Abstract
Accumulating evidence suggests that the central locus for the progression of chronic kidney disease (CKD) is the renal proximal tubule. As injured tubular epithelial cells dedifferentiate in attempted repair they stimulate inflammation and recruit myofibroblasts. At the same time, tissue loss stimulates remnant nephron hypertrophy. Increased tubular transport workload eventually exceeds the energy-generating capacity of the hypertrophied nephrons, leading to anerobic metabolism, acidosis, hypoxia, endoplasmic reticulum stress and the induction of additional inflammatory and fibrogenic responses. The result is a vicious cycle of injury, misdirected repair, maladaptive responses and more nephron loss. Therapy that might be advantageous at one phase of this progression pathway could be deleterious during other phases. Thus, interrupting this downward spiral requires narrowly targeted approaches that promote healing and adequate function without generating further entry into the progression cycle.
The primary anatomical locus driving progressive chronic kidney disease (CKD) remains controversial, with debate cycling through different segments of the nephron. It is likely that each part of the nephron contributes. But a prominent role is played by the proximal tubule. Even in primary glomerular disease, where recent research emphasis has focused on the podocyte dysfunction that initiates glomerular injury,1 the resulting proteinuria2,3 and the formation of glomerular synechiae that lead to extrusion of the plasma contents into the tissue;4 the only pathologic processes that have been strongly implicated in progression relate to the tubulointerstitium. Indeed, the best clinical marker for progression of focal segmental glomerulosclerosis is tubulointerstitial inflammation.5,6 Further, a widely accepted mechanism of progression involves a lesion at the glomerulotubular junction that interrupts the passage of filtrate from the glomerulus into the tubule.4 In a number of diseases of either glomerular or tubular origin, the presence of atubular glomeruli7 suggests that the critical event is the demise of the proximal tubule.8 Anatomical studies of Bright’s disease by Oliver9 implicated proximal tubule hypertrophy, consistent with more recent studies of diabetic nephropathy.10 Given these findings in varied conditions, it is appropriate to consider the role of the tubulointerstitium in a progression pathway that is common to all CKD.
Clinical clues to the pathogenesis of progression
Insight into the pathogenesis of CKD can be derived from risk factors that are not modifiable, those that can be modified by medical intervention, and additional, environmental factors that could contribute to progression (reported by other authors11–15 and reviewed by this author in more detail elsewhere16). Non-modifiable risk factors include fetal programming/low nephron number; poor kidney function at the time of clinical presentation; and, in children, significant somatic growth in the presence of kidney dysfunction or decreased renal mass. These factors have in common that they involve increased amounts of work by the nephrons that remain after the initial injury. Potentially modifiable risk factors include obesity, hypertension, acidosis, proteinuria, anemia, vascular dysfunction and cigarette smoking. Obesity17 may contribute to progression by increasing per-nephron load, as is the case for the non-modifiable risk factors listed above, or it may reflect metabolic factors that affect kidney function. Hypertension18,19 remains a complex issue. The influence of high blood pressure has been attributed to modified circulation,20 hyperfiltration21 or proteinuria.22 Both experimental23 and clinical24 data support acidosis as a modifiable progression factor. It has been suggested that acidosis plays a role in the activation of the terminal complement pathway;25 other effects on metabolism remain to be tested. The consideration of proteinuria as a potentially modifiable progression factor is widely accepted by nephrologists,13,18,19 although the mechanism by which proteinuria engenders progression remains poorly understood (see below). Finally, the impacts of anemia,26 cigarette smoking27 and direct effects of uremia on vascular function28,29 support a role for renal perfusion in the maintenance of renal function.
A risk factor that may or may not relate to perfusion, acute kidney injury (AKI), has received considerable attention recently. Clinical and epidemiological data indicate that CKD is much more common in individuals who have experienced an episode of AKI,14 and experimental models support this observation.30 AKI also is a progression factor in patients who have CKD.31 Experimental studies have defined a cascade of events that are initiated by AKI.32 Many of these events, and the contributing factors that are listed above, can be placed into a common schema wherein tubulointerstitial mechanisms involved in either normal function or attempted repair, usually beneficial, generate a vicious cycle that leads to the ultimate demise of the kidney. These mechanisms will be discussed here.
Mechanisms of renal injury and repair may lead to CKD
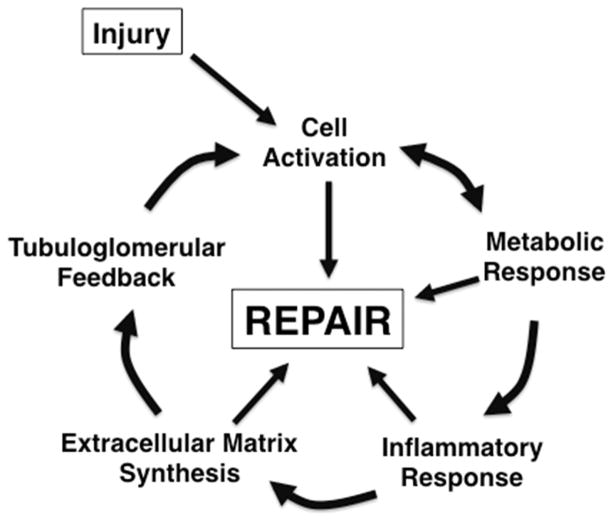
In adults, the primary causes of renal impairment are diabetes and hypertension.33 In children, these actually are rare causes of CKD, with more common causes being developmental abnormalities and genetically determined disorders, supplemented by acquired causes such as glomerular disease.34–36 Recent data suggest that, even in adult disorders, genetics may play a significant role in determining which patients are more likely to develop CKD.37,38 Regardless of the stimulus in adults or children, renal injury initiates a repair process that involves five components that are mutually reinforcing (Figure 1). Cell activation occurs to permit tissue cell precursors to multiply and migrate into areas where repair is required. This process actually involves multiple events, including the stimulation of cell division, production of chemokines and adhesion molecules to recruit cells to the area of need, and re-differentiation of the precursor cells into functional tissue. Altered metabolism is needed to respond to the changing needs for different cell populations as they undergo dedifferentiation, proliferation, repair and redifferentiation. Inflammation occurs to remove debris in order to permit healing to occur. To promote cell trafficking and subsequent structural integrity, extracellular matrix (ECM) production is required.39 ECM provides a provisional matrix for cell migration and the assembly of structures, and offers new material to support these structures and maintain requisite cell phenotype. Finally, throughout this process, the nephron must maintain a relatively controlled balance among physiological parameters in order to protect body homeostasis. It does so through the production and regulation of a number of hormonal mediators including those that regulate not only renal function but also calcium/phosphorus homeostasis, erythropoiesis and blood pressure. Bricker and colleagues proposed that these hormonal mediators, produced to maintain normal renal physiology, have extension effects on non-target tissues that underlie the pathogenesis of the uremic state.40,41 Within the kidney, a critical factor in homeostasis is the tubuloglomerular feedback that maintains body fluid and electrolyte balance.
