ABSTRACT
Background: Blast-related ocular injuries sustained by military personnel have led to rigorous efforts to elucidate the effects of blast exposure on neurosensory function. Recent studies have provided some insight into cognitive and visual deficits sustained following blast exposure; however, limited data are available on the effects of blast on pain and inflammatory processes. Investigation of these secondary effects of blast exposure is necessary to fully comprehend the complex pathophysiology of blast-related injuries. The overall purpose of this study is to determine the effects of single and repeated blast exposure on pain and inflammatory mediators in ocular tissues.
Methods: A compressed air shock tube was used to deliver a single or repeated blast (68.0 ± 2.7 kPa) to anesthetized rats daily for 5 days. Immunohistochemistry was performed on ocular tissues to determine the expression of the transient receptor potential vanilloid 1 (TRPV1) channel, calcitonin gene-related peptide (CGRP), substance P (SP), and endothelin-1 (ET-1) following single and repeated blast exposure. Neutrophil infiltration and myeloperoxidase (MPO) expression were also assessed in blast tissues via immunohistochemistry and enzyme-linked immunosorbent assay (ELISA) analysis, respectively.
Results: TRPV1 expression was increased in rat corneas exposed to both single and repeated blast. Increased secretion of CGRP, SP, and ET-1 was also detected in rat corneas as compared to control. Moreover, repeated blast exposure resulted in neutrophil infiltration in the cornea and stromal layer as compared to control animals.
Conclusion: Single and repeated blast exposure resulted in increased expression of TRPV1, CGRP, SP, and ET-1 as well as neutrophil infiltration. Collectively, these findings provide novel insight into the activation of pain and inflammation signaling mediators following blast exposure.
KEYWORDS: Blast injury, cornea, inflammation, ocular trauma, pain
Introduction
Improvised explosive devices (IEDs) have drastically transformed modern warfare and more importantly how medicine cares for wounded military personnel. The use of IEDs by insurgents in Iraq and Afghanistan has consequently led to a significant increase in blast-related injuries.1,2 Blast injuries are generally categorized as primary to quaternary and can evoke a myriad of effects on multiple body systems. 3 – 5 Importantly, ocular injuries are the fourth most common battlefield injury, 2 constituting approximately 13% of all combat injuries. 6 Despite the fact that the eye is relatively small, being responsible for only 0.1% of the frontal surface area, 7 it is the most important sensor available to military personnel. Unfortunately, even with protective gear the eye is frequently exposed and susceptible to penetrating blast-related injuries that can result in vision loss. Importantly, closed-eye or non-penetrating ocular and sensory injuries, which may not be readily apparent, are of great concern as well.
Current research efforts are predominantly focused on cognitive and visual deficits sustained following blast exposure. 8 – 10 Unfortunately, research to investigate the secondary effects of blast injuries, such as chronic pain and inflammation, are somewhat limited. A comprehensive understanding of both primary and secondary effects of blast exposure is crucial for the effective care and treatment of blast-related injuries, to include ocular trauma. Exposure to a blast-wave is a well-established mechanism of traumatic injury that results in ocular damage and visual deficits. 11 – 16 Recently, our group developed a rodent model to assess the effects of single and repeated low-level blast-wave exposure. Our recent data revealed that low-level blast exposure induced apoptosis in the retina 16 as well as increased inflammation in the optic nerve. 17 Based on these findings, we sought to utilize this model to assess the effects of low-level blast exposure on pain and inflammatory signaling mediators in ocular tissues.
The TRPV1 channel is well characterized for its role in inflammation and pain transmission. TRPV1 is a non-selective, polymodal cation channel activated by multiple stimuli, to include noxious heat (>42°C), acidic pH, and capsaicin18,19 and is predominantly expressed in nociceptive neurons of the peripheral and central nervous system. Recent literature has demonstrated TRPV1 expression in various ocular tissues, to include the cornea20,21 and retina.22,23 Of interest, a critical role of TRPV1 in neuronal injury and wound healing has also been identified in both the cornea and retina. 24 – 26 Previous studies have also demonstrated co-expression of TRPV1 with the neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP) in the cornea.20,21 Importantly, following activation of TRPV1, CGRP and SP are released from primary sensory neurons, resulting in the activation of pain and inflammatory signaling pathways.27,28 In addition to these peptides, emerging evidence also implicates ET-1, a well-characterized vasoconstrictive peptide, in pain transmission via potentiation of TRPV1. 29 – 32 Altogether, these data provide the rationale to investigate TRPV1 and these associated peptides in blast-related pain and inflammation processes in ocular tissues. To determine the effect of blast exposure on pain and inflammatory mediator expression, we performed immunohistochemical analysis on rat corneal sections obtained from animals exposed to either a single or repeated blast (68.0 ± 2.7 kPa). We proposed to investigate whether blast exposure alters expression of these proteins in ocular tissues. Increased expression of pain and inflammatory mediators following blast exposure may provide critical insight into the pathophysiology of secondary blast-related injuries.
Our findings presented herein revealed that both single and repeated blast-wave exposure results in increased expression of the TRPV1 receptor, CGRP, SP, and ET-1 in the cornea epithelial and stromal layers. Collectively, the results presented herein indicate that low-level blast exposure stimulates the up-regulation and secretion of pain and inflammatory signaling mediators. Further investigation is necessary to elucidate the functional and behavioral effects of blast exposure on TRPV1 activity, which may identify therapeutic treatment options for the treatment of blast-related injuries, to include chronic pain and inflammation.
