the multidisciplinary team.34 The pooled values for the sensitivity and specificity of the ICDSC are 74% and 81.9%, respectively.34 The CAM-ICU is composed by four features 1) acute onset of mental status changes or fluctuating course; 2) inattention; 3) disorganized thinking; and 4) altered level of consciousness. The patient is considered CAM positive and, so delirious, if he/she manifests both features 1 and 2, plus either feature 3 or 4.35 Overall accuracy of the CAM-ICU is excellent, with pooled values for sensitivity and specificity of 80% and 95.9%, respectively.34 The CAM-ICU has been modified and validated in pediatric, emergency department, and neurocritical care populations, as well as translated in over 25 languages36–40.
Figure 5.
(A) Confusion Assessment Method for the ICU (CAM-ICU) (B) Intensive Care Delirium Screening checklist (ICDSC)
Copyright E. Wesley, MD, MPH and Vanderbilt University
Normal 0; Delirium4–8: Subsyndromal Delirium 1–3
Score your patient over the entire shift. Components don't all need to be present at the same time. Components 1 through 4 cannot be completed when the patient is deeply sedated or comatose (ie. SAS= 1 or 2; RASS = −4 or -5); Components 5 through 8 are based on observations throughout the entire shift. Information from the prior 24 hrs. should be obtained for components 7 and 8.
Adapted from Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001 May;27(5):859–64; Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intens CareMed 2007;33:1007–13. Epub 2007 Apr 3; with permission.
Delirium can be categorized into subtypes according to psychomotor behavior. Hyperactive delirium (CAM positive, RASS positive range) is associated with a better overall prognosis and it is characterized by agitation, restlessness, and emotional lability.41 Hypoactive delirium (CAM positive, RASS negative range), which is very common and often more deleterious in the long term, is characterized by decreased responsiveness, withdrawal, and apathy and remains unrecognized in 66 to 84% of hospitalized patients.42 Another categorization based on the ICDSC score assigns patients with a score of 0 to have no delirium, those with a score ≥ 4 to have clinical delirium, and those with a score of 1–3 to have subsyndromal delirium.43 Whichever delirium metric is used, the best picture of the patient’s mental status comes from assessing delirium serially throughout the day.
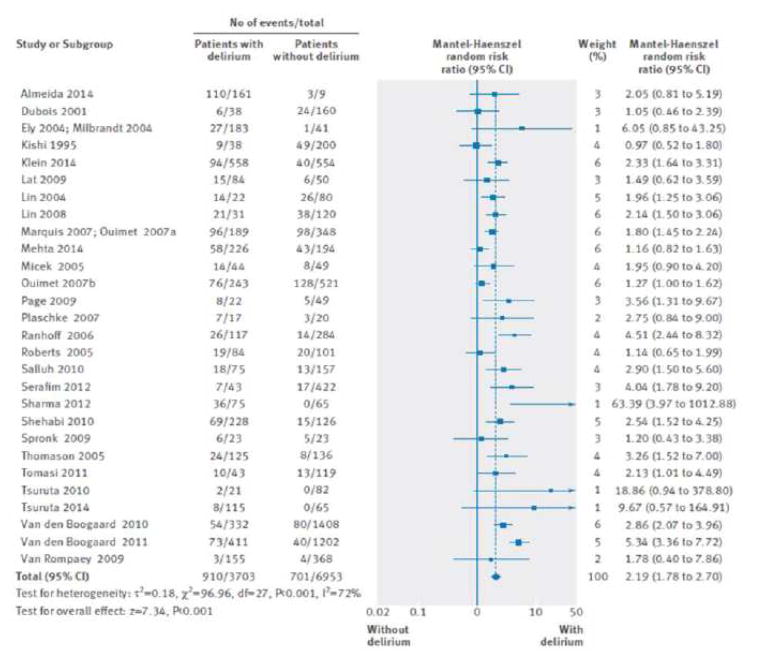
Evidence shows that delirium is a strong predictor of increased length of mechanical ventilation, longer ICU stays, increased cost, long-term cognitive impairment, and mortality (Figure 6).19,44–47 The cumulative effect of multiple days of delirium on mortality may be multiplicative, rather than additive.48
Figure 6.