FIGURE 1.
Multiple biological processes contribute to repair of the injured kidney.
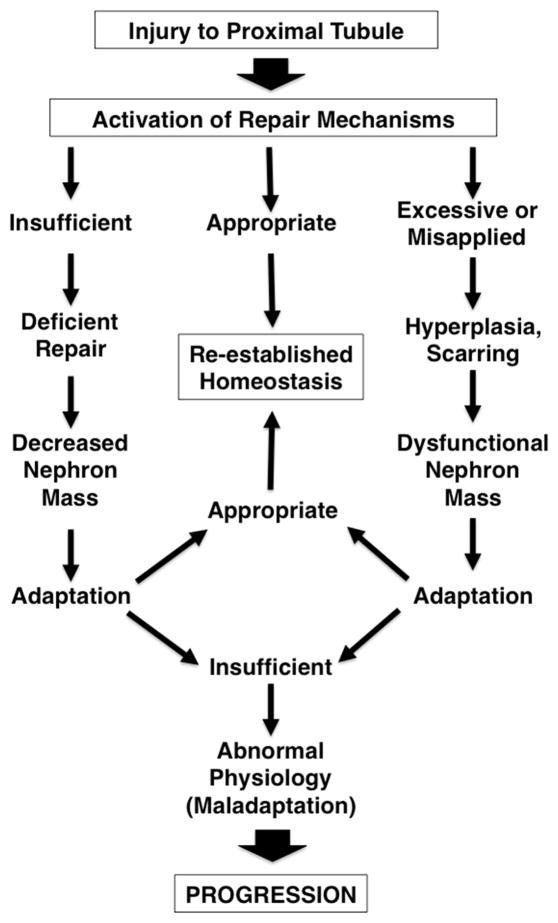
While repair functions are essential, they must be tightly regulated. If they are applied to an inappropriate target or in an unbalanced manner, these same processes promote misdirected repair, leading to renal dysfunction, scarring and CKD (Figure 2). Given the delicate balance and structure-function relationships in the kidney, insufficient, excessive or inappropriately applied repair mechanisms yield decreased functional renal mass. The kidney must respond to these changes. If there is appropriate adaptation, homeostasis is reestablished, even if at a level of renal function that may be somewhat below the previous steady-state. If, however, adaptation requires ongoing compensatory mechanisms, these mechanisms may cause further injury to the remaining nephrons in a vicious cycle of injury, maladaptation and misdirected repair. This latter series of events defines the course of progressive CKD.
FIGURE 2.
Possible outcomes of repair after injury. If repair is appropriately applied and adequate, normal function is re-established. But if repair is insufficient, nephron mass is decreased; if it is excessive or misapplied, dysfunctional tissue results. In either of these latter cases, the remnant kidney must adapt. Successful adaptation also re-establishes homeostasis, but maladaptation leads to further cycles of injury and repair and chronic, progressive disease.
Many cells and proteins that contribute to normal homeostasis in the tubulointerstitial milieu also may contribute to progression. Table 1 lists many such cells and their functions. In particular, the renal tubular cell produces, among other proteins, endothelin-1 (vasoconstriction42), hypoxia-inducible factors HIFs (profibrotic and altering metabolism43,44, kidney-injury molecule (KIM)-1 (adhesion and regeneration45,46), macrophage chemoattractant protein (MCP)-1 (chemokine47) and transforming growth factor (TGF)-β (Smad protein48). Two examples of how the same processes may be involved in both repair and progression are offered here, involving the roles of the hypoxia-inducible factors (HIFs) and kidney injury-molecule (KIM)-1. HIFs have long been suspected of being involved in progression. HIF α-chains are rapidly destabilized by prolyl hydroxylases, so that the α/β HIF heterodimer is short-lived under normal conditions. Under conditions of hypoxia, the HIF α-chain is stabilized. HIFs act as transcription factors and provide a central component of the response to hypoxia by promoting the expression of erythropoietin and vascular endothelial growth factor (VEGF), as well as multiple genes involved in the regulation of metabolism.49 However, the HIFs also promote the expression of numerous genes that could contribute to fibrosis. Genetic manipulation of HIF-1α expression in mice has demonstrated that HIF is profibrotic in several mouse models of progressive CKD.43,50,51 However, inhibition of HIF exacerbates injury in several other models.52–55 While it is possible that these results are model dependent, the general trend suggests that HIFs play a role in protecting the kidney against acute hypoxic injury, whereas they may play a deleterious role in more chronic, fibrotic injury. Given that repeated episodes of AKI presage CKD32,56 and that the tubulointerstitium is a region of relatively low oxygen tension,57 elucidating how the outcome of HIF signaling is determined remains an important consideration in approaching CKD.
Table 1.
Tubulointerstitial cells that may contribute to progression*
| Cell type | Product or function |
|---|---|
| Endothelium | NO production, Tissue perfusion |
| Tubule cell | Endothelin, HIF, KIM-1, MCP-1, TGF-β, others |
| Juxtaglomerular cells | Renin |
| Interstitial cells | Erythropoietin |
| Pericyte | Differentiation into fibroblasts |
| Resident fibroblast | ECM production |
| Myofibroblast | ECM production/scarring |
| Macrophage | Phagocytosis, cytokines, ROS |
| Dendritic cell | DAMP pattern sensing; T cell activation |
| T lymphocytes | Cytokine production |
| Platelets | Endothelial dysfunction |
This list is not complete for either cell types or functions, but is provided to offer an indication that multiple cell types participate in progression. DAMP, danger/damage-associated molecular patterns; ECM, extracellular matrix; HIF, hypoxia-inducible-factor; KIM-1, kidney injury molecule-1; MCP-1, macrophage chemoattractant protein-1; NO, nitric oxide; ROS, reactive oxygen species; TGF-β, transforming growth factor-β.
