Abstract
Vitellogenin (Vtg), a large lipoglycophosphoprotein, is the most important precursor of the yolk proteins, and the major source of nutrients for the developing embryo in oviparous species. After its uptake by the oocytes, Vtg is converted into lipovitellins (high and light) and phosvitin, which are deposited into crystalline yolk platelets. We describe here the presence of two high molecular mass lipovitellins isoforms in Bufo arenarum mature oocytes with masses of 113 and 100 kDa, respectively. The amino acid sequence analysis of p113 and p100 peptides showed a high sequence homology between both polypeptides and the complete reported sequences of Xenopus laevis vitellogenin. Using specific antibodies, we determined that the Vtg uptake begins early during oogenesis, at the previtellogenic stage, and continues until oocytes have reached their mature status. In addition, we found that large endocytic vesicles mediate Vtg uptake in stage I oocytes, and that the size of the endocytic vesicles declines with oogenesis progression. In terms of the Vtg protein trafficking, we detected the Vtg precursor (190 kDa) in the liver of estradiol-injected females. Finally, we propose a subclassification of B. arenarum stage-II oocytes into three physiologically and morphologically distinct periods (early, mid and late).
Keywords: Vitellogenesis, oogenesis, lipovitellin, yolk platelets, amphibian
1. INTRODUCTION
In egg-laying species, the developing embryo is dependent on the egg components for its nutritional requirements. The major source of nutrients for the developing embryo is the egg yolk. In amphibians, vitellogenin (Vtg), a large lipoglycophosphoprotein, is the most important precursor of the yolk proteins. Vtg is synthesized in the liver in response to estrogen, transported via the bloodstream to the ovary and internalized by the growing oocytes through receptor-mediated endocytosis (Tata and Smith, 1979; Wallace et al., 1973) After its uptake by the oocyte, Vtg is converted into lipovitellins (a high molecular mass lipovitellin –LvH- and a light lipovitellin -LvL-) and phosvitins, which are deposited into crystalline yolk platelets (Opresko et al., 1980). Xenopus laevis (Mesobatrachia) has been a suitable model for studies on vitellogenesis (Wallace, 1970; Wallace and Ho, 1972; Yoshitome et al., 2003). Although the existence of two families (A and B) and four subtypes (A1, A2, B1 and B2) of vitellogenin has been shown in Xenopus sp., the complete sequence has been determined only for vitellogenins A2 (Gerber-Huber et al., 1987; Wahli et al., 1982; Wallace et al., 1990) and B1 (Yoshitome et al., 2003). X. laevis LvH is derived from the amino-terminal of its precursor and has an apparent molecular mass of 115 kDa (Molla et al., 1983). Using higher-resolution analytical procedures, three apoLvH proteins with molecular masses of 121, 116, 111 kDa have been characterized in Xenopus laevis (Wiley and Wallace, 1981). In species closely related to Bufo arenarum, like Odontophrynus americanus (Neobatrachia), two isoforms, LvHα and β, with molecular masses of 104.6 kDa and 92.6 kDa, respectively, have been also identified (Winter et al., 1985). Several studies have reported on the mechanism of the Vtg internalization in amphibians (Wall and Patel, 1987; Ward, 1978). However, there is scarce information on Vtg protein processing during the oogenesis in these species. It is known that the growth rate of oocytes is closely related to the rate of the vitellogenin uptake. The fastest rate of growth in Xenopus sp. oocytes occurs from mid-stage IV (approximately 0.8 mm diameter) until midstage V (1.2 mm diameter), which corresponds to the period of the most pronounced vitellogenin uptake. In the final stages of the oogenesis, the amphibian oocytes acquire an animal-vegetal polarity, showing pigment granules in the animal pole and the yolks platelets localized in the vegetal hemisphere (Danilchik and Gerhart, 1987). Bufo arenarum (Chaunus arenarum, Frost et al., 2006) is a common toad (Anura: Bufonidae) in the South American region and has a yearly reproductive cycle. Its ovulation occurs during the spring season. During natural hibernation large populations of uniform sized hormone-responsive follicles exist in arrested meiosis within the ovary. In the present work, two polypeptides, p113 and p110, were identified as two different isoforms of LvH by MS/MS analysis in B. arenarum oocytes. Our work focuses on their biochemical characterization and localization during the oogenesis, and demonstrates that the Vtg uptake begins early during the oogenesis and continues until the oocyte reaches its full, mature size.
