Abstract
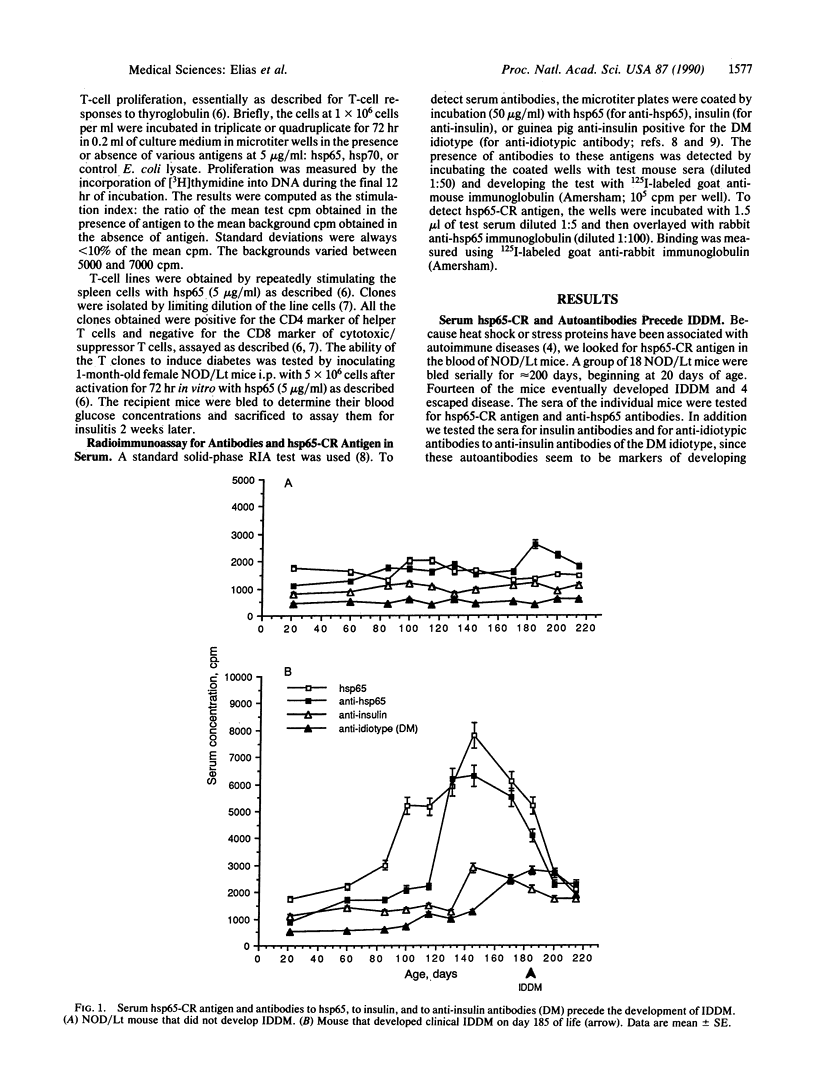
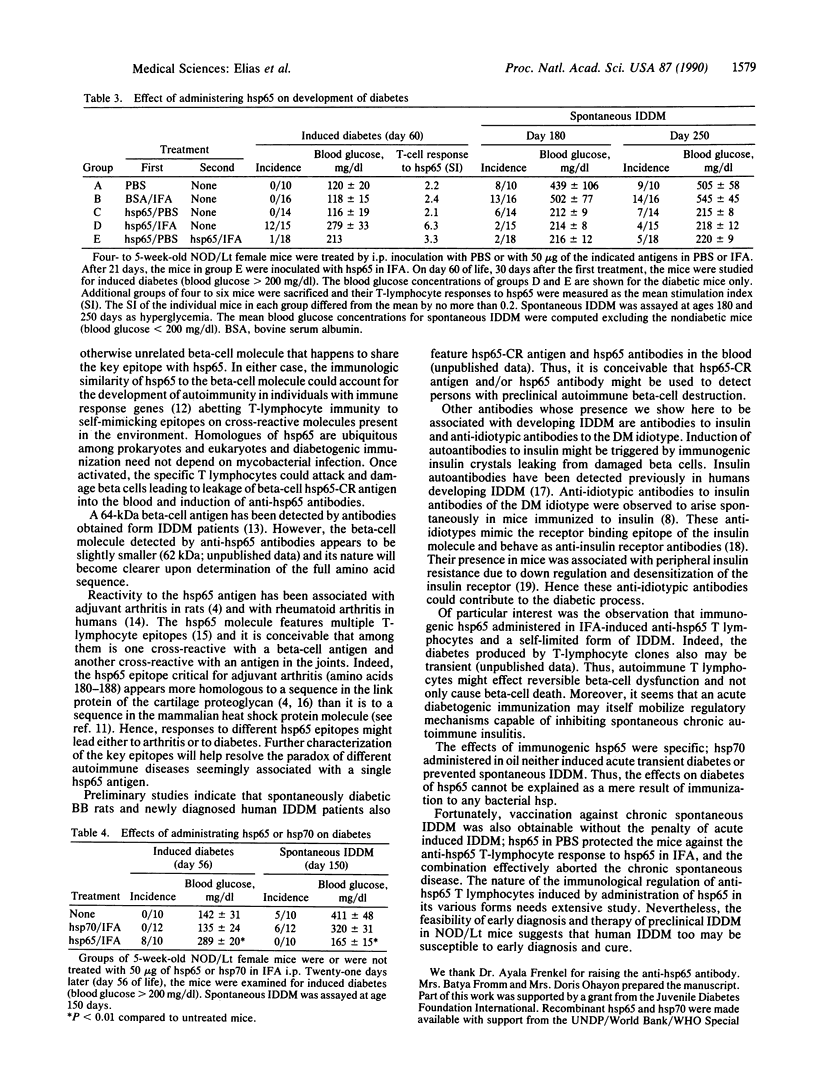
Insulin-dependent diabetes mellitus is caused by autoimmune destruction of the insulin-producing beta cells of the pancreas. The results described here indicate that a beta-cell target antigen in non-obese diabetic (NOD/Lt) mice is a molecule cross-reactive with the 65-kDa heat shock protein (hsp65) of Mycobacterium tuberculosis. The onset of beta-cell destruction is associated with the spontaneous development of anti-hsp65 T lymphocytes. Subsequently hsp65 cross-reactive antigen becomes detectable in the sera of the prediabetic mice and some weeks later anti-hsp65 antibodies, anti-insulin antibodies, and anti-idiotypic antibodies to insulin antibodies become detectable. The hsp65-cross-reactive antigen, the autoantibodies, and the T-cell reactivity then decline with the development of overt insulin-dependent diabetes. The importance of hsp65 in the pathogenesis of insulin-dependent diabetes was confirmed by the ability of clones of anti-hsp65 T cells to cause insulitis and hyperglycemia in young NOD/Lt mice. Moreover, hsp65 antigen could be used either to induce diabetes or to vaccinate against diabetes, depending on the form of its administration to prediabetic NOD/Lt mice. Other antigens such as the 70-kDa heat shock protein (hsp70) had no effect on the development of diabetes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baekkeskov S., Nielsen J. H., Marner B., Bilde T., Ludvigsson J., Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein M. B., Miller A., Slagle S., Weitzman M., Crystal H., Drexler E., Keilson M., Merriam A., Wassertheil-Smoller S., Spada V. A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. N Engl J Med. 1987 Aug 13;317(7):408–414. doi: 10.1056/NEJM198708133170703. [DOI] [PubMed] [Google Scholar]
- Cohen I. R. The self, the world and autoimmunity. Sci Am. 1988 Apr;258(4):52–60. doi: 10.1038/scientificamerican0488-52. [DOI] [PubMed] [Google Scholar]