Similarly. KIM-1 may have different roles under different conditions. Originally described as a sensitive biomarker for AKI,46 it was subsequently determined to be an adhesion molecule that promotes renal regeneration.45 It also confers a phagocytic phenotype on tubular cells58 that reduces the extent of renal injury in AKI.59 However, chronic overexpression of KIM-1 induces renal fibrosis.60 It is likely that such events as activation, phagocytosis and immune stimulation are important for repair after AKI but, misapplied chronically, contribute to progression.
The pathophysiology of progressive CKD
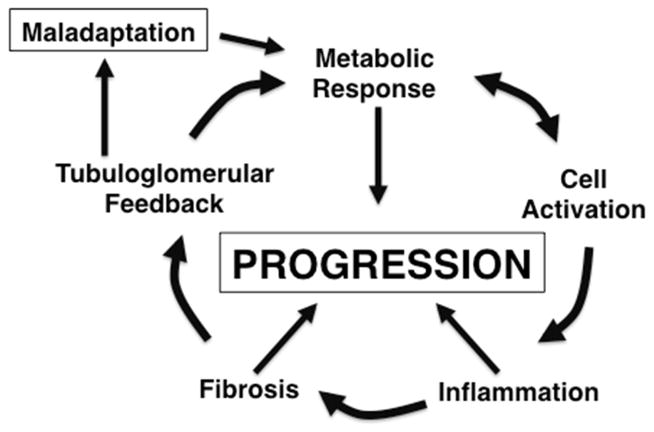
Accepting the premise underlying evolution, pathogenic mechanisms per se represent “normal” physiological mechanisms that are dysregulated for some reason. To illustrate this principle, Figure 3 represents a modification of Figure 1; the same processes are now referred to by the manner in which they contribute to CKD progression. Each will be considered here.
FIGURE 3.
Biological process involved in progression are parallel to those involved in repair. Compare this figure with Figure 1. Although activated cells show increased metabolic activity, for progression the metabolic response is placed before activation to emphasize the role of altered metabolism after the compensatory hypertrophic response of the nephron.
Cell activation
The chemotactic and cytokine activity mediating both repair and progression is likely derived from multiple sources. The renal tubular cell itself may have immunologic properties when appropriately activated, including phagocytosis and subsequent antigen presentation,61 as well as co-stimulation of dendritic cells62,63 or lymphocytes.64 The proximal tubular cell is activated to migrate and proliferate in order to replenish the tubular structure, but it also produces chemoattractants and fibrogenic factors (reviewed in65,66). Some examples are listed in Table 1. Cells in the tubulointerstitium produce a variety of proinflammatory and profibrotic agents. The origin of the tubulointerstitial myofibroblast will be discussed below.
Importantly, in response to injury, cells that normally are stably differentiated to promote homeostasis may instead dedifferentiate into a phenotype to support the reorganization of new functional units. For example, in AKI renal tubular epithelial cells may undergo a phenotypic switch from a columnar epithelium with a brush border and tight intercellular junctions that facilitate electrolyte transport, to a cell that may divide, migrate and take on a secretory phenotype, producing chemokines and inflammatory mediators. This process of epithelial-to-mesenchymal transition (EMT), a normal part of the response to injury that promotes healing, also may be an important contributor to repeated, ongoing cycles of tissue damage and misdirected repair.
Inflammation
As described above, cellular infiltrates are a hallmark of chronic progression. With injury, macrophages are recruited through the production of inflammatory cytokines such as MCP-167 and fractalkine68 to remove debris and permit regeneration.69 Interrupting adhesion molecule expression decreases this recruitment.65,70 The macrophages themselves produce a number of inflammatory molecules including mediators of further inflammation and fibrogenesis47 such as tumor necrosis factor (TNF)-α, platelet-derived growth factor (PDGF), basic fibroblast growth factor (FGF2), transforming growth factor (TGF)-β and reactive oxygen species (ROS). This process has been likened to that involved in sepsis. In sepsis, both a spectrum of endogenous inflammatory and other mediators called danger/damage-associated molecular patterns (DAMPs) and a similar spectrum of pathogen-associated molecular patterns (PAMPs) activate Toll-like receptors (TLRs) and NOD-like receptors (NLRs) to disrupt cellular metabolism, alter vascular perfusion and activate a number of metabolic processes in the kidney, centered upon mitochondria and energy metabolism.71 A similar set of responses may be mediated by DAMPs in CKD progression (reviewed in72).
Fibrosis
Fibrosis involves the replacement of normal, functioning tissue with scar. The accumulating extracellular matrix (ECM) in the scarred kidney includes both increased amounts of “normal” renal ECM73,74 and abnormal types or locations of ECM.75,76 For example, fibronectin is present in small quantities in the normal kidney77 but increases in disease sa part of the local response to injury. Changes in the quantity and type of ECM that are present may affect the cell-matrix interactions that regulate cell function and phenotype.78,79 As cells are injured, they produce atypical forms and amounts of ECM. This response alters the signals transmitted into the cell from the ECM, which in turn leads to further dedifferentiation of the cells in a vicious cycle that promotes EMT and maintenance of the mesenchymal phenotype.
Continued debate surrounds the origin of the actual scar-producing cell(s) in CKD.80 The myofibroblast (MFb) has been attributed to (1) activation of quiescent, resident fibroblasts in the kidney,81 (2) recruitment from the bone marrow or other distant sites82,83 or (3) EMT of other cells that already reside in the kidney. Although it has been suggested that the tubular epithelium is the precursor of the MFb,84,85 recent attention has focused on the vascular pericyte,86,87 a multipotential cell that may differentiate into an adipocyte as well as to a fibroblast. To a degree, this debate is important mostly to determine whether there is a specific cell that might be targeted to directly inhibit the excess production of ECM. In actuality, multiple cell types participate in the pathogenesis of renal fibrosis. These include not only the MFb, but also tubular cells that recruit inflammatory cells and activate fibroblasts, macrophages that induce further immune responses and promote ROS generation, and local cells that mediate the production of renin, VEGF, chemokines, TGF-β, etc. Metaphorically speaking, the MFb could be viewed as the “soloist” in an orchestra of cells that generate the “symphony” of fibrosis. All parts are necessary for the whole.