2. MATERIALS AND METHODS
2.1 Experimental Animals
Sexually mature B. arenarum specimens were collected in the neighborhoods of Rosario City and kept in a moist chamber at 12 ºC until used. Experiments were performed in accordance with the guide for the care and use of laboratory animals of Facultad de Ciencias Bioquimicas y Farmacéuticas, Universidad Nacional de Rosario.
2.2 Preparation of protein extracts from B. arenarum oocytes
Female specimens were kept in a moist chamber at 20–22 ºC for 1 day before stimulation, which was done by intracoelomical injection of a homologous pituitary extract of sexually mature animals. After 10–12 h, oocyte strings were collected from ovisacs (Valz-Gianinet et al., 1991). Degelling was then performed as previously reported (Barisone et al., 2002). Oocytes were washed with 10% v/v Ringer-Tris buffer, homogenized with a Potter-Elvehjem homogenizer, and the vitelline envelopes were separated by filtering the protein extract through a double sheet of a 30-mesh screen. In order to improve the yolk protein recovery, ovulated oocytes were solubilized in a variety of high-ionic strength buffers. Once treated, the samples were centrifuged and supernatants were analyzed by SDS/PAGE (data not shown) to determine the presence or not of the vtg related bands. We found that yolk proteins solubility was highest in 6 M guanidine + 5% w/v CHAPS, 6 M guanidine + 50 mM DTT, 2% w/v SDS, or 8 M urea + 2% w/v CHAPS + 50 mM DTT.
2.3 Collagenase – dissociation of ovarian oocytes
Females were anesthetized and pieces of ovary were carefully dissected and incubated during 15 minutes in PBS buffer containing 4 mM EDTA, 25 mM sucrose and 1mg/mL of collagenase.
2.4 Staging of B. arenarum oocytes
After collagenase treatment, oocytes, freed from follicular cells, were staged in accordance to Valdez Toledo and Pisanó (1980) as follows: stage I or previtellogenic B. arenarum oocytes (45–200 μm), stage II or primary vitellogenic (200–600 μm), stage III or late vitellogenic (600–1200 μm) and stage IV or full-grown (> 1200 μm). The oocytes diameter was measured with a micrometer fitted into the eyepiece of a dissecting microscope. In some cases, ovarian oocytes were resuspended directly in Laemmli (1970) sample buffer prior to analysis by SDS-PAGE.
2.5 Protein analysis by 1D and 2D PAGE
Protein analysis by 1D SDS-PAGE was performed essentially according to the method of Laemmli (1970). Two dimension gel electrophoresis (2D PAGE) was performed on Protean IEF cell (Bio-Rad) using pI 3–10 strips (Amersham Biosciences) (first dimension). The strip was then rehydrated in buffer 8 M Urea, 2% CHAPS and 50 mM dithiothreitol, and run in a 8% SDS/PAGE (second dimension). Gels were either stained with Silver (Gradilone et al., 1998) or with Coomassie Blue. Apparent molecular masses were estimated with molecular mass standards (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Protein concentration was estimated by gel densitometry using the GelPro32 software.
2.6 Isolation of p113 and p100
Oocytes protein extracts were analyzed by SDS/PAGE, followed by Coomassie Blue staining. Bands of interest were then excised and electroeluted with an Electro-Eluter Assembly (Model 422, BioRad) to obtain purified p113 and p100.