Methods
Blast exposure experimental design
Adult male Long Evans rats (300–350 g) were randomly divided into one of two blast-exposed groups (single and repeated) or a control group (n = 5 per group). Animals were anesthetized with a ketamine-xylazine cocktail and positioned into a mesh holder (Figure 1A–B). Rats were placed into the end of an expansion chamber of an air-driven shock tube facing into the incident blast wave (Figure 1C), then subjected to a peak overpressure of 68.0 ± 2.7 kPa for a positive pressure peak duration of 2.8 ± 0.1 ms as previously described. 17 Figure 1D is a representative shock wave produced by the Institute of Surgical Research (ISR) shock tube as measured by the reference and reflected pressure sensors indicated in Figure 1B–C. Control animals were handled in the same manner, anesthetized and placed in the end of the expansion chamber in the shock tube without receiving a blast exposure. Blast-exposed rats received either a single-blast exposure on the first day of experiments or repeated blast exposure once daily for five consecutive days. All blast-exposed groups were euthanized on day 5 of the experiment, with the repeat blast group euthanized 1 h following the last blast exposure, and the group receiving only a single exposure was euthanized 96 h after blast exposure. Frozen, paraffin-embedded sections of rat corneas, collected at euthanization, were utilized for all immunohistochemistry experiments. This study was conducted in compliance with the Animal Welfare Act, implementing Animal Welfare Regulations, the principles of the Guide for the Care and Use of Laboratory Animals (IACUC), and approved by the Institute of Surgical Research (ISR) IACUC committee.
Figure 1.
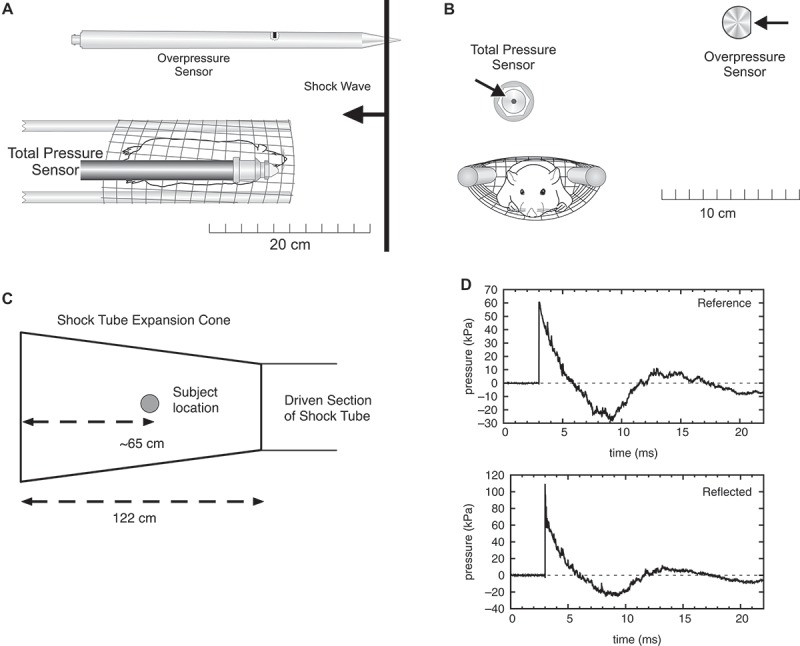
(A) Top view of rat in mesh holder, with positions of the reference overpressure and reflected pressure sensors indicated. (B) Front view of rat in mesh holder, with positions of pressure sensors indicated. (C) Location of rat within the expansion cone of the shock tube during blast-wave exposures. (D) Representative shock wave produced by the ISR shock tube as measured by the reference and reflected pressure sensors as indicated in (A–B).
Tissue histopathology
Rat ocular tissue samples were carefully collected to prevent surgical damage and fixed for 24 h with modified Davidson’s solution (64133-50, EMS, PA), followed by immersion in formalin. The fixed samples were then moved through increasing percentages of alcohol to 100% and then blocked in paraffin. About 5-mm thick sections were cut from the paraffin-embedded tissue. These sections were de-paraffinized in xylene and rehydrated in descending grades of ethanol (100%, 70%, and 45%) and then water. Staining for hematoxylin and eosin (H&E) was performed using a standardized protocol. 33
Immunohistochemistry (IHC)
Protein expression was determined on frozen, de-paraffinized sections using antibodies directed against TRPV1, CGRP (GP14100, RA24112; Neuromics, Edina, MN), SP, and ET-1 (ab14184, ab2786; Abcam, Cambridge, MA). Following primary antibody incubation, tissue sections were incubated with the appropriate Alexa-Fluor secondary antibodies (Life Technologies, Grand Island, NY). Sections receiving only secondary antibody were included as negative controls. Sections of spinal cord and dorsal root ganglia (DRGs) were also performed as positive controls for TRPV1, CGRP, and SP staining.
Microscopy
Immunofluorescence images of rat corneas were taken at 60× using an oil immersion objective in an Olympus BX 53 microscope. Scale bar on images represents 10 μM. For neutrophil infiltration analysis in ocular tissues, bright field images were obtained using a 40× magnification. Images are representative of five to six animals for each group. Two tissues per animal from the center cut of the cornea were assessed for neutrophil infiltration. Red arrows indicate specific expression of neutrophils in rat cornea following repeat blast.