Impact of delirium on hospital mortality in critically ill patients.
From Salluh JI, Wang H, Schneider EB, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015 Jun 3;350:h2538. doi: 10.1136/bmj.h2538.
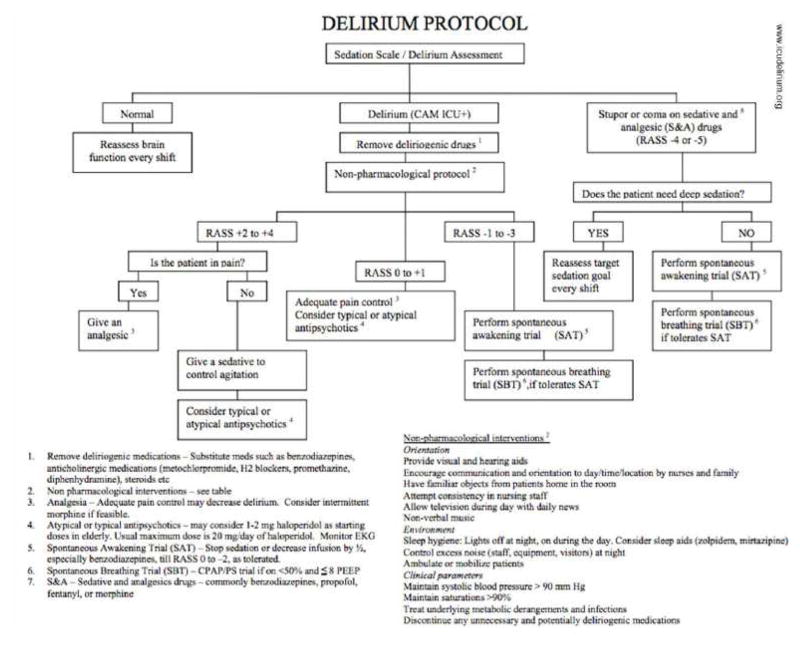
Numerous risk factors for delirium have been identified, including preexisting cognitive impairment, advanced age, use of psychoactive drugs, mechanical ventilation, untreated pain, and a variety of medical conditions such as heart failure, prolonged immobilization, abnormal blood pressure, anemia, sleep deprivation, and sepsis.42,49 The most frequent risk factor was the use of benzodiazepines or narcotics (98%).44 The mean number of identified risk factors for delirium in these patients was 11±4 with a range of 3–17 risk factors present. Patients with 3 or more risk factors were considered at high risk for delirium.42,49,50 In delirious patients, a systematic protocolized search for all reversible precipitants is the first line of action and symptomatic treatment should be considered when available and not contraindicated (Figure 7).51
Figure 7.
Sample Delirium Protocol.
From ICU Delirium, Vanderbilt University. Available at www.ICUdelerium.org.
Antipsychotics, especially haloperidol, are commonly administered for the treatment of delirium in critically ill patients. However, evidence for the safety and efficacy of antipsychotics in this patient population is lacking. Moreover, the 2013 PAD Guidelines include no specific recommendations for using any particular medication.6 Ely et al. are conducting the MIND-USA (Modifying the Impact of ICU-Induced Neurological Dysfunction-USA) Study (ClinicalTrials.gov Identifier NCT01211522) to define the role of antipsychotics in the management of delirium in vulnerable critically ill patients.
