Abstract
Increased listerial barotolerance at elevated osmolarity is attributed, in part, to the presence of accumulated betaine and l-carnitine. The percentage of listerial survival following exposure to 400 MPa for 5 min increased from 0.008 to 0.02% with added l-carnitine (5 mM) and to 0.05% with added betaine (5 mM). Furthermore, listerial cells incapable of transporting compatible solutes fail to adapt to high pressure at elevated osmolarity.
Consumer demand for safe, minimally processed, additive-free foods with an extended shelf life has resulted in considerable developments in modern food-processing technology (1). One technique in particular, high-pressure (HP) processing, is gaining importance as an alternative and effective method of food preservation (11). However, the efficacy of HP-induced microbial inactivation is dependent on a number of external factors, including the chemical composition of the extracellular environment. While the solutes salt (NaCl) and sugar (sucrose) have previously been shown to offer an effect that is protective with respect to microbes in foods subjected to HP (13), the underlying mechanism has yet to be elucidated. In the present study we investigated the phenomenon of increased microbial barotolerance at elevated osmolarity and demonstrated that, in addition to their previously defined roles in osmo- and cryotolerance (9, 15, 16), it is the trimethyl ammonium compounds glycine betaine and, to a lesser extent, l-carnitine which are primarily responsible for the observed increase in barotolerance at elevated osmolarities.
The compatible solutes betaine and l-carnitine contribute to increased listerial barotolerance at elevated osmolarity.
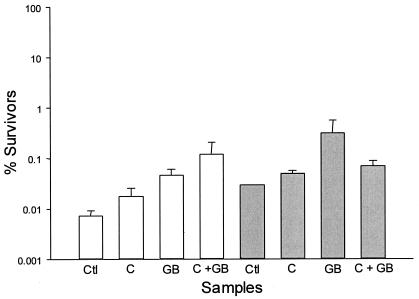
To directly assess the individual contribution of each osmolyte to listerial barotolerance, the efficacy of HP inactivation was determined in DM (6)-3% added NaCl (DMS) with and without added osmolytes (Fig. 1). The percentage of survival following HP treatment of Listeria innocua at 400 MPa increased from 0.008% in DMS alone to 0.02% in DMS with 5 mM added l-carnitine, to 0.05% with added betaine (5 mM), and to 0.12% in DMS containing both betaine and l-carnitine (2.5 mM each) (P < 0.5). These results prove not only that both betaine and l-carnitine contribute to listerial barotolerance but also that betaine is more effective than l-carnitine in protection of the microbial cell from the detrimental effects of HP treatment. Furthermore, no additive effect was observed when samples containing both betaine and l-carnitine were held at 20°C for 1 h prior to HP treatment, a phenomenon that most likely reflects transinhibition due to the presence of preaccumulated betaine (14). However, storing samples for 1 h at 4°C prior to HP treatment did result in an additive effect, betaine and l-carnitine together being considerably more effective than either osmolyte alone. It is conceivable that the added stress of cold storage may induce osmolyte uptake, as described previously (15), to sufficiently high levels to relieve the transinhibitory effect of preaccumulated solute. Interestingly, in support of this proposal Noma and Hayakawa (5) also observed an approximately 2-log increase in survival of Staphylococcus aureus following HP treatment when samples were preincubated at temperatures < 0°C.
FIG. 1.
HP-induced inactivation of L. innocua in defined medium (Ctl) or in the same medium containing the compatible solute glycine betaine (GB) or l-carnitine (C) or both, stored for 1 h at 4°C (white bars) or 20°C (grey bars) and then HP treated at 400 MPa for 5 min at 20°C.