2.7 Antibody preparation
In order to study Vtg uptake and localization in the oocytes, purified p113 and p100 proteins were used to produce specific antiserum in rabbits using the method described by Arranz et al. (1997). Preimmune serum was obtained from the same animals before the first injection. Anti p113 and anti p100 were affinity purified according to Plaxton (1989).
2.8 Western-blot analysis
After 1D or 2D PAGE, proteins were electrotransferred to nitrocellulose membranes, washed twice with PBS and the membrane was blocked with PBS buffer supplemented with 5% nonfat dried milk (Molico, Nestlé). Membranes were incubated for 1.5 h with each rabbit antiserum (anti-p113 or anti-p100) at different dilutions. Bound antibodies were detected with a goat anti-rabbit secondary antibody coupled to horseradish peroxidase (1:5000 dilution in PBS, Amersham). Antiserum against Mozambique tilapia (Oreochromis mossambicus) Vtg was generous provided by Dr. Jennifer Specker (University of Rhode Island, RI, USA).
2.9 Immunohistochemistry
Immunohistochemical staining was performed according to Armas et al. (2001). Five μm thick sections were dehydrated and coincubated with p113 and p100 antiserum (1:500 dilution each). Secondary anti-rabbit Cy3 conjugated antibody (Chemicon, International) was used to detect the first antibody. Slides were examined under light and fluorescence microscopy with an Olympus BH-2 microscope (Olympus, Tokyo, Japan).
2.10 Immuno electron microscopy
For ultrastructural studies, pieces of preovulatory stage ovary from sexually mature females were fixed in 2.5% v/v glutaraldehyde in 0.1M phosphate buffer (pH=4) for 4 h at 4 °C. Samples were then washed twice in the same buffer and post-fixed overnight at 4 °C in 1% osmium tetroxide, 0.1M phosphate buffer (pH=7.4). After dehydration in an ethanol-acetone series, and inclusion in Arakdite 6007, specimens were sectioned. P113 and p100 were evidenced by embedding sections with antibodies against p113 and p100 (1:500 dilution each), and detected by a secondary goat anti-rabbit immunoglobulin G antibody, conjugated to 18 nm gold particles (Sigma). Sections were then observed under a Zeiss 109 electron microscope.
2.11 B. arenarum vitellogenin precursor
In order to enhance synthesis of Vtg in the liver, females of B. arenarum were injected with 4 mg of oestradiol-17 β per 100 g body mass (Wallace and Selman, 1980). After four days, the animals were anaesthetized and the liver removed. Control animals received an injection of Ringer solution without oestradiol-17 β. Tissues were homogenized in 1mM EDTA, 1mM PMSF in PBS buffer with a Potter-Elvehjem homogenizer. The homogenate was centrifuged at 12000 g for 30 min at 4 °C and supernatants were stored at −20 °C until further analysis.
2.12 Protein Identification and Sequence Analysis
Protein identification was carried out by tandem mass spectrometry. Gel pieces containing p113 and p100 polypeptides respectively, were submitted to the Proteomic Mass Spectrometry Laboratory in the University of Massachusetts Medical School (Worcester, MA, USA) for peptide sequencing. The peptides were derivatized according to Wang et al. (2004) to produce easily interpreted MS/MS spectra. The most abundant signals in the spectra were sequenced. Then, a manual BLAST search for “short nearly exact matches” was performed (www.ncbi.nlm.nih). Multiple amino acid alignments of Vtg homologues were achieved using Clustal X software (Chenna et al., 2003; Thompson et al., 1997).