Delirium prophylaxis with medications is discouraged in the PAD guidelines. Recently, a prospective, randomized, multicenter trial compared a low-dose haloperidol infusion administered for 12 hours (0.5 mg intravenous bolus injection followed by continuous infusion at a rate of 0.1 mg/h, n=229 patients) against placebo (n = 228 patients) in the immediate postoperative period. This study provided evidence that haloperidol could reduce the incidence of delirium within the first 7 days postoperatively in patients undergone noncardiac surgery (15.3% in the haloperidol group versus 23.2% in the control group, p=.031).52 By contrast, another ICU study showed no benefit of early administration of intravenous haloperidol in a mixed population of medical and surgical adult ICU patients.53 In this double-blinded, placebo-controlled randomized trial, 142 patients were randomized to receive haloperidol or placebo intravenously every 8 hours irrespective of coma or delirium status. Patients in the haloperidol group spent about the same number of days alive, without delirium or coma, as did patients in the placebo group (median 5 days [IQR 0–10] versus 6 days [0–11] days; p=0.53).
The only strategy strongly recommended in the PAD Guidelines, to reduce the incidence and duration of ICU delirium and to improve functional outcomes, is promoting sleep hygiene to prevent sleep disruption and the use of early and progressive mobilization and in these patients.
E: Early mobility
Early mobility is an integral part of the ABCDEF bundle and has been the only intervention resulting in a decrease in days of delirium.54 During ICU stay critically ill patients can lose up to 25% peripheral muscle weakness within 4 days when mechanically ventilated and 18% in body weight by the time of discharge and this process is higher in the first 2–3 weeks of immobilization.55 The consequence of physical dysfunction in critically ill patients can be profound and long-term with significant reduction in functional status being observed even 1 year and 5 years after ICU discharge.56–58
ICU-acquired weakness is caused by many different pathophysiological mechanisms that are not mutually exclusive given the diverse diseases that precipitate critical illness, the drugs used during its management, and the consequences of protracted immobility.54 The reported incidence of ICU-acquired weakness ranges from 25 to 100%.59,60 The diagnosis of ICU-acquired weakness is made by the Medical Research Council (MRC) scale for grading the strength (i.e., 0, total palsy to 5, normal strength) of various muscle groups in the upper and lower extremities. The scale ranges from 0 (complete tetraplegia) to 60 (normal muscle strength), with a score < 48 is diagnostic of ICU-acquired weakness.61 Patients with ICU-acquired weakness should undergo serial evaluations, and if persistent deficits are noted, electrophysiological studies, muscle biopsy, or both are warranted.54
Although clinical providers may have fears about early mobilization, there is good evidence regarding the strategy of minimizing sedation and increasing the physical activity of ICU patients to the point of getting up and out of bed.54 Physical therapy has shown to be feasible, safe, even in the most complicated patients receiving the most advanced medical therapies (e.g., continuous renal replacement therapy, extracorporeal cardiopulmonary support).62,63 Early activity can be done without increases in usual ICU staffing and with a low risk (<1%) of complications.64 Studying patients early in the their course of mechanical ventilation (<3 days), Schweickert et al. showed that a daily SAT combined with physical and occupational therapy, versus SAT alone, resulted in an improved return to independent functional status at hospital discharge, shorter duration of ICU-delirium, higher survival, and more days breathing without assistance.65 However, in a study where ICU patients were enrolled 4 days after the initiation of mechanical ventilation (average 8 days), an intensive physical therapy program did not improve long-term physical functioning when compared to a standard of care program 66. Although both these studies demonstrated feasibility of physical therapy, it may more effective to embark on physical therapy early in the ICU course, rather than later when it is much more challenging to improve ICU-acquired weakness.65,66
The focus on rehabilitation of critically ill patients should begin in the ICU and continue all the way to recovery at home. The close collaboration and coordination with medicine, nursing, and physical therapists is fundamental for an efficacy and safe strategy.62 This is particularly important because the burden of illness affects not only the patient but his or her family or other caregivers as well.54
F: Family engagement