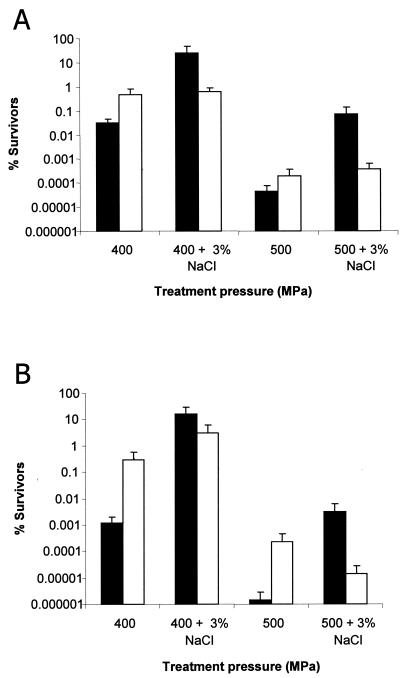
To further assess the significance of betaine and l-carnitine in listerial barotolerance in a complex undefined environment, DM was replaced with tryptone soya broth (Sigma) supplemented with 0.6% yeast extract (TSBYE). In this instance the compatible solute uptake mutant strain LO28ΔBCGBH was used as the test strain. Having deletions in BetL, OpuC and Gbu, the three primary osmolyte uptake systems (described previously) (10), this mutant is incapable of transporting either betaine or l-carnitine (15), thus providing an ideal background for studying the effects of osmolyte uptake on listerial barotolerance in a complex undefined environment. Furthermore, LO28ΔBCGBH and HSOE, the control strain to which it was compared, were rendered nonpathogenic by deletion of the hly gene by use of constructs and procedures described previously (2). Survival rates of strain HSOE in TSBYE differed considerably from those seen with TSBYES (TBSYE plus 3% added NaCl). Treatment at 400 and 500 MPa resulted in 25.6% survivors (∼0.75-log reduction) and 0.07% survivors (∼3.3-log reduction) in TSBYES, respectively, compared to only 0.03% survivors (∼3.7-log reduction) following treatment at 400 MPa and 0.00004% survivors (∼6.5-log reduction) following treatment at 500 MPa in TSBYE (Fig. 2A).
FIG. 2.
HP-induced inactivation of L. monocytogenes HSOE (black bars) and the osmolyte uptake mutant LO28ΔBCGB (white bars) in TSBYE with and without 3% added NaCl. Survivors were plated on TSBYE (A) and LSA (B). All treatments were carried out for 5 min at 20°C.
In comparison, survival rates of the osmolyte uptake mutant LO28ΔBCGBH were not significantly increased at elevated osmolarity; treatment at 400 and 500 MPa resulted in 0.45% (∼0.5-log reduction) and 0.0002% (∼3.8-log reduction) survivors, respectively, in TSBYE, while in TSBYES, treatment at 400 and 500 MPa resulted in 0.64% (∼0.5-log reduction) and 0.0004% (∼3.5-log reduction) survivors. Similar trends were observed when samples were plated on Listeria selective agar (LSA) as opposed to TSBYE (Fig. 2B). An interesting observation was the increased survival of the osmolyte uptake mutant LO28ΔBCGBH relative to that of the control strain HSOE when exposed to HP under low-salt conditions. We suggest that this increased barotolerance at low salt concentrations may result from an increase in osmolyte synthesis (most likely proline synthesis mediated by the ProBA/C pathway) (7) to compensate for the lack of osmolyte uptake and to generate turgor for continued cell growth and division. Treatment at ≥600 MPa resulted in complete inactivation of both strains in complex and defined media (data not shown), indicating that 600 MPa is the upper limit for compatible solute-mediated barotolerance in Listeria.
These results suggest that baroprotection at elevated osmolarity is not directly the result of the osmotic stressor itself but instead results from uptake of the compatible solutes betaine and l-carnitine at elevated osmolarity. Interestingly, in support of this proposal, Yancey et al. (17) recently reported that the tissues of certain marine organisms contain high concentrations of compatible solutes. In particular, trimethylamine N-oxide (TMAO), found in muscles of deep-sea teleosts, is thought to offset the inhibitory effects of high hydrostatic pressures. Indeed, greater levels of TMAO are found in deeper-living sea organisms which are subjected to the greatest hydrostatic pressure (3). Thus, it appears that, as is the case with osmotolerance, compatible solute-mediated barotolerance is not only restricted to prokaryotic species but may represent a global response to survival in HP environments. Furthermore, studies of HP-induced inactivation of bacteriophage have revealed little difference in the degrees of inactivation as a function of osmolarity (M. Smiddy, unpublished data). Given that phages lack the capacity to accumulate compatible solutes, these findings further support the hypothesis that it is compatible solutes that protect bacteria from HP inactivation at elevated osmolarity.
Possible mechanisms of compatible solute function.
While the exact mechanisms by which compatible solutes protect the bacterial cell from the damaging effects of HP treatment have yet to be determined, a number of possibilities exist. Firstly, the hydration shell formed by the preferential exclusion of compatible solutes from the immediate surface of proteins may shield essential enzymes from unfolding at HPs (8). In addition to protein stabilization, the compatible solutes betaine and l-carnitine have previously been suggested to play a role in maintaining membrane fluidity (8). Indeed, a previous study by MacDonald (4) led to the finding that salt and other solutes influence the fatty acid composition of membrane lipids and that an increase in membrane fluidity increases resistance to HP. This mechanism of membrane stiffening through changes in fatty acid saturation was postulated by Smelt et al. (12) as a possible mechanism of HP-induced inactivation of microorganisms. L. plantarum, grown in the presence of salt and betaine, exhibits increased levels of lysy-phosphatidylglycerol (LPG) at the expense of phosphatidylglycerol (PG) and diphosphatidylglycerol (DPG), increasing membrane fluidity and making the membrane less stiff and, in turn, more resistant to HP treatment. However, when bacteria are grown in saltalone, the LPG levels of L. plantarum decrease (12), indicating that it is betaine rather than salt which is responsible for triggering increased levels of LPG, membrane fluidity, and the concomitant increase in barotolerance.