Myeloperoxidase (MPO) ELISA assay
While under ketamine-xylazine sedation, blood samples were collected from rats by cardiac puncture at the experimental end time point (97 h). Blood samples were immediately placed into tubes containing 200 mM sodium citrate for plasma collection. Concentrations of MPO were measured according to manufacturer’s instructions (MPO; HK105, HyCult Biotechnology, Uden, the Netherlands). This method utilizes a biotinylated capture antibody generated against MPO and a peroxidase-labeled reporter antibody directed against MPO.
Statistical analysis
Quantified TRPV1 expression in the rat cornea was performed via one-way ANOVA with Bonferroni post-hoc analysis, **, p < 0.01, NS not significant. MPO results were analyzed via one-way ANOVA with Newman–Keuls multiple comparison test with significance set at p < 0.05. All data are expressed as the mean ± SEM.
Results
Single and repeat blast exposure results in increased TRPV1 expression in the rat cornea
In agreement with previous studies, TRPV1 expression was detected in the rat cornea in non-blast, control animals.20,25,34 Interestingly, rats exposed to either a single or repeated blast demonstrated a substantial increase in TRPV1 expression in the cornea as compared to control animals (Figure 2A). No discernible differences in TRPV1 expression were observed in single versus repeat blast-exposed ocular tissues (Figure 2B). Although TRPV1 expression was detected in the retinal ganglion cell (RGC) layer of the retina, no difference in expression was observed in control animals versus single or repeated blast-exposed animals (data not shown) in the posterior segment of the eye.
Figure 2.
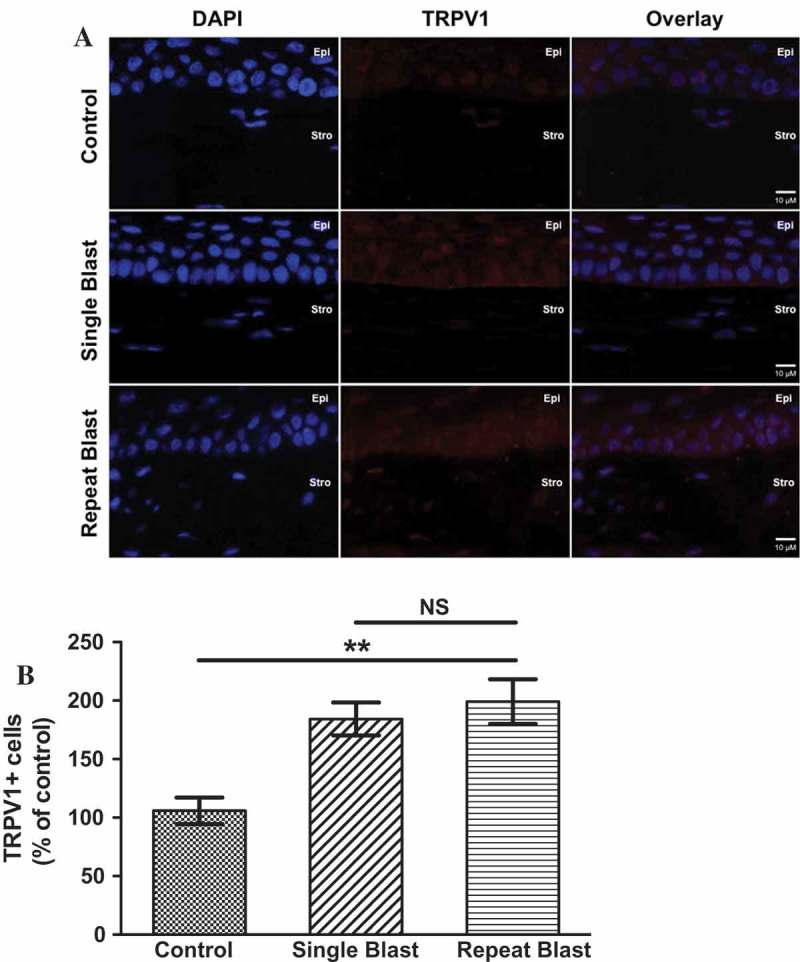
(A) Immunofluorescence analysis was performed on rat corneal sections exposed to either a single or repeated blast (70 KPa). Tissues were subjected to staining with an anti-TRPV1 (1:250) antibody and probed with an Alexa Fluor 568 secondary antibody. Nuclei were visualized with DAPI staining (1:1000). Images were captured at 60× magnification, oil immersion. (B) Quantification of TRPV1-positive cells as a percent of control animals. One-way ANOVA, Bonferroni post-hoc analysis, **p < 0.01, NS not significant. Cornea layers are indicated as Epi = epithelial, Str = Stromal.
Blast exposure leads to increased CGRP, SP, and ET-1 secretion in the rat cornea
Expression of specific pain and inflammation-associated peptides was assessed for co-expression with TRPV1 by immunohistochemistry. Single and repeated blast exposure resulted in substantial increases in CGRP (Figure 3) and SP (Figure 4) expression and/or secretion in the cornea and stromal layer. Of interest, increased ET-1 expression was observed in rat corneas following either a single or repeated low-level blast (Figure 5). Importantly, all of these increases in peptide expression coincided with increased expression of the TRPV1 receptor. Increased expression of CGRP, SP, and ET-1 was also observed in the stromal layer of the cornea; however, no significant differences in this layer were observed following single and repeated blast-wave exposure. These results indicate that low-level blast exposure, both single and repeated, resulted in increased peptide expression in the cornea as compared to control non-blast tissues.
Figure 3.