3 RESULTS
3.1 Biochemical characterization of the Bufo arenarum oocyte yolk proteins
In a first attempt to identify the oocyte yolk proteins, we used an antiserum against the Oreochromis mossambicus Vtg (omVtg) that was available in our laboratory. A multiple alignment of Vtg protein sequences showed that many peptides were conserved in fish and amphibians (data not shown). Protein extracts from the B. arenarum ovulated oocytes were fractionated by SDS/PAGE and Western blot (Fig. 1 A and B respectively). Major bands with molecular masses of 113, 100 kDa (hereafter termed p113 and p100, respectively) and between 25–30 kDa were detected in a silver stained gels (Fig. 1 A). The anti-omVtg antibodies recognized p113 and p100 from B. arenarum oocytes (see Fig. 1 B arrows). A 30 kDa protein was also recognized. In addition, the analysis of the homogenate by 2D-gel and Western blot using the anti–omVtg antiserum showed two distinct protein spots (see Fig 1 C, arrows) corresponding to p113 and p100, with isoelectric points of 5.5 in each other. This analysis also showed a lower molecular weight spot, corresponding to the 30 kDa yolk component previously recognized by the antibodies. The isoelectric point of the 30 kDa spot was pI 6.
Fig. 1. Yolk proteins in B. arenarum oocytes and specific antibodies production.

A. Oocytes extracts (1 and 0.5 μg of total protein) were analyzed on 8% gel under reducing and denaturing conditions and Silver stained. B. and C. Western Blot analysis of oocytes extracts using anti-Vtg antiserum from Mozambique tilapia (anti-omVtg, 1:2000). B. Oocyte extracts (1 ug) was loaded in a 8% SDS/PAGE and then transferred to a nitrocellulose membrane. Left blot represents the Ponceau staining of the membrane. C. Analysis of mature oocyte (1 μg total protein) by 2D gels. D. Specificity of anti-p113 and anti-p100 antiserums. Oocyte extracts (0.1 μg total protein) were analyzed in 10% SDS/PAGE and Western blots using anti-p113 (lane I) or anti-p100 (lane II) sera (1:1000 each). Black arrows show p113 and p100 and molecular mass markers (kDa) are displayed on the left.
3.2 Identification and sequence analyses of B. arenarum p113 and p100 proteins
Identification of p113 and p100 from B. arenarum was carried out after in gel trypsinolysis of the silver stained bands followed by a tandem mass spectrometry analysis. The peptides obtained were derivatized to produce easily interpreted MS/MS spectra since the genome of Bufo arenarum has not been sequenced as yet. Table 1 shows the sequences of four abundant peptides from p113 and seven peptides from p100. A manual protein BLAST search for “short nearly exact matches” of the eleven peptides (http://www.ncbi.nlm.nih.gov/BLAST) showed that five of them presented high homology with X. laevis vitellogenin A2 and B1 (Table 1). Peptides 2, 4 and 11 presented high homology with Vtg X. laevis B1, while peptides 3 and 10 presented homology with both A2 and B1. A protein sequence alignment was done between the peptides and X. laevis Vtgs A2 and B1 (Fig. 2). Alignment studies showed that all the peptides that matched with X. laevis Vtgs protein sequences were situated in the N-terminal region from the consensus putative cleavage sites (Finn, 2007; Kristoffersen et al., 2009), suggesting that p113 and p100 may be different isoforms of LvH. Additionally, we found high sequence homology between the peptides and other amphibian vitellogenins. B. gargarians Vtg (GenBank accession no. AAX84828) was partially sequenced and peptides 3 and 10 showed 70 % and 100 % sequence similarity respectively (data not shown).
Table 1.
Analyses of B. arenarum yolk peptides by MS/MS spectrometry.
| p133 | |||
| Peptide | Amino acid sequence | Homology | % of identity–similarity |
| 1 | TQVFAVSR | NH | |
| 2 | AEGiQEViR | B1 | 100–100 |
| 3 | VQVDAVMAiR | A2 and B1 | 80–90 |
| 4 | GYAAGASTDiFEFGVR | B1 | 44–50 |
| p100 | |||
| Peptide | Sequence | Homology | % of identity–similarity |
| 5 | iQiSPYSQR | NH | |
| 6 | iiPiAiHYTR | NH | |
| 7 | SWiiQAiPVTR | NH | |
| 8 | VQETiMNVYMNR | NH | |
| 9 | GSQQAiQiAQDiiR | NH | |
| 10 | FiPGFSSSAQQiPVR | A2 and B1 | 86–93/66–86 |
| 11 | iiYQMiHDGDiTNAEANR | B1 | 44–61 |
P113 and P100 were analyzed by MS/MS spectrometry. Table shows amino acid sequences of eleven abundant derivatized peptides from 1 to 11. The homology with X. laevis vitellogenin A2 (GenBank accession no. CAA68433) and B1 (GenBank accession no. NP_001094403) is presented. The percentages represent sequence identity (pain) and similarity (bold). Notice that peptide number 10 presented different percentages in the analysis with VtgA2 or Vtg B1. In De Novo sequencing i = I or L. NH indicates no homology.