Acknowledgments
This work was supported by safefood, the Food Safety Promotion Board. R.D.S. is funded by an Irish Research Council for Science, Engineering and Technology postdoctoral fellowship (under the Embark Initiative) and a joint European Society of Clinical Microbiology and Infectious Diseases-Federation of European Microbiological Societies research fellowship.
REFERENCES
- 1.Abee, T., and J. A. Wouters. 1999. Microbial stress response in minimal processing. Int. J. Food Microbiol. 50:65-91. [DOI] [PubMed] [Google Scholar]
- 2.Gahan, C. G. M., and C. Hill. 2000. The use of listeriolysin to identify in vivo induced genes in the gram-positive intracellular pathogen Listeria monocytogenes. Mol. Microbiol. 36:498-507. [DOI] [PubMed] [Google Scholar]
- 3.Kelly, R. H., and P. H. Yancey. 1999. High contents of trimethylamine oxide correlating with depth in deep-sea teleost fishes, skates and decapod crustaceans. Biol. Bull. 196:18-25. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald, A G. 1992. Effects of high pressures on natural and artificial membranes, p. 67-75. In C. Balny, R. Hayashi, K. Heremans, and P. Mason (ed.), High pressure and biotechnology. Libby Eurotext, London, United Kingdom.
- 5.Noma, S., and I. Hayakawa. 2003. Barotolerance of Staphylococcus aureus is increased by incubation at below 0°C prior to hydrostatic pressure treatment. Int. J. Food Microbiol. 80:261-264. [DOI] [PubMed] [Google Scholar]
- 6.Premaratne, R. J., W.-J. Lin, and E. A. Johnson. 1991. Development of an improved chemically defined medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sleator, R. D., C. G. M. Gahan, and C. Hill. 2001. Identification and disruption of the proBA locus in Listeria monocytogenes: role of proline biosynthesis in salt tolerance and murine infection. Appl. Environ. Microbiol. 67:2571-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sleator, R. D., and C. Hill. 2002. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26:49-71. [DOI] [PubMed] [Google Scholar]
- 9.Sleator, R. D., C. G. M. Gahan, and C. Hill. 2003. A postgenomic appraisal of osmotolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 69:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sleator, R. D., H. H. Wemekamp-Kamphuis, C. G. M. Gahan, and C. Hill. A PrfA-regulated bile exclusion system (BilE) is a novel virulence factor in Listeria monocytogenes. Mol. Microbiol., in press. [DOI] [PubMed]
- 11.Smelt, J. P. P. M. 1998. Recent advances in the microbiology of high pressure processing. Trends Food Sci. Technol. 9:152-158. [Google Scholar]
- 12.Smelt, J. P. P. M., A. G. F. Rikje, and A. Hayhurst. 1994. Possible mechanism of high pressure inactivation of microorganisms. High Pressure Res. 12:199-203. [Google Scholar]
- 13.Smiddy, M., L. O'Gorman, R. D. Sleator, A. L. Kelly, and C. Hill. Increased high pressure resistance of bacteria in oysters compared to in buffer. Innovative Food Sci. Emerg. Technol., in press.
- 14.Verheul, A., E. Glaasker, B. Poolman, and T. Abee. 1997. Betaine and l-carnitine transport by Listeria monocytogenes ScottA in response to osmotic signals. J. Bacteriol. 179:6979-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wemekamp-Kamphuis, H. H., J. A. Wouters, R. D. Sleator, C. G. M. Gahan, C. Hill, and T. Abee. 2002. Multiple deletions of the osmolyte transporters BetL, Gbu, and OpuC of Listeria monocytogenes affect virulence and growth at high osmolarity. Appl. Environ. Microbiol. 68:4710-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wemekamp-Kamphuis, H. H., R. D. Sleator, J. A. Wouters, C. Hill, and T. Abee. 2004. Molecular and physiological analysis of the role of the osmolyte transporters BetL, Gbu and OpuC in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 70:2912-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yancey, P. H., A. L. Fyfe-Johnson, R. H. Kelly, V. P. Walker, and M. T. Aunon. 2001. Trimethylamine oxide counteracts effects of hydrostatic pressure on proteins of deep-sea teleosts. J. Exp. Zool. 289:172-176. [DOI] [PubMed] [Google Scholar]