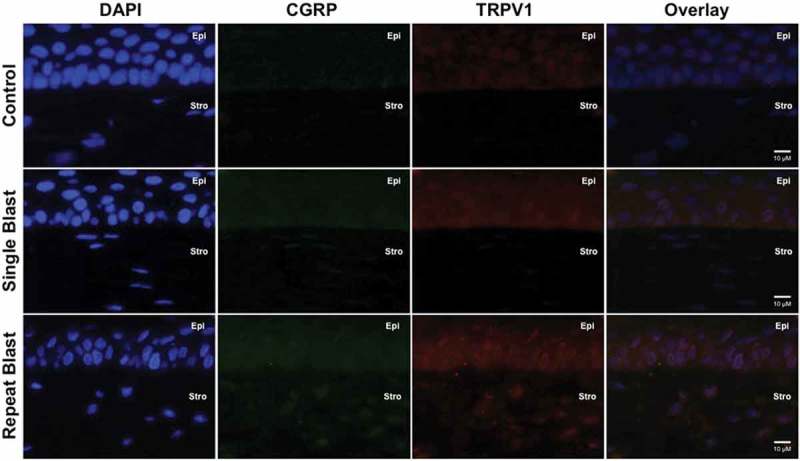
Immunofluorescence analysis was performed on rat corneal sections exposed to either a single or repeated blast (70 KPa). Tissues were subjected to staining with anti-TRPV1 (1:250) and anti-CGRP (1:600) antibodies and probed with Alexa Fluor 568 and 488 secondary antibodies, respectively. Nuclei were visualized with DAPI staining (1:1000). Images were captured at 60× magnification, oil immersion. Cornea layers are indicated as Epi = epithelial, Str = Stromal.
Figure 4.
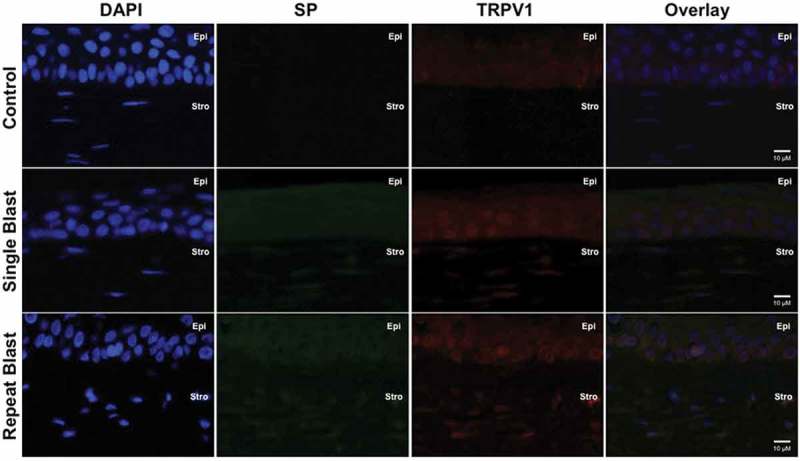
Immunofluorescence analysis was performed on rat corneal sections exposed to either a single or repeated blast (70 KPa). Tissues were subjected to staining with anti-TRPV1 (1:250) and anti-SP (1:500) antibodies and probed with Alexa Fluor 568 and 488 secondary antibodies, respectively. Nuclei were visualized with DAPI staining (1:1000). Images were captured at 60× magnification, oil immersion. Cornea layers are indicated as Epi = epithelial, Str = Stromal.
Figure 5.
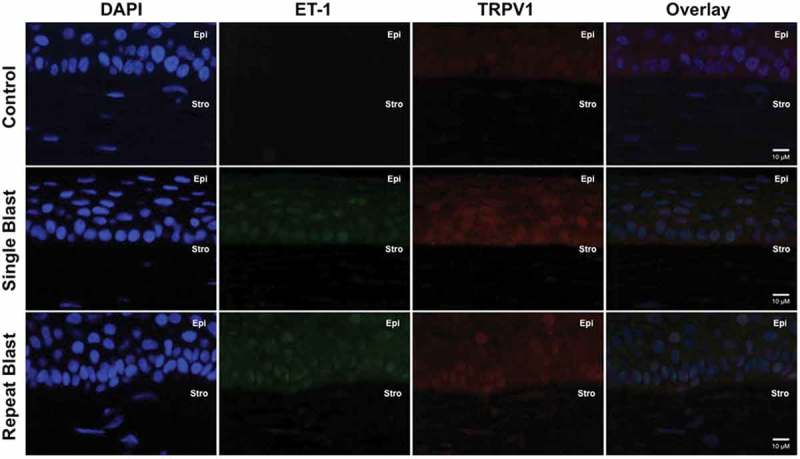
Immunofluorescence analysis was performed on rat corneal sections exposed to either a single or repeated blast (70 KPa). Tissues were subjected to staining with anti-TRPV1 (1:250) and anti-ET-1 (1:250) antibodies and probed with Alexa Fluor 568 and 488 secondary antibodies, respectively. Nuclei were visualized with DAPI staining (1:1000). Images were captured at 60× magnification, oil immersion. Cornea layers are indicated as Epi = epithelial, Str = Stromal.
Blast exposure and neutrophil infiltration
To determine the effect of blast exposure on inflammation, H&E staining was conducted to assess neutrophil expression in the cornea. Neutrophil infiltrates were observed in the corneal stromal layer of rats exposed to both a single and repeated blast (Figure 6). A significant number of neutrophils were observed in the stromal layer closest to the ciliary body of repeat, blast-exposed rats. Repeated blast tissues demonstrated 10.6 ± 1.9 neutrophils/mm 2 in the cornea, proximal to the limbus, as compared to single-blast tissue, which was negligible (Table 1). Increased neutrophil infiltration was further substantiated by increased MPO levels in the blood from repeated blast animals (5.09 μg/mL) as compared to control (3.08 μg/mL) (Figure 7).