The ABCDE bundle has evolved to include Family Engagement, as no ICU treatment plan is complete without incorporation of the family’s wishes, concerns, questions, and participation. Family members and surrogate decision makers must become active partners in multi-professional decision-making and treatment planning. Through this partnership, patients’ preferences can be identified, the anxiety of families can be lessened, and physicians can have appropriate input into decisions.67
Family presence on ICU rounds is beneficial, and it does not interfere with education and communication process.68 Families have reported increased feelings of inclusion, respect, and having a better understanding of their loved one's care. Nurses have indicated satisfaction with team communication and facilitation of family relationships.69 Several studies suggested that increased focus on communication with family members, through routine ICU family conferences, palliative care consultation, or ethics consultation can reduce ICU length of stay for those patients whose trajectory is ultimately mortal.70–73 One study of communication occurring during ICU family conferences sought to understand how ICU clinicians conduct communication concerning withdrawing life-sustaining treatments or the delivery of bad news, and how this communication might be improved.74 Most clinicians failed to listen and respond appropriately, failed to acknowledge the expression of family members’ emotions, and failed to explain key tenets of palliative care. An important missed opportunity when communicating with families is exploring patient treatment preferences that are key to clinical decision making in the ICU setting.74
Ethics and palliative care consultations have been introduced into the practice of medicine during the past several decades as a way to help health care professionals, patients, and surrogates come to a decision about medical treatment ensuring that the process of decision making is inclusive, educational, respectful of cultural values, and reflect appropriate resource utilization. When ethics consultation have been used, they have been associated with reductions in hospital and ICU lengths of stay, and more frequent decisions to forgo life-sustaining treatment.72,75 When tackling treatment conflicts, the majority (87%) of ICU physicians, nurses, and patients/surrogates agreed that ethics consultations are helpful. However, in a recent randomized study in 4 medical ICUs in those receiving mechanical ventilation for greater than one week, family discussions conducted by palliative care specialists (intervention) versus standard ICU led family discussions (control) did not alter anxiety or depression symptoms in surrogate decision makers.76
Beyond sharing of communication, family presence has been encouraged in traumatizing medical events and procedures, such as Cardiopulmonary Resuscitation (CPR). In some studies, the family presence during CPR is associated with positive results on psychological variables, and did not interfere with medical efforts, increase stress in the health care team, or result in medicolegal conflicts. In fact, relatives who did not witness CPR had symptoms of anxiety and depression more frequently than those who did witness CPR.77
Critical illness usually impacts not only an individual, but their entire support system, which may or may not be their nuclear family, or some combination of family and friends or other caregivers who are actively engaged in supportive roles. In light of this, it is crucial not only to recognize the needs of the identified patient but the needs of their family as well.
Summary
We have reviewed the core evidence and features behind the ABCDEF bundle, which was created to combat the adverse effects of critical illness related to acute and chronic brain dysfunction. The ABCDEF bundle represents one method of approaching the organizational changes that create a culture shift in our treatment of ICU patients. The multifold potential benefits of these recommended strategies outweigh minimal risks of costs and coordination. Ultimately, the ABCDEF bundle is one path to well-rounded patient care and optimal resource utilization resulting in more interactive ICU patients with better pain control, who can safely participate with their families and healthcare providers in higher-order physical and cognitive activities at the earliest point in their critical illness.
KEY POINTS.
The ABCDEF bundle is an evidence-based guide for clinicians to coordinate multidisciplinary patient care in the intensive care unit (ICU).
Assessment of pain is the first step before administering pain relief. The Behavioral Pain Scale (BPS) and the Critical-Care Pain Observation Tool (CPOT) are the most valid and reliable behavioral pain scales for ICU patients unable to communicate.
Coordination of Spontaneous Awakening Trials (SAT) with Spontaneous Breathing Trials (SBT) is associated with decreases in sedative use, delirium, time on mechanical ventilation, and ICU and hospital lengths of stay.