The fibrogenic role of urine or glomerular filtrate also should be considered. Kriz and colleagues have put forth a model4 in which loss of podocytes permits synechiae between the glomerular tuft and Bowman’s capsule, permitting the extrusion of plasma contents directly into the adjacent tubulointerstitium. The resulting inflammation precipitates scarring. A similar phenomenon could explain the apparent relationship between severity of proteinuria and progression of tubulointerstitial disease. Intratubular delivery of plasma proteins, lipids and metals would lead to their reabsorption by the tubule. Intracellular or interstitial accumulation of these moieties would then stimulate all of the pathogenic processes described here.42 The exact nature of the fibrogenic signal in urine remains uncertain.
Tubuloglomerular feedback
As damage to the kidney causes nephron loss, physical forces, altered processing of glomerular filtrate and changes in distal tubular delivery of fluid and solute lead to altered renin production at the macula densa. The renin-angiotensin-aldosterone system (RAS) appears to play a significant role in nephron hypertrophy after kidney injury88 and stimulates the production of fibrogenic factors.89 It is possible that the RAS also pathologically modulates blood flow to the nephron. Decreased perfusion could be a significant maintenance factor in CKD progression.20 Alternatively, the role of renin may not be based solely on blood pressure, perfusion or proteinuria. The ESCAPE trial in children found that the effects of RAS antagonism on progression remain even if blood pressure and proteinuria have returned to baseline levels after 2–3 years of treatment.90
Metabolic changes
This additional effect of RAS antagonism could be explained on the basis of nephron hypertrophy. Cell activation and metabolic responses occur in tandem in both AKI and CKD. However, whereas in Figure 1 cell activation precedes metabolic changes, for conceptual purposes the order is reversed in the cycle shown in Figure 3. This change accounts for the central role of tubuloglomerular feedback in progression. Although overall glomerular filtration decreases in CKD, selective glomerular filtration may increase. As nephrons are lost, a combination of physical forces and active regulation (e.g., through the RAS) leads to increases in perfusion, single-nephron plasma flow and SNGFR. In order to maintain glomerulotubular balance, tubular reabsorption must become more aggressive. More energy production is required to support the consequently increased tubular transport activity, requiring changes in tubular metabolism. The increased consumption of oxygen and substrate causes hypoxia and metabolic stress that are pro-inflammatory91 and profibrotic. Paradoxically, the essential physiological mechanism of glomerulotubular balance, by meeting the need to absorb locally increased amounts of filtrate, stimulates hypertrophy, placing an increased metabolic load on the tubule and forcing the tubular cell to and beyond the limits of its capacity. Increased protein synthesis and relative hypoperfusion cause endoplasmic reticulum stress92 that may lead to tubular cell apoptosis, further fibrosis,93 and more nephron loss.94 Notably, this concept places a new perspective on the classical work of Brenner and others, in which such factors as increased glomerular protein load were noted to cause hyperfiltration95 and subsequent glomerulosclerosis. Arguably, the sequence of events is that hyperfiltration causes increased per-nephron workload, leading to tubular hypertrophy and the metabolic changes that are described here. Glomerulosclerosis could be a secondary event.
The tubule as a major determinant of progression
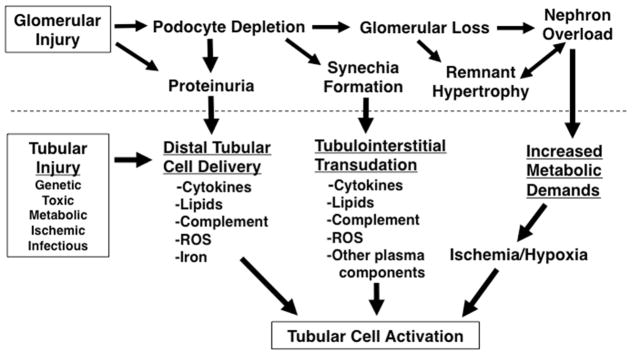
A model can therefore be proposed in which tubular injury represents the ultimate, final common pathway for CKD progression. As shown in Figure 4, even primarily glomerular disease contributes to progression via the tubule. Podocyte depletion causes misdirected filtration, with transudation of plasma causing inflammation and nephron loss. Similarly, proteinuria activates tubular cells to mediate fibrogenic responses. As nephrons are lost, remnant nephron hypertrophy causes increased metabolic demand. Primary tubular injury more directly activates this deleterious tubular response.
FIGURE 4.
Both glomerular and tubular injury lead to tubulointerstitial responses and renal tubular cell activation, potentially initiating progressive CKD. Reprinted with permission from.89
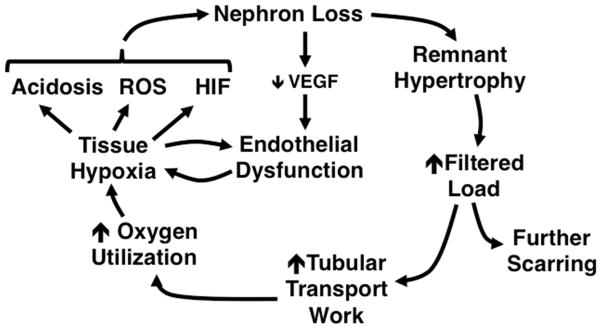
The result is the cycle of progression depicted in Figure 5. Acutely upon tubular loss, physical factors provide the same amount of blood to a lesser number of nephrons, increasing single-nephron perfusion. The renin-angiotensin system system is activated and, in part, alters autoregulation to maintain perfusion rates. However, within a matter of hours, this same system also triggers an increase in renal DNA synthesis and protein expression, resulting in more permanent, hypertrophic changes.96 Increased perfusion places an increased filtered load on the nephron. This has two effects. The first is delivery of biologically active molecules to the tubule, as described above, causing further scarring. Secondly, this increased filtration necessitates increased tubular transport work to maintain glomerulotubular balance. The kidney receives up to 25% of the cardiac output at rest, and expends large quantities of energy for the active transport that is needed to reabsorb 99% of the filtrate. The kidney consumes about 400 μMol oxygen per minute to provide the energy source (ATP) needed to meet this demand.97 Although the total oxygen utilization by the CKD kidney is decreased, the per-nephron oxygen utilization is increased.97 Because blood flow is relatively sluggish in the tubulointerstitium, it is at baseline a somewhat hypoxic microenvironment,57 and decreased perfusion may further deny oxygen and substrate, depressing ATP synthesis.