Figure 6.
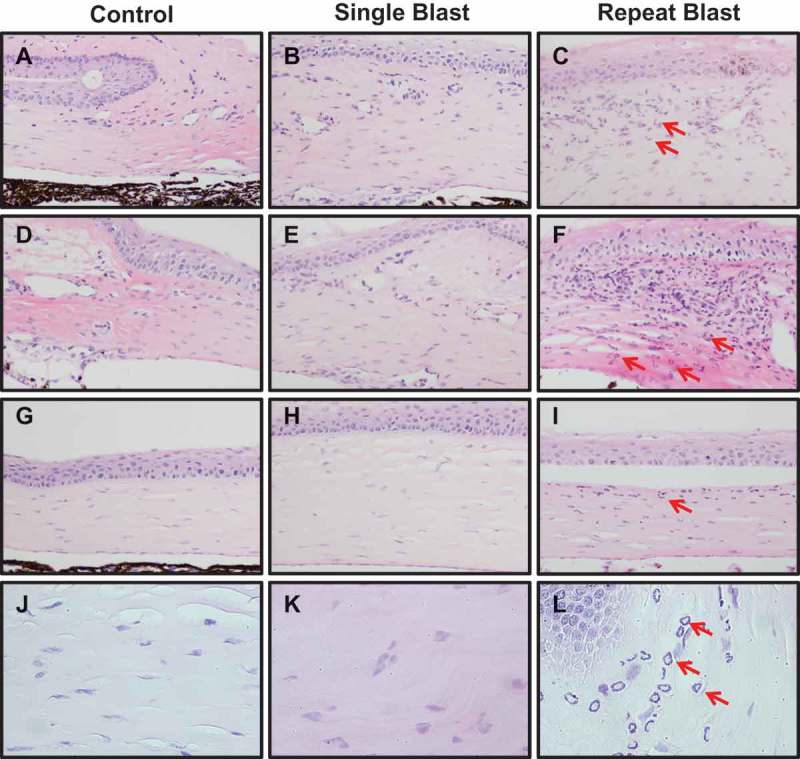
Neutrophil infiltration in the cornea close to the limbus (A–F) and the center of cornea (G–I) of control, single, or repeat blast-exposed rats (red arrows indicate neutrophils), 20× magnification. (J–L) Images of all groups obtained with 40× magnification. Images are representative of five to six animals for each group. Two tissues per animal from the center cut of the cornea were assessed for neutrophil infiltration.
Table 1.
Neutrophil infiltration was quantitated in the corneas of single and repeated blast-exposed tissues.
| Control | Single blast | Repeat blast | |
|---|---|---|---|
| Number of neutrophils/mm 2 | – | Negligible | 10.6 ± 1.9 |
Values represented denote number of neutrophils/mm 2 close to both sides of the limbus.
Figure 7.
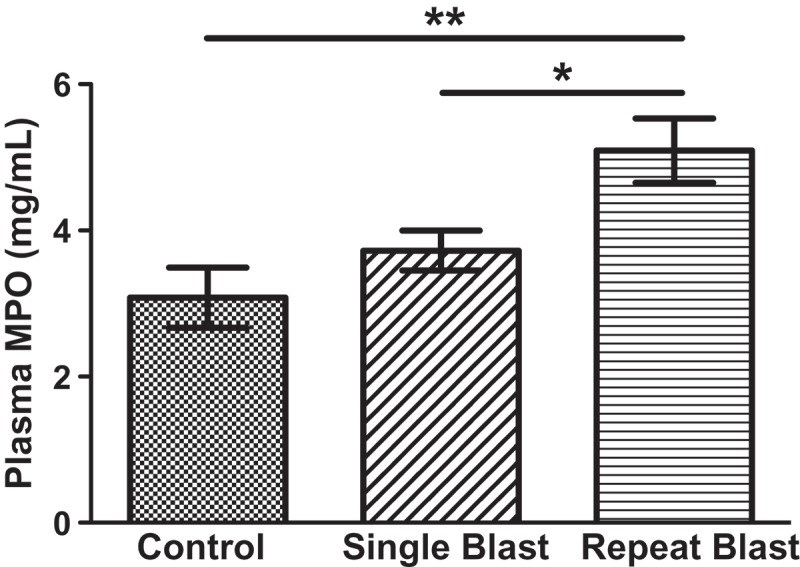
Blood samples from rats exposed to single and repeated low-level blast were collected at time of euthanization. Concentrations of MPO (ng/mL) were calculated via ELISA analysis using a biotinylated capture antibody and a peroxidase-labeled reporter directed against MPO. One-way ANOVA with Newman–Keuls multiple comparison test, statistical significance is indicated as *p < 0.05, **p < 0.01. Data are expressed as the mean ± SEM.