Delirium monitoring and management is critically important since it is a strong risk factor for increased time on mechanical ventilation, length of ICU and hospital stay, cost of hospitalization, long term cognitive impairment, and mortality.
Early mobility is the only currently known intervention associated with a decrease in delirium duration. Physical therapy is safe and feasible in the ICU, even while on mechanical ventilation, renal replacement therapy, and/or circulatory support.
Acknowledgments
Funding Sources: EWE, PPP, and MBP are supported by National Institutes of Health HL111111 (Bethesda, MD). EWE is supported by the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (Nashville, TN). EWE and PPP are supported by the VA Clinical Science Research and Development Service (Washington, DC) and the National Institutes of Health AG027472 and AG035117 (Bethesda, MD). MBP is supported by the Vanderbilt Faculty Research Scholars Program. This project was supported by REDCap, a secure online database, supported in part by the National Institutes of Health TR000445. EWE has received honoraria from Abbott Laboratories, Hospira, Inc., and Orion Corporation, and research grants from Abbott Laboratories. PPP and EWE have received research grants from Hospira, Inc. AM has received research grants from Massimo.
Footnotes
Disclosures: The Authors have no other disclosures relevant to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel MB, Jackson JC, Morandi A, et al. Incidence and Risk Factors for Intensive Care Unit-related Post-traumatic Stress Disorder in Veterans and Civilians. Am J Respir Crit Care Med. 2016;193(12):1373–1381. doi: 10.1164/rccm.201506-1158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. The lancet. Respiratory medicine. 2014;2(5):369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "She was probably able to ambulate, but I'm not sure". JAMA. 2011;306(16):1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 6.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 7.Payen JF, Chanques G, Mantz J, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007;106(4):687–695. doi: 10.1097/01.anes.0000264747.09017.da. [DOI] [PubMed] [Google Scholar]
- 8.Chanques G, Viel E, Constantin JM, et al. The measurement of pain in intensive care unit: comparison of 5 self-report intensity scales. Pain. 2010;151(3):711–721. doi: 10.1016/j.pain.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Puntillo KA, Max A, Timsit JF, et al. Determinants of procedural pain intensity in the intensive care unit. The Europain(R) study. Am J Respir Crit Care Med. 2014;189(1):39–47. doi: 10.1164/rccm.201306-1174OC. [DOI] [PubMed] [Google Scholar]
- 10.Gelinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;15(4):420–427. [PubMed] [Google Scholar]
- 11.Chanques G, Jaber S, Barbotte E, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34(6):1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- 12.Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J Investigators D. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post Hoc analysis of the DOLOREA study. Anesthesiology. 2009;111(6):1308–1316. doi: 10.1097/ALN.0b013e3181c0d4f0. [DOI] [PubMed] [Google Scholar]
- 13.Erstad BL, Puntillo K, Gilbert HC, et al. Pain management principles in the critically ill. Chest. 2009;135(4):1075–1086. doi: 10.1378/chest.08-2264. [DOI] [PubMed] [Google Scholar]
- 14.Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 15.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka LM, Azevedo LC, Park M, et al. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Crit Care. 2014;18(4):R156. doi: 10.1186/cc13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balzer F, Weiss B, Kumpf O, et al. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Crit Care. 2015;19:197. doi: 10.1186/s13054-015-0929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandharipande P, Banerjee A, McGrane S, Ely EW. Liberation and animation for ventilated ICU patients: the ABCDE bundle for the back-end of critical care. Crit Care. 2010;14(3):157. doi: 10.1186/cc8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335(25):1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 20.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 21.Mehta S, Burry L, Cook D, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012;308(19):1985–1992. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 22.Hughes CG, Girard TD, Pandharipande PP. Daily sedation interruption versus targeted light sedation strategies in ICU patients. Crit Care Med. 2013;41(9 Suppl 1):S39–45. doi: 10.1097/CCM.0b013e3182a168c5. [DOI] [PubMed] [Google Scholar]