Fig. 2. Sequences comparison between X. laevis A2 and B1 Vtg proteins and B. arenarum LvH peptides.

A. Schematic representation of vertebrates Vitellogenin protein, composed of a signal peptide, a heavy chain lipovitellin (LvH), a phosvitin (Pv), a light chain lipovitellin (LvL), and von Willebrand factor type D domains (Wf). B. Alignment of the amino acid sequences of A2 (GenBank accession no. CAA68433) (upper) and B1 (NP_001094403) (lower) X. laevis vitellogenin (number of residues 1 to 1280 are indicated). Asterisk (*) indicate identical amino acid residues, double dots (:) highly similar residues and single dots similar residues. Two, 3, 4, 10 and 11 homologous peptides are represented by black boxes. Consensus sequence of the putative subdomain cleavage sites (“KKIL”, “KIIL” and “KMIL”) is shown with dotted line.
3.3 BaLvH presence during the oogenesis
In order to study the Vtg uptake and localization in the oocytes, polyclonal antibodies were prepared against p113 and p100 respectively. Both antisera presented high specificity with dilution titles of 1:5000 for each one using 0.5 μg of protein. Antisera (anti-p113 and anti-p100) were highly reactive against the specific antigen (Fig. 1 D). However, some cross-reactivity was noted between them. In order to rule out cross-contamination during the antiserum preparation, each antiserum was tested after antisera purification and the same cross-reactivity was observed (data not shown) indicating that p113 and p100 share some common epitopes. In order to determine the presence of Bufo arenarum LvH (baLvH) in ovarian oocytes, we first subclassified stage II oocytes in Early, Mid and Late based on morphological differences between these oocytes. Fig. 3 A shows the morphology of seven collagenase - dissociated oocytes (1–7). The stage I oocytes are transparent with a visible germinal vesicle (Fig. 3A 1) In the early stage II oocytes, the cytoplasm is translucent (Fig. 3A 2 and detail). In the mid stage II, the oocyte acquires a characteristic white and opaque color (Fig. 3A 3 and detail), and in the late stage II, oocytes appear light brown and opaque (Fig. 3A 4 and detail). Oocytes in stage III present a uniform pigmentation. They are grey (Fig. 3A 5) or brown (Fig. 3A 6) depending on the pigment concentration. The stage IV oocytes show the typical differentiation of animal and vegetal hemispheres (Fig. 3 A 7). To obtain a representative pattern of the oogenesis progression and the Vtg uptake, equal volumes of proteins extracts, obtained from different stages of the collagenase– dissociated oocytes, were analyzed by SDS/PAGE and Western blot (Fig. 3, B and C respectively). The presence of the baLvHs was initially observed between stage II and IV (Fig. 3 B and C). In the early-stage II, p100 band was detected as a weak signal in Western blots (Fig. 3 C, lane 2). In the mid-stage II, both bands were clearly detected (Fig. 3 B and C, lane 3). The baLvHs bands were the main proteins of the crude extracts from late stage II to stage IV. Both lipovitellins bands were detected also in stage I oocytes if higher quantities of total protein extracts were used for Western blot analysis (Fig. 3 D). In Stage I oocytes extract we observed a double band with higher molecular weight that were not recognized by the anti-p100/p133 antibodies (see Ponceau stain and western blot essay of Fig. 3 D lane I). To rule out the possibility that the addition of collagenase could cause Vtg proteolysis, we obtained oocytes from the ovaries with watch-makers forceps and performed a Western blot essay using the oocyte extracts. In all the cases, both lipovitellins bands were observed (data not shown).