Discussion
Pain complaints are highly prevalent among military veterans and importantly are co-morbid with more severe conditions, to include post-traumatic stress disorder (PTSD) and traumatic brain injury (TBI). 35 A recent study evaluated pain treatment outcomes of military personnel following blast exposure and revealed that this unique patient population experienced higher levels of hospital admission as well as reduced improvements in pain intensity. 36 As such, a greater understanding of blast-related injuries is required to effectively manage and treat secondary blast-related injuries. The findings presented herein are the first to demonstrate blast exposure effects on pain and inflammatory mediator expression in ocular tissues. Rats subjected to either a low-level single or repeated blast demonstrated substantial increases in TRPV1 expression in the cornea (Figure 2). Up-regulated TRPV1 expression coincided with increased CGRP, SP, and ET-1 expression following blast exposure (Figures 3–5). Crosstalk with various proteins and receptor systems is known to modulate TRPV1 and potentiate its activity.31,37 Specifically, ET-1 is known to enhance TRPV1-mediated nociception and thermal hyperalgesic responses. 29 – 31 Interestingly, a receptor splice variant of TRPV1 was recently found to modulate receptor activity. To date, at least three rat TRPV1 splice variants have been identified in neuronal and non-neuronal tissues. 38 – 40 The splice variant, TRPV1VAR, was identified to physically interact with canonical TRPV1 and differentially modulate the receptor depending on the cellular context. 39 Further studies to characterize proteins that modulate TRPV1-mediated behavioral pain responses may identify potential therapeutics to alleviate pain and inflammatory processes associated with blast-related injuries.
TRPV1 is primarily expressed on small, unmyelinated sensory neurons in the trigeminal ganglia and is well characterized for its role in mediating various types of pain. The trigeminal nerve is the principal sensory nerve of the head that provides dense sensory input to the cornea, the most richly innervated surface tissue in the body. 41 Moreover, the receptor is expressed in various tissues of the eye, to include corneal epithelial, endothelial layers, stromal fibroblasts, and nerve fibers26,34. Of interest, increased TRPV1, CGRP, and SP expression levels are correlated with patients suffering from chronic migraines.42,43 TRPV1 antagonists have also been evaluated in the treatment of this disorder. 44 Of direct relevance to our research, a significant percentage of military veterans with blast-related injuries and confirmed TBI have also been diagnosed with migraine headaches and/or chronic, daily headaches. 45 Inflammatory cytokines are known to play a significant role in trigeminal nerve sensitization regulation in migraines.34,46 Interleukin-6 (IL-6), produced in the initial phase of acute and chronic inflammation by fibroblasts, macrophages, and neutrophils, is heavily implicated in migraines.47,48 A recent study revealed that TRPV1-mediated secretion of IL-6 in corneal fibroblasts enhanced wound healing and resulted in corneal opacification. 25 Collectively, these findings suggest that receptor expression in corneal epithelial and stromal layers appears to possess diverse functions as compared to ocular sensory fibers. The differential roles of TRPV1 in wound healing in the cornea and pain transmission in ocular sensory fibers highlight the functional complexity of the receptor in ocular tissues.31,37,38–49
Our rodent model of blast injury, which utilizes a compressed air-driven shock tube to deliver a single or repeated blast, has recently demonstrated that low-level exposure induced apoptosis in the retina 16 and increased inflammation in the optic nerve. 17 These data correlate with our findings presented herein, in which we demonstrate significant neutrophil infiltration in the cornea following blast exposure (Figure 5). Recruitment of neutrophils from the circulating blood to the site of tissue injury is an early event and one of the important elements of innate immunity. 50 MPO is a peroxidase enzyme abundantly expressed in the azurophilic granules of neutrophils and is released into the extracellular space during degranulation. 51 Accordingly, increased expression of MPO was detected in the blood of rats following low-level repeated blast exposure (Figure 7). Importantly, differences in MPO expression of single and repeated blast tissues may be an artifact of sample collection times.50,51 Single-blast samples were taken 4 days following blast and repeated blast samples were taken 1 h post the final blast. Future studies are planned to appropriately determine these effects; however these data demonstrate that low-level blast leads to an inflammatory response as evident by the recruitment of neutrophils to ocular tissue. Neutrophil migration to the site of damage/injury leads to the release of additional chemical mediators and amplification of the inflammatory response. Initiation of an inflammatory response coupled with increased TRPV1 and associated peptide expression provide crucial evidence, which highlight the detrimental effects of blast. Studies to evaluate the physiological consequences are necessary to comprehend the contribution of these pain and inflammatory mediators in blast pathophysiology, specifically with regard to ocular and sensory injuries.
In conclusion, both low-level single and repeated blast exposure resulted in increased expression of TRPV1, CGRP, SP, and ET-1 in the cornea. Low-level blast exposure in a rat model also resulted in increased neutrophil infiltration in the cornea and stromal layer, which was supported by increased levels of blood MPO. The results of this study provide novel insight into the impact of blast exposure on protein expression, which may underlie chronic pain and inflammation experienced by wounded military personnel. Future animal studies to investigate the functional activity of TRPV1 and behavioral pain responses following blast exposure will enable a greater understanding of blast pathophysiology and related secondary injuries.
Acknowledgments
We would like to give special thanks to Dr. Heuy-Ching Wang for providing rat ocular tissue samples. We would also like to thank Dr. Joseph Novak for expert technical assistance with tissue histopathology experiments and Mr. Shenouda Zarif-Matta for technical assistance with immunohistochemistry experiments.
Funding Statement
This work was supported by U.S. Army Military Operational Medicine Research Program (MOMRP) and Clinical Rehabilitative Medicine Research Program (CRMRP).
Funding
This work was supported by U.S. Army Military Operational Medicine Research Program (MOMRP) and Clinical Rehabilitative Medicine Research Program (CRMRP).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense. This study was conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals.