- 23.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375(9713):475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 24.Khan BA, Guzman O, Campbell NL, et al. Comparison and agreement between the Richmond Agitation-Sedation Scale and the Riker Sedation-Agitation Scale in evaluating patients' eligibility for delirium assessment in the ICU. Chest. 2012;142(1):48–54. doi: 10.1378/chest.11-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 26.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114(2):541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 27.Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Annals of the American Thoracic Society. 2014;11(3):367–374. doi: 10.1513/AnnalsATS.201306-210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Seymour CW, Pandharipande PP, Koestner T, et al. Diurnal sedative changes during intensive care: impact on liberation from mechanical ventilation and delirium. Crit Care Med. 2012;40(10):2788–2796. doi: 10.1097/CCM.0b013e31825b8ade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 31.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 32.Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Association. AP. Diagnostic and statistical manual of mental disorders: DSM–5. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 34.Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC. The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and Intensive Care Delirium Screening Checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16(4):R115. doi: 10.1186/cc11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 36.Mitasova A, Kostalova M, Bednarik J, et al. Poststroke delirium incidence and outcomes: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2012;40(2):484–490. doi: 10.1097/CCM.0b013e318232da12. [DOI] [PubMed] [Google Scholar]
- 37.Naidech AM, Beaumont JL, Rosenberg NF, et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am J Respir Crit Care Med. 2013;188(11):1331–1337. doi: 10.1164/rccm.201307-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han JH, Wilson A, Graves AJ, et al. Validation of the Confusion Assessment Method for the Intensive Care Unit in older emergency department patients. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2014;21(2):180–187. doi: 10.1111/acem.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith HA, Boyd J, Fuchs DC, et al. Diagnosing delirium in critically ill children: Validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit Care Med. 2011;39(1):150–157. doi: 10.1097/CCM.0b013e3181feb489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith HAB, Gangopadhyay M, Goben CM, et al. The Preschool Confusion Assessment Method for the ICU: Valid and Reliable Delirium Monitoring for Critically Ill Infants and Children*. Crit Care Med. 2016;44(3):592–600. doi: 10.1097/CCM.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624–636. doi: 10.1378/chest.06-1795. [DOI] [PubMed] [Google Scholar]
- 42.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–139. [PubMed] [Google Scholar]
- 43.Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 2007;33(6):1007–1013. doi: 10.1007/s00134-007-0618-y. [DOI] [PubMed] [Google Scholar]
- 44.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 45.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31(4):1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 46.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 47.Salluh JI, Wang H, Schneider EB, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857. [PubMed] [Google Scholar]
- 50.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119(6):474–481. doi: 10.7326/0003-4819-119-6-199309150-00005. [DOI] [PubMed] [Google Scholar]
- 51.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, Li HL, Wang DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Crit Care Med. 2012;40(3):731–739. doi: 10.1097/CCM.0b013e3182376e4f. [DOI] [PubMed] [Google Scholar]
- 53.Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. The lancet Respiratory medicine. 2013;1(7):515–523. doi: 10.1016/S2213-2600(13)70166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;371(3):287–288. doi: 10.1056/NEJMc1406274. [DOI] [PubMed] [Google Scholar]