Fig. 3. B. arenarum LvH presence during oogenesis.

A. Photomicrographs of collagenase–dissociated ovarian oocytes from Bufo arenarum. 1: 180 μm, 2: 290 μm, 3: 340 μm, 4: 430 μm, 5: 635 μm, 6: 870 μm, 7: 1380 μm in diameter. Stages were named according to Valdez Toledo and Pisano (I, II, III and IV) and the long arrow represents the progress of oogenesis. Barr represents 200 μm. B. Collagenase–dissociated ovarian oocytes were homogenated with sample buffer, loaded in 8% SDS/PAGE and stained with silver. C. Western blot analysis using baLvH antiserum (anti-p113 and anti-p100 coincubation, 1:1000 each antiserum). In each lane, the volume corresponding to three stage I oocytes (170 μm in diameter) were loaded. D. Twenty collagenase–dissociated ovarian stage I (lane I) and stage IV (lane IV) oocyte extracts were analyzed in 8% SDS/PAGE and Western blot. Left panel represents the Ponceau stain of the membrane. Black arrows indicate the immunoreactive bands. Numbers at the right side indicate molecular mass markers (kDa).
3.4 Immunohistochemistry localization and Ultrastructural studies of baLvH during oogenesis
Based on our previous results, we next studied the LvHs localization in the ovary of B. arenarum by immunohistochemistry and immunoelectron microscopy of ultrathin sections. Sections from ovaries were incubated with polyclonal antibodies against p113 and p100, alternatively. Since p113 and p100 colocalized (data not shown), all immunolocalization essays were further done using a combined antiserum. In the stage I previtellogenic oocytes (Fig. 4), a membrane-associated fluorescence was observed in the border of the oocyte and in vesicles attached to the membrane (Fig. 4, panels C, E, F and H,). In electron microscopic images from the ovarian previtellogenic oocytes (Fig. 4 K to M), the vitelline envelope is absent (Cabada et al., 1996) and the plasma membrane presented pronounced invaginations with black gold particles inside them (see Fig. 4 L). The electro-dense particles were also observed in vesicles into the cytoplasm near the plasma membrane (Fig. 4 M). In stage II primary vitellogenic oocytes (Fig. 5) the fluorescence was observed in the border of the oocyte and in some cytoplasmatic patches with a peripheral location (Fig. 5, C, E, F and H). Figure 5 K showed oocyte microvilli and a growing vitelline envelope organized into a fiber bundles-like network (Cabada et al., 1996). Plasma membrane invaginations appeared less pronounced compared with previtellogenic oocytes ones and a abundant vesicles with different electro-dense particles content fusing to each other were observed in the oocyte cortex (see detail inside a black square, highlighted by black arrow in Fig. 5 K). These images suggest that the content of the early endosomes may be discharged into denser bodies. We also observed multivesicular bodies in the oolemma of the primary vitellogenic oocyte (see magnifications highlighted by white arrows in Fig. 5 K). In full-grown oocytes (Fig. 6), p113/p100 staining was observed between the vitelline envelope and the plasma membrane of the oocyte (Fig. 6, C, E and G). Immune reactivity was not polarized since the fluorescence was detected in both, the animal and vegetal hemispheres of the full-grown oocytes (see Fig. 6, panels C and E, respectively). P113/p100 staining was also detected inside the yolk platelets (see Fig. 6, panel E). In the immunoelectron micrograph, vitelline envelope is completely formed (Cabada et al., 1996) and it is difficult to distinguish its components. Gold particles could be seen within the microvilli and in the perivitelline space (Fig. 6 K) suggesting late massive yolk precursor incorporation. At this stage of the oogenesis, typical yolk platelets are found in the peripheral cytoplasm and it is possible to observe the characteristic yolk platelet architecture (Fig. 6 L and M).