References
- Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, et al. Prospectively assessed clinical outcomes in concussive blast vs. nonblast traumatic brain injury among evacuated US military personnel. JAMA Neurol 2014;71(8):994–1002. [DOI] [PubMed] [Google Scholar]
- Weichel ED, Colyer MH.. Combat ocular trauma and systemic injury. Curr Opin Ophthalmol 2008;19(6):519–525. [DOI] [PubMed] [Google Scholar]
- Bass CR, Panzer MB, Rafaels KA, Wood G, Shridharani J, Capehart B.. Brain injuries from blast. Ann Biomed Eng 2012;40(1):185–202. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Cernak I, Xu L, Song Y, Savonenko A, Crain BJ, et al. A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J Neuropathol Exp Neurol 2011;70(5):399–416. [DOI] [PubMed] [Google Scholar]
- Mathews ZR, Koyfman A.. Blast Injuries. J Emerg Med 2015;49(4):573–87. [DOI] [PubMed] [Google Scholar]
- Weichel ED, Colyer MH, Bautista C, Bower KS, French LM.. Traumatic brain injury associated with combat ocular trauma. J Head Trauma Rehabil 2009;24(1):41–50. [DOI] [PubMed] [Google Scholar]
- Thomas R, McManus JG, Johnson A, Mayer P, Wade C, Holcomb JB.. Ocular injury reduction from ocular protection use in current combat operations. J Trauma 2009;66(4 Suppl):S99–S103. [DOI] [PubMed] [Google Scholar]
- Bricker-Anthony C, Hines-Beard J, Rex TS.. Molecular changes and vision loss in a mouse model of closed-globe blast trauma. Invest Ophthalmol Vis Sci 2014;55(8):4853–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magone MT, Kwon E, Shin SY.. Chronic visual dysfunction after blast-induced mild traumatic brain injury. J Rehabil Res Dev 2014;51(1):71–80. [DOI] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J.. Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj 2001;15(7):593–612. [DOI] [PubMed] [Google Scholar]
- Neuroscience Bhattacharjee Y. Shell shock revisited: solving the puzzle of blast trauma. Science 2008;319(5862):406–408. [DOI] [PubMed] [Google Scholar]
- Choi JH, Greene WA, Johnson AJ, Chavko M, JM Cleland, RM McCarron, et al. Pathophysiology of blast-induced ocular trauma in rats after repeated exposure to low-level blast overpressure. Clin Exp Ophthalmol; 2014;43(3):239–46. [DOI] [PubMed] [Google Scholar]
- Goodrich GL, Flyg HM, Kirby JE, Chang CY, Martinsen GL.. Mechanisms of TBI and visual consequences in military and veteran populations. Optom Vis Sci 2013;90(2):105–112. [DOI] [PubMed] [Google Scholar]
- Huber BR, Meabon JS, Martin TJ, Mourad PD, Bennett R, Kraemer BC, et al. Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J Alzheimers Dis 2013;37(2):309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke S, Cockerham GC, Glynn-Milley C, Cockerham KP.. Visual quality of life in veterans with blast-induced traumatic brain injury. JAMA Ophthalmol 2013;131(12):1602–1609. [DOI] [PubMed] [Google Scholar]
- Wang HC, Choi JH, Greene WA, Plamper ML, Cortez HE, Chavko M, et al. Pathophysiology of blast-induced ocular trauma with apoptosis in the retina and optic nerve. Mil Med 2014;179(8 Suppl):34–40. [DOI] [PubMed] [Google Scholar]
- Choi JH, Greene WA, Johnson AJ, Chavko M, Cleland JM, McCarron RM, et al. Pathophysiology of blast-induced ocular trauma in rats after repeated exposure to low-level blast overpressure. Clin Exp Ophthalmol 2015;43(3):239–246. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D.. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389(6653):816–824. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998;21(3):531–543. [DOI] [PubMed] [Google Scholar]
- Murata Y, Masuko S.. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res 2006;1085(1):87–94. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Hayakawa T, Kuwahara S, Maeda S, Tanaka K, Seki M, et al. Morphological and immunohistochemical characterization of the trigeminal ganglion neurons innervating the cornea and upper eyelid of the rat. J Chem Neuroanat 2007;34(3–4):95–101. [DOI] [PubMed] [Google Scholar]
- Leonelli M, Martins DO, Kihara AH, Britto LR.. Ontogenetic expression of the vanilloid receptors TRPV1 and TRPV2 in the rat retina. Int J Dev Neurosci 2009;27(7):709–718. [DOI] [PubMed] [Google Scholar]
- Sappington RM, Sidorova T, Long DJ, Calkins DJ.. TRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Invest Ophthalmol Vis Sci 2009;50(2):717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Kuroki T, Okuno Y, Sekiya H, Watanabe A, Sagawa T, et al. Activation of the TRPV1 channel attenuates N-methyl-D-aspartic acid-induced neuronal injury in the rat retina. Eur J Pharmacol 2014;733:13–22. [DOI] [PubMed] [Google Scholar]
- Sumioka T, Okada Y, Reinach PS, Shirai K, Miyajima M, Yamanaka O, et al. Impairment of corneal epithelial wound healing in a TRPV1-deficient mouse. Invest Ophthalmol Vis Sci 2014;55(5):3295–3302. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang Z, Yang H, Wang L, Gillespie SR, Wolosin JM, et al. TRPV1 potentiates TGFbeta-induction of corneal myofibroblast development through an oxidative stress-mediated p38-SMAD2 signaling loop. PLoS One 2013;8(10):e77300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, et al. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol 2003;138(5):977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicho R, Donnerer J, Liebmann I, Lippe IT.. Nociceptive transmitter release in the dorsal spinal cord by capsaicin-sensitive fibers after noxious gastric stimulation. Brain Res 2005;1039(1–2):108–115. [DOI] [PubMed] [Google Scholar]