FIGURE 5.
Remnant nephron hypertrophy may create a vicious cycle in which processes that preserve functional homeostasis drive further nephron loss. Adapted with permission from.16
The result is tissue hypoxia, which has multiple effects. A shift in metabolism from oxidative phosphorylation to glycolysis98 promotes the development of acidosis, which, as described in the clinical section of this article, accelerates the progression of CKD. Because of its high metabolic needs, the renal tubular cell contains large amounts of mitochondria to generate ATP.99 In response to hypoxia, mitochondrial complex 3 is stabilized,100 generating superoxide that ultimately raises the levels of various reactive oxygen species (ROS) in the cytoplasm. At low concentrations ROS function as intracellular signaling molecules, but at high concentrations they may alter the structure of receptors or other signaling proteins, interfering with the normal regulation of these molecules. Hypoxia exacerbates ER stress, leading to the autophagy of proteins and even mitophagy of mitochondria.92 The decrease in mitochondria further decreases ATP generation. While the source of intracellular ROS remains somewhat controversial,101–103 one downstream mediator of ROS actions is the stabilization and generation of HIF, which promotes extracellular matrix expression50 and decreases cell metabolic rates further. With decreased tubular function, less local generation of pro-angiogenic factors such as VEGF leads to decreased health of the peritubular vasculature,104 further promoting hypoxia and HIF expression.55 HIF itself stimulates the expression of numerous profibrotic factors.105 The result is further nephron loss and continuance of this vicious cycle.106
Based on this model, it is reasonable to propose that the renal tubule plays a central role in the progression of CKD. It sends signals to other tissues in the kidney and more distantly, recruiting inflammatory cells and ECM-producing cells. Locally, it activates other cells to participate in the perpetuation of renal injury and the replacement of healthy tissue with scar. Remnant nephron hypertrophy triggers a series of events by which normal physiological functions of the nephron, applied to maintain that function, lead to its demise.
Clinical and research implications of this paradigm
Given this set of circumstances, a primary goal for research and treatment should be to attempt to resolve a paradox: treatments that enhance kidney function may also accelerate progression and, conversely, treatments to delay progression may also require diminishing remnant-nephron adaptive responses. Thus, identifying the means to promote oxygen and substrate delivery to the nephron would moderate the negative impact of hypertrophy on metabolism. A better understanding of disease mechanisms might also enhance our ability to treat certain conditions. In the examples mentioned earlier, KIM-1 and HIF appear to contribute significantly to favorable outcome in AKI. Addressing the way in which these molecules advance progression, rather than blocking these molecules directly, would leave the favorable effects intact but delay the progression of CKD.
Even if such optimal treatments are identified, their application will be complicated. Therapy that might be advantageous early in the disease course—such as facilitating repair—could be deleterious later, after misdirected or unbalanced repair becomes a major mechanism of scarring. Unfortunately, we lack accurate markers for these different phases of response to injury.107 The situation is further complicated by the heterogeneity of the kidney; at any given time, different areas of the kidney may be undergoing repair, scarring or the physiological progression of nephron loss. The key issues are thus identifying (1) appropriate and rationally-designed therapies, (2) specific targets for those treatments and (3) the means to direct treatments to their intended targets. Alternatively, this conundrum illustrates the importance of continuing study to understand and inhibit the pathogenesis of primary diseases, before tubular maladaptation and progression supervene.
CLINICAL SUMMARY.
The tubulointerstitium contributes to CKD progression in all kidney diseases.
Activation and dedifferentiation of proximal tubular cells mediates multiple components of the fibrogenic response.
A critical factor in the pathophysiology of progression is remnant nephron hypertrophy.
All of the components of this pathophysiology represent normal, beneficial tubular functions that are misapplied in a maladaptive response to injury.
Acknowledgments
HWS effort and related research were supported in part by grants R01-DK49362 from the National Institute of Diabetes Digestive and Kidney Diseases, UL1TR001422 from the National Center for Advancing Translational Sciences, and grants from the Feinberg School of Medicine and the Stanley Manne Children’s Research Institute.