- 55.Morris PE. Moving our critically ill patients: mobility barriers and benefits. Crit Care Clin. 2007;23(1):1–20. doi: 10.1016/j.ccc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 57.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 58.Sacanella E, Perez-Castejon JM, Nicolas JM, et al. Functional status and quality of life 12 months after discharge from a medical ICU in healthy elderly patients: a prospective observational study. Crit Care. 2011;15(2):R105. doi: 10.1186/cc10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit - A prospective multicenter study. Jama-J Am Med Assoc. 2002;288(22):2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 60.Bednarik J, Vondracek P, Dusek L, Moravcova E, Cundrle I. Risk factors for critical illness polyneuromyopathy. J Neurol. 2005;252(3):343–351. doi: 10.1007/s00415-005-0654-x. [DOI] [PubMed] [Google Scholar]
- 61.Kleyweg RP, van der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve. 1991;14(11):1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 62.Dammeyer J, Dickinson S, Packard D, Baldwin N, Ricklemann C. Building a protocol to guide mobility in the ICU. Critical care nursing quarterly. 2013;36(1):37–49. doi: 10.1097/CNQ.0b013e3182750acd. [DOI] [PubMed] [Google Scholar]
- 63.Freeman R, Maley K. Mobilization of intensive care cardiac surgery patients on mechanical circulatory support. Critical care nursing quarterly. 2013;36(1):73–88. doi: 10.1097/CNQ.0b013e31827532c3. [DOI] [PubMed] [Google Scholar]
- 64.Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 65.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moss M, Nordon-Craft A, Malone D, et al. A Randomized Trial of an Intensive Physical Therapy Program for Acute Respiratory Failure Patients. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201505-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davidson JE, Powers K, Hedayat KM, et al. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004–2005. Crit Care Med. 2007;35(2):605–622. doi: 10.1097/01.CCM.0000254067.14607.EB. [DOI] [PubMed] [Google Scholar]
- 68.Phipps LM, Bartke CN, Spear DA, et al. Assessment of parental presence during bedside pediatric intensive care unit rounds: effect on duration, teaching, and privacy. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2007;8(3):220–224. doi: 10.1097/01.PCC.0000262798.84416.C5. [DOI] [PubMed] [Google Scholar]
- 69.Cameron MA, Schleien CL, Morris MC. Parental presence on pediatric intensive care unit rounds. J Pediatr. 2009;155(4):522–528. doi: 10.1016/j.jpeds.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 70.Lilly CM, De Meo DL, Sonna LA, et al. An intensive communication intervention for the critically ill. Am J Med. 2000;109(6):469–475. doi: 10.1016/s0002-9343(00)00524-6. [DOI] [PubMed] [Google Scholar]
- 71.Campbell ML, Guzman JA. Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest. 2003;123(1):266–271. doi: 10.1378/chest.123.1.266. [DOI] [PubMed] [Google Scholar]
- 72.Schneiderman LJ, Gilmer T, Teetzel HD, et al. Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: a randomized controlled trial. JAMA. 2003;290(9):1166–1172. doi: 10.1001/jama.290.9.1166. [DOI] [PubMed] [Google Scholar]
- 73.Schneiderman LJ, Gilmer T, Teetzel HD. Impact of ethics consultations in the intensive care setting: a randomized, controlled trial. Crit Care Med. 2000;28(12):3920–3924. doi: 10.1097/00003246-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 74.Curtis JR, Engelberg RA, Wenrich MD, Shannon SE, Treece PD, Rubenfeld GD. Missed opportunities during family conferences about end-of-life care in the intensive care unit. Am J Respir Crit Care Med. 2005;171(8):844–849. doi: 10.1164/rccm.200409-1267OC. [DOI] [PubMed] [Google Scholar]
- 75.Dowdy MD, Robertson C, Bander JA. A study of proactive ethics consultation for critically and terminally ill patients with extended lengths of stay. Crit Care Med. 1998;26(2):252–259. doi: 10.1097/00003246-199802000-00020. [DOI] [PubMed] [Google Scholar]
- 76.Carson SS, Cox CE, Wallenstein S, et al. Effect of Palliative Care-Led Meetings for Families of Patients With Chronic Critical Illness: A Randomized Clinical Trial. JAMA. 2016;316(1):51–62. doi: 10.1001/jama.2016.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jabre P, Belpomme V, Azoulay E, et al. Family presence during cardiopulmonary resuscitation. N Engl J Med. 2013;368(11):1008–1018. doi: 10.1056/NEJMoa1203366. [DOI] [PubMed] [Google Scholar]