- Elias D., Maron R., Cohen I. R., Schechter Y. Mouse antibodies to the insulin receptor developing spontaneously as anti-idiotypes. II. Effects on glucose homeostasis and the insulin receptor. J Biol Chem. 1984 May 25;259(10):6416–6419. [PubMed] [Google Scholar]
- Elias D., Rapoport M., Cohen I. R., Shechter Y. Desensitization of the insulin receptor by antireceptor antibodies in vivo is blocked by treatment of mice with beta-adrenergic agonists. J Clin Invest. 1988 Jun;81(6):1979–1985. doi: 10.1172/JCI113546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Matitiau A., Cohen I. R. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984 Jan;73(1):211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron R., Zerubavel R., Friedman A., Cohen I. R. T lymphocyte line specific for thyroglobulin produces or vaccinates against autoimmune thyroiditis in mice. J Immunol. 1983 Nov;131(5):2316–2322. [PubMed] [Google Scholar]
- Mehlert A., Young D. B. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71kD antigen, a member of the 70kD heat-shock protein family. Mol Microbiol. 1989 Feb;3(2):125–130. doi: 10.1111/j.1365-2958.1989.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Asplin C. M., Clemons P., Lyen K., Tatpati O., Raghu P. K., Paquette T. L. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983 Dec 23;222(4630):1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Rossini A. A., Mordes J. P., Like A. A. Immunology of insulin-dependent diabetes mellitus. Annu Rev Immunol. 1985;3:289–320. doi: 10.1146/annurev.iy.03.040185.001445. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Elias D., Maron R., Cohen I. R. Mouse antibodies to the insulin receptor developing spontaneously as anti-idiotypes. I. Characterization of the antibodies. J Biol Chem. 1984 May 25;259(10):6411–6415. [PubMed] [Google Scholar]
- Steinman L., Cohen I. R., Teitelbaum D., Arnon R. Regulation of autosensitisation to encephalitogenic myelin basic protein by macrophage-association and soluble antigen. Nature. 1977 Jan 13;265(5590):173–175. doi: 10.1038/265173a0. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]