Footnotes
The author has no conflicts to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney international. 2007;71(12):1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 2.Fried LF, Lewis J. Albuminuria is Not an Appropriate Therapeutic Target in Patients with CKD: The Con View. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(6):1089–1093. doi: 10.2215/CJN.10681014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambers Heerspink HJ, Gansevoort RT. Albuminuria Is an Appropriate Therapeutic Target in Patients with CKD: The Pro View. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(6):1079–1088. doi: 10.2215/CJN.11511114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney international. 1998;54(3):687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonsib SM. Focal-segmental glomerulosclerosis. The relationship between tubular atrophy and segmental sclerosis. Am J Clin Pathol. 1999;111(3):343–348. doi: 10.1093/ajcp/111.3.343. [DOI] [PubMed] [Google Scholar]
- 6.Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. II. The correlations. Human pathology. 1970;1(4):631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- 7.Forbes MS, Thornhill BA, Park MH, Chevalier RL. Lack of endothelial nitric-oxide synthase leads to progressive focal renal injury. The American journal of pathology. 2007;170(1):87–99. doi: 10.2353/ajpath.2007.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. American journal of physiology. Renal physiology. 2016;311(1):F145–161. doi: 10.1152/ajprenal.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver J. Architecture of the Kidney in Chronic Bright’s Disease. New York: Hoeber; 1939. [Google Scholar]
- 10.Tang SC, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(8):3049–3056. doi: 10.1093/ndt/gfs260. [DOI] [PubMed] [Google Scholar]
- 11.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney international. 2011;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herget-Rosenthal S, Dehnen D, Kribben A, Quellmann T. Progressive chronic kidney disease in primary care: modifiable risk factors and predictive model. Preventive medicine. 2013;57(4):357–362. doi: 10.1016/j.ypmed.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim F, Hamzah L, Jones R, et al. Baseline kidney function as predictor of mortality and kidney disease progression in HIV-positive patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012;60(4):539–547. doi: 10.1053/j.ajkd.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. Journal of the American Society of Nephrology: JASN. 2009;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Annals of internal medicine. 2003;139(4):244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 16.Schnaper HW. Pathophysiology of progressive renal disease. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Emma F, Goldstein SL, editors. Pediatric Nephrology. 4. Vol. 2016. Berlin: Springer; 2016. pp. 2171–2206. [Google Scholar]
- 17.McMahon GM, Preis SR, Hwang SJ, Fox CS. Mid-adulthood risk factor profiles for CKD. Journal of the American Society of Nephrology: JASN. 2014;25(11):2633–2641. doi: 10.1681/ASN.2013070750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2013;185(11):949–957. doi: 10.1503/cmaj.121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Perna A, Benini R, et al. In chronic nephropathies prolonged ACE inhibition can induce remission: dynamics of time-dependent changes in GFR. Investigators of the GISEN Group. Gruppo Italiano Studi Epidemiologici in Nefrologia. Journal of the American Society of Nephrology: JASN. 1999;10(5):997–1006. doi: 10.1681/ASN.V105997. [DOI] [PubMed] [Google Scholar]
- 20.Kheder-Elfekih R, Yannoutsos A, Blacher J, London GM, Safar ME. Hypertension and chronic kidney disease: respective contribution of mean and pulse pressure and arterial stiffness. J Hypertens. 2015;33(10):2010–2015. doi: 10.1097/HJH.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 21.Fabris B, Candido R, Armini L, et al. Control of glomerular hyperfiltration and renal hypertrophy by an angiotensin converting enzyme inhibitor prevents the progression of renal damage in hypertensive diabetic rats. J Hypertens. 1999;17(12 Pt 2):1925–1931. doi: 10.1097/00004872-199917121-00023. [DOI] [PubMed] [Google Scholar]
- 22.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P. Sodium intake, ACE inhibition, and progression to ESRD. Journal of the American Society of Nephrology: JASN. 2012;23(1):165–173. doi: 10.1681/ASN.2011040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goraya N, Wesson DE. Acid-base status and progression of chronic kidney disease. Current opinion in nephrology and hypertension. 2012;21(5):552–556. doi: 10.1097/MNH.0b013e328356233b. [DOI] [PubMed] [Google Scholar]
- 24.Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney international. 2014;86(5):1031–1038. doi: 10.1038/ki.2014.83. [DOI] [PubMed] [Google Scholar]
- 25.Nangaku M, Pippin J, Couser WG. Complement membrane attack complex (C5b-9) mediates interstitial disease in experimental nephrotic syndrome. Journal of the American Society of Nephrology: JASN. 1999;10(11):2323–2331. doi: 10.1681/ASN.V10112323. [DOI] [PubMed] [Google Scholar]
- 26.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Archives of internal medicine. 2009;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orth SR. Smoking--a renal risk factor. Nephron. 2000;86(1):12–26. doi: 10.1159/000045708. [DOI] [PubMed] [Google Scholar]
- 28.Fujimi-Hayashida A, Ueda S, Yamagishi S, et al. Association of asymmetric dimethylarginine with severity of kidney injury and decline in kidney function in IgA nephropathy. American journal of nephrology. 2011;33(1):1–6. doi: 10.1159/000322367. [DOI] [PubMed] [Google Scholar]
- 29.Rossert J, Levin A, Roger SD, et al. Effect of early correction of anemia on the progression of CKD. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;47(5):738–750. doi: 10.1053/j.ajkd.2006.02.170. [DOI] [PubMed] [Google Scholar]
- 30.Basile DP, Leonard EC, Tonade D, Friedrich JL, Goenka S. Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion. American journal of physiology. Renal physiology. 2012;302(5):F625–635. doi: 10.1152/ajprenal.00562.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okusa MD, Chertow GM, Portilla D Acute Kidney Injury Advisory Group of the American Society of N. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clinical journal of the American Society of Nephrology: CJASN. 2009;4(3):520–522. doi: 10.2215/CJN.06711208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. Journal of the American Society of Nephrology: JASN. 2015 doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chronic Kidney Disease (CKD) in the United States. 2014 [Google Scholar]
- 34.Ardissino G, Dacco V, Testa S, et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111(4 Pt 1):e382–387. doi: 10.1542/peds.111.4.e382. [DOI] [PubMed] [Google Scholar]