- Chichorro JG, Fiuza CR, Bressan E, Claudino RF, Leite DF, Rae GA.. Endothelins as pronociceptive mediators of the rat trigeminal system: role of ETA and ETB receptors. Brain Res 2010;1345:73–83. [DOI] [PubMed] [Google Scholar]
- Motta EM, Chichorro JG, Rae GA.. Role of ET(A) and ET(B) endothelin receptors on endothelin-1-induced potentiation of nociceptive and thermal hyperalgesic responses evoked by capsaicin in rats. Neurosci Lett 2009;457(3):146–150. [DOI] [PubMed] [Google Scholar]
- Plant TD, Zollner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, et al. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain 2007;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Ono K, Hitomi S, Harano N, Sago T, Yoshida M, et al. Endothelin receptor-mediated responses in trigeminal ganglion neurons. J Dent Res 2013;92(4):335–339. [DOI] [PubMed] [Google Scholar]
- Avwioro G. Histochemical uses of haematoxylin – a review. JPCS 2011;1(April–June):24–34. [Google Scholar]
- Mergler S, Valtink M, Takayoshi S, Okada Y, Miyajima M, Saika S, et al. Temperature-sensitive transient receptor potential channels in corneal tissue layers and cells. Ophthalmic Res 2014;52(3):151–159. [DOI] [PubMed] [Google Scholar]
- Stratton KJ, Hawn SE, Amstadter AB, Cifu DX, Walker WC.. Correlates of pain symptoms among Iraq and Afghanistan military personnel following combat-related blast exposure. J Rehabil Res Dev 2014;51(8):1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ME, Walker RL, Gironda RJ, Scholten JD.. Comparison of pain and emotional symptoms in soldiers with polytrauma: unique aspects of blast exposure. Pain Med 2009;10(3):447–455. [DOI] [PubMed] [Google Scholar]
- Rowan MP, Bierbower SM, Eskander MA, Szteyn K, Por ED, Gomez R, et al. Activation of mu opioid receptors sensitizes transient receptor potential vanilloid type 1 (TRPV1) via beta-arrestin-2-mediated cross-talk. PLoS One 2014;9(4):e93688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Moff I, Sudanagunta SP, Levine JD.. Molecular cloning of an N-terminal splice variant of the capsaicin receptor. Loss of N-terminal domain suggests functional divergence among capsaicin receptor subtypes. J Biol Chem 2000;275(4):2756–2762. [DOI] [PubMed] [Google Scholar]
- Tian W, Fu Y, Wang DH, Cohen DM.. Regulation of TRPV1 by a novel renally expressed rat TRPV1 splice variant. Am J Physiol Renal Physiol 2006;290(1):F117–F126. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sato J, Kutsuwada K, Ooki G, Imai M.. Cloning of a stretch-inhibitable nonselective cation channel. J Biol Chem 1999;274(10):6330–6335. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Cox J, Deek S, Dvorscak L.. Anatomy of the human corneal innervation. Exp Eye Res 2010;90(4):478–492. [DOI] [PubMed] [Google Scholar]
- Del Fiacco M, Quartu M, Boi M, Serra MP, Melis T, Boccaletti R, et al. TRPV1, CGRP and SP in scalp arteries of patients suffering from chronic migraine. J Neurol Neurosurg Psychiatry 2015;86(4):393–397. [DOI] [PubMed] [Google Scholar]
- Silberstein SD. TRPV1, CGRP and SP in scalp arteries of patients suffering from chronic migraine. Some like it hot! Chronic migraine increases TRPV1 receptors in the scalp. J Neurol Neurosurg Psychiatry 2015;86(4):361. [DOI] [PubMed] [Google Scholar]
- Meents JE, Hoffmann J, Chaplan SR, Neeb L, Schuh-Hofer S, Wickenden A, et al. Two TRPV1 receptor antagonists are effective in two different experimental models of migraine. J Headache Pain 2015;16:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil VK, St Andre JR, Crisan E, Smith BM, Evans CT, Steiner ML, et al. Prevalence and treatment of headaches in veterans with mild traumatic brain injury. Headache 2011;51(7):1112–1121. [DOI] [PubMed] [Google Scholar]
- Bruno PP, Carpino F, Carpino G, Zicari A.. An overview on immune system and migraine. Eur Rev Med Pharmacol Sci 2007;11(4):245–248. [PubMed] [Google Scholar]
- Yan J, Melemedjian OK, Price TJ, Dussor G.. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6). Mol Pain 2012;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, He Q, Ren Z, Li F, Chen W, Lin X, et al. Association of serum levels of intercellular adhesion molecule-1 and interleukin-6 with migraine. Neurol Sci: Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 2015;36(4):535–540. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang H, Wang Z, Mergler S, Wolosin JM, Reinach PS.. Functional TRPV1 expression in human corneal fibroblasts. Exp Eye Res 2013;107:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AR, Li Z, Smith CW.. Neutrophil migration in the wounded cornea: the role of the keratocyte. Ocul Surf 2005;3(4 Suppl):S173–S176. [DOI] [PubMed] [Google Scholar]
- Deby-Dupont G, Grulke S, Caudron I, Mathy-Hartert M, Benbarek H, Deby C, et al. Equine neutrophil myeloperoxidase in plasma: design of a radio-immunoassay and first results in septic pathologies. Vet Immunol Immunopathol 1998;66(3–4):257–271. [DOI] [PubMed] [Google Scholar]