Fig. 4. B. arenarum LvH localization in stage I oocytes.

Five μm thick ovarian sections were deparaffinized and stained by hematoxylin and eosin (panels A and B). Oocytes are close associated with follicle cells (FC) layers. With black arrows are highlighted the pronounced invaginations of the plasma membrane. Immunohistochemistry and immuno electron studies was performed according to M&M using baLvH antiserum (anti – 113 and anti – p100 co-incubation, 1:500 each one). Immunofluorescent micrographs and their light micrograph of stage I oocytes (C and F correspond to a 150 μm and 167 μm in diameter, respectively). A fluorescent stain is highlighted with white arrows in higher magnification of C and F (E and H). Control essay was performed using anti-rabbit pre-immune serum (I and its light micrograph in J). In immunoelectron microscopy sections the theca cells (TC) are close to the folded plasma membrane of the oocyte (O) which presents pronounced invaginations (K, white arrows) with black gold particles inside them (L and M).
Fig. 5. B. arenarum LvH localization in stage II oocytes.

Five μm thick ovarian sections were deparaffinized and stained by hematoxylin and eosin (A and B). Immunofluorescent micrographs (C corresponds to an oocyte with 205 μm and F corresponds to an oocyte with 240 μm in diameter) and their light micrograph (D and G, respectively). A fluorescent stain is highlighted with white arrows in higher magnification (E and H). Control essay was performed using anti-rabbit pre-immune serum (I and its light micrograph in J). K. Immunoelectron micrograph of late stage II oocyte follicle. At this stage, oocyte microvilli (mv) and a growing vitelline envelope (VE) were observed. Oocyte cortex shows many electro-dense vesicles (see the magnification of an electro-dense structure with gold particles inside them highlighted with black arrow) and multivesicular bodies (see higher magnifications highlighted with white arrows).
Fig. 6. B. arenarum LvH localization in full-grown oocytes.

Five μm thick ovarian sections were deparaffinized and stained by hematoxylin and eosin (A and B). The oocyte acquires a distinctive animal-vegetal polarity; an asymmetric distribution of cortical pigments granules (cpg) in the animal hemisphere (AH) and the yolk mass in the vegetal hemisphere (VH). Nucleus of follicular cell (NFC) and vitelline envelope (VE) are easy recognized (see black arrows). Immunofluorescence micrographs of animal (C and its light micrograph D) and vegetal (E and its light micrograph F) hemispheres of a fully-grown oocyte follicle. A higher magnification of a full-grown oocyte, vegetal hemisphere detail (G) and the light micrograph (H). Control essay was performed using anti-rabbit pre-immune serum (I and its light micrograph in J). Immunoelectron micrograph of full-grown oocyte surface (K). Gold particles within the oocyte (O) microvilli (mv, see dot line under the micrograph) and perivitellin space (ps). Yolk platelets are fusing (M) and a typical crystalline structure with gold particles inside (L).
3.5 Detection and biochemical characterization of B. arenarum vitellogenin precursor
It is known that the precursor of the yolk proteins is synthesized in the liver under estradiol-17β stimulation. In order to characterize the estrogenic dependence of Vtg precursor synthesis, we injected B. arenarum females with estradiol. Liver extracts obtained from control and estradiol-injected females were analyzed by Western blot using anti-BaLvH combined antiserum. A protein of ~190 kDa was detected only in estradiol-injected female liver extracts (see arrow in Fig. 7 B, lane SL) but neither in control liver extracts nor in mature oocytes. These results suggest that the Vtg precursor from liver is processed soon after the uptake by the oocyte and that both lipovitellins isoforms belong to Vtg precursors of similar molecular weights.
Fig. 7. Analysis of B. arenarum vitellogenin precursor upon stimulation with estradiol.