- 35.Furth SL, Abraham AG, Jerry-Fluker J, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clinical journal of the American Society of Nephrology: CJASN. 2011;6(9):2132–2140. doi: 10.2215/CJN.07100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staples AO, Greenbaum LA, Smith JM, et al. Association between clinical risk factors and progression of chronic kidney disease in children. Clinical journal of the American Society of Nephrology: CJASN. 2010;5(12):2172–2179. doi: 10.2215/CJN.07851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonomo JA, Palmer ND, Hicks PJ, et al. Complement factor H gene associations with end-stage kidney disease in African Americans. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(7):1409–1414. doi: 10.1093/ndt/gfu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer ND, Ng MC, Hicks PJ, et al. Evaluation of candidate nephropathy susceptibility genes in a genome-wide association study of African American diabetic kidney disease. PloS one. 2014;9(2):e88273. doi: 10.1371/journal.pone.0088273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaissling B, Lehir M, Kriz W. Renal epithelial injury and fibrosis. Biochimica et biophysica acta. 2013;1832(7):931–939. doi: 10.1016/j.bbadis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Bricker NS. On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. The New England journal of medicine. 1972;286(20):1093–1099. doi: 10.1056/NEJM197205182862009. [DOI] [PubMed] [Google Scholar]
- 41.Bricker NS, Fine LG, Kaplan M, Epstein M, Bourgoignie JJ, Light A. “Magnification phenomenon” in chronic renal disease. The New England journal of medicine. 1978;299(23):1287–1293. doi: 10.1056/NEJM197812072992307. [DOI] [PubMed] [Google Scholar]
- 42.Theilig F, Kriz W, Jerichow T, et al. Abrogation of protein uptake through megalin-deficient proximal tubules does not safeguard against tubulointerstitial injury. Journal of the American Society of Nephrology: JASN. 2007;18(6):1824–1834. doi: 10.1681/ASN.2006111266. [DOI] [PubMed] [Google Scholar]
- 43.Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7(9):1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW. Interdependence of HIF-1alpha and TGF-beta/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. American journal of physiology. Renal physiology. 2011;300(4):F898–905. doi: 10.1152/ajprenal.00335.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. The Journal of biological chemistry. 2002;277(42):39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 46.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney international. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 47.Hogaboam CM, Steinhauser ML, Chensue SW, Kunkel SL. Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney international. 1998;54(6):2152–2159. doi: 10.1046/j.1523-1755.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- 48.Schiffer M, von Gersdorff G, Bitzer M, Susztak K, Bottinger EP. Smad proteins and transforming growth factor-beta signaling. Kidney international. Supplement. 2000;77:S45–52. doi: 10.1046/j.1523-1755.2000.07708.x. [DOI] [PubMed] [Google Scholar]
- 49.Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Research. 2009 doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumann BC, Hayashida T, Liang X, Schnaper HW. Hypoxia-inducible factor 1-alpha promotes glomerulosclerosis and regulates COL1A2 expression through interactions with Smad3. Kidney international. 2016 doi: 10.1016/j.kint.2016.05.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura K, Iwano M, Higgins DF, et al. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. American journal of physiology. Renal physiology. 2008;295(4):F1023–1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang Y, Yu X, Liu Y, et al. miR-29c is downregulated in renal interstitial fibrosis in humans and rats and restored by HIF-alpha activation. American journal of physiology. Renal physiology. 2013;304(10):F1274–1282. doi: 10.1152/ajprenal.00287.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nordquist L, Friederich-Persson M, Fasching A, et al. Activation of hypoxia-inducible factors prevents diabetic nephropathy. Journal of the American Society of Nephrology: JASN. 2015;26(2):328–338. doi: 10.1681/ASN.2013090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka T, Matsumoto M, Inagi R, et al. Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney international. 2005;68(6):2714–2725. doi: 10.1111/j.1523-1755.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. American journal of physiology. Renal physiology. 2014;307(11):F1187–1195. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 56.Takaori K, Nakamura J, Yamamoto S, et al. Severity and Frequency of Proximal Tubule Injury Determines Renal Prognosis. Journal of the American Society of Nephrology: JASN. 2016;27(8):2393–2406. doi: 10.1681/ASN.2015060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nordquist L, Palm F. Diabetes-induced alterations in renal medullary microcirculation and metabolism. Current diabetes reviews. 2007;3(1):53–65. doi: 10.2174/157339907779802120. [DOI] [PubMed] [Google Scholar]
- 58.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. The Journal of clinical investigation. 2008;118(5):1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang L, Brooks CR, Xiao S, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. The Journal of clinical investigation. 2015;125(4):1620–1636. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Humphreys BD, Xu F, Sabbisetti V, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. The Journal of clinical investigation. 2013;123(9):4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelley VR, Singer GG. The antigen presentation function of renal tubular epithelial cells. Experimental nephrology. 1993;1(2):102–111. [PubMed] [Google Scholar]
- 62.Macconi D, Chiabrando C, Schiarea S, et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. Journal of the American Society of Nephrology: JASN. 2009;20(1):123–130. doi: 10.1681/ASN.2007111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weisheit CK, Engel DR, Kurts C. Dendritic Cells and Macrophages: Sentinels in the Kidney. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(10):1841–1851. doi: 10.2215/CJN.07100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, Yang C, Xie Z, et al. Expression of the novel co-stimulatory molecule B7-H4 by renal tubular epithelial cells. Kidney international. 2006;70(12):2092–2099. doi: 10.1038/sj.ki.5001867. [DOI] [PubMed] [Google Scholar]
- 65.Eddy AA. Progression in chronic kidney disease. Advances in chronic kidney disease. 2005;12(4):353–365. doi: 10.1053/j.ackd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Eddy AA. Serine proteases, inhibitors and receptors in renal fibrosis. Thrombosis and haemostasis. 2009;101(4):656–664. [PMC free article] [PubMed] [Google Scholar]
- 67.Lin Q, Chen Y, Lv J, et al. Kidney injury molecule-1 expression in IgA nephropathy and its correlation with hypoxia and tubulointerstitial inflammation. American journal of physiology. Renal physiology. 2014;306(8):F885–895. doi: 10.1152/ajprenal.00331.2013. [DOI] [PubMed] [Google Scholar]
- 68.Kassianos AJ, Wang X, Sampangi S, Afrin S, Wilkinson R, Healy H. Fractalkine-CX3CR1-dependent recruitment and retention of human CD1c myeloid dendritic cells by in vitro-activated proximal tubular epithelial cells. Kidney international. 2015 doi: 10.1038/ki.2014.407. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell CA, McGeachie JK, Grounds MD. Cellular differences in the regeneration of murine skeletal muscle: a quantitative histological study in SJL/J and BALB/c mice. Cell and tissue research. 1992;269(1):159–166. doi: 10.1007/BF00384736. [DOI] [PubMed] [Google Scholar]
- 70.Bonventre JV, Colvin RB. Adhesion molecules in renal disease. Current opinion in nephrology and hypertension. 1996;5(3):254–261. doi: 10.1097/00041552-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 71.Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. Journal of the American Society of Nephrology: JASN. 2011;22(3):416–425. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergijk EC, Van Alderwegen IE, Baelde HJ, et al. Differential expression of collagen IV isoforms in experimental glomerulosclerosis. The Journal of pathology. 1998;184(3):307–315. doi: 10.1002/(SICI)1096-9896(199803)184:3<307::AID-PATH5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 74.Setty S, Michael AA, Fish AJ, et al. Differential expression of laminin isoforms in diabetic nephropathy and other renal diseases. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25(6):859–868. doi: 10.1038/modpathol.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morel-Maroger Striker L, Killen PD, Chi E, Striker GE. The composition of glomerulosclerosis. I. Studies in focal sclerosis, crescentic glomerulonephritis, and membranoproliferative glomerulonephritis. Laboratory investigation; a journal of technical methods and pathology. 1984;51(2):181–192. [PubMed] [Google Scholar]
- 76.Vleming LJ, Baelde JJ, Westendorp RG, Daha MR, van Es LA, Bruijn JA. Progression of chronic renal disease in humans is associated with the deposition of basement membrane components and decorin in the interstitial extracellular matrix. Clinical nephrology. 1995;44(4):211–219. [PubMed] [Google Scholar]
- 77.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Seminars in cancer biology. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 78.Zeisberg M, Maeshima Y, Mosterman B, Kalluri R. Renal fibrosis. Extracellular matrix microenvironment regulates migratory behavior of activated tubular epithelial cells. The American journal of pathology. 2002;160(6):2001–2008. doi: 10.1016/S0002-9440(10)61150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schnaper HW, Kleinman HK. Regulation of cell function by extracellular matrix. Pediatr Nephrol. 1992;7:96–104. doi: 10.1007/BF00861587. [DOI] [PubMed] [Google Scholar]
- 80.LeBleu VS, Taduri G, O’Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nature medicine. 2013;19(8):1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hutchison N, Fligny C, Duffield JS. Resident mesenchymal cells and fibrosis. Biochimica et biophysica acta. 2013;1832(7):962–971. doi: 10.1016/j.bbadis.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jang HS, Kim JI, Jung KJ, Kim J, Han KH, Park KM. Bone marrow-derived cells play a major role in kidney fibrosis via proliferation and differentiation in the infiltrated site. Biochimica et biophysica acta. 2013;1832(6):817–825. doi: 10.1016/j.bbadis.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 83.Reich B, Schmidbauer K, Rodriguez Gomez M, et al. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney international. 2013;84(1):78–89. doi: 10.1038/ki.2013.84. [DOI] [PubMed] [Google Scholar]
- 84.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. The Journal of clinical investigation. 2002;110(3):341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Venkov CD, Link AJ, Jennings JL, et al. A proximal activator of transcription in epithelial-mesenchymal transition. The Journal of clinical investigation. 2007;117(2):482–491. doi: 10.1172/JCI29544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. The American journal of pathology. 2010;176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren S, Duffield JS. Pericytes in kidney fibrosis. Current opinion in nephrology and hypertension. 2013;22(4):471–480. doi: 10.1097/MNH.0b013e328362485e. [DOI] [PubMed] [Google Scholar]
- 88.Wolf G, Wenzel UO. Angiotensin II and Cell Cycle Regulation. Hypertension. 2004;43(4):693–698. doi: 10.1161/01.HYP.0000120963.09029.ca. [DOI] [PubMed] [Google Scholar]
- 89.Hodgkins KS, Schnaper HW. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatric nephrology. 2012;27(6):901–909. doi: 10.1007/s00467-011-1992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.ESCAPE Group ET. Wuhl E, et al. Strict blood-pressure control and progression of renal failure in children. The New England journal of medicine. 2009;361(17):1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 91.Tang C, Dong Z. Mitochondria in Kidney Injury: When the Power Plant Fails. Journal of the American Society of Nephrology: JASN. 2016;27(7):1869–1872. doi: 10.1681/ASN.2015111277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inagi R, Ishimoto Y, Nangaku M. Proteostasis in endoplasmic reticulum--new mechanisms in kidney disease. Nature reviews. Nephrology. 2014;10(7):369–378. doi: 10.1038/nrneph.2014.67. [DOI] [PubMed] [Google Scholar]
- 93.Jang HS, Padanilam BJ. Simultaneous deletion of Bax and Bak is required to prevent apoptosis and interstitial fibrosis in obstructive nephropathy. American journal of physiology. Renal physiology. 2015;309(6):F540–550. doi: 10.1152/ajprenal.00170.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300(5):R1009–1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brenner BM. Nephron adaptation to renal injury or ablation. Am J Physiol. 1985;249:F324. doi: 10.1152/ajprenal.1985.249.3.F324. [DOI] [PubMed] [Google Scholar]
- 96.Komers R, Meyer TA, Anderson S. Pathophysiology and nephron adaptation in chronic kidney disease. In: Coffman TM, Falk RJ, Molitoris BA, Neilson EG, Schrier RW, editors. Schrier’s Diseases of the Kidney, Ninth Edition. Philadelphia: Lippincott, Williams & Wilkins; 2013. pp. 2214–2237. [Google Scholar]
- 97.Kurnik BR, Weisberg LS, Kurnik PB. Renal and systemic oxygen consumption in patients with normal and abnormal renal function. Journal of the American Society of Nephrology: JASN. 1992;2(11):1617–1626. doi: 10.1681/ASN.V2111617. [DOI] [PubMed] [Google Scholar]
- 98.Hall AM, Unwin RJ, Parker N, Duchen MR. Multiphoton imaging reveals differences in mitochondrial function between nephron segments. Journal of the American Society of Nephrology: JASN. 2009;20(6):1293–1302. doi: 10.1681/ASN.2008070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol. 1986;48:9–31. doi: 10.1146/annurev.ph.48.030186.000301. [DOI] [PubMed] [Google Scholar]
- 100.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Experimental physiology. 2006;91(5):807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 101.Coughlan MT, Sharma K. Challenging the dogma of mitochondrial reactive oxygen species overproduction in diabetic kidney disease. Kidney international. 2016;90(2):272–279. doi: 10.1016/j.kint.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 102.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxidants & redox signaling. 2009;11(10):2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 103.Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes. 2015;64(3):663–672. doi: 10.2337/db14-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. Journal of the American Society of Nephrology: JASN. 2015;26(5):1027–1038. doi: 10.1681/ASN.2014010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kushida N, Nomura S, Mimura I, et al. HIF-1α activates the TGF-β/SMAD3 pathway in kidney tubular epithelial cells. American journal of nephrology. doi: 10.1159/000449323. In press. [DOI] [PubMed] [Google Scholar]
- 106.Schnaper HW. Remnant nephron physiology and the progression of chronic kidney disease. Pediatric nephrology. 2014;29(2):193–202. doi: 10.1007/s00467-013-2494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. American journal of physiology. Cell physiology. 2013;304(3):C216–225. doi: 10.1152/ajpcell.00328.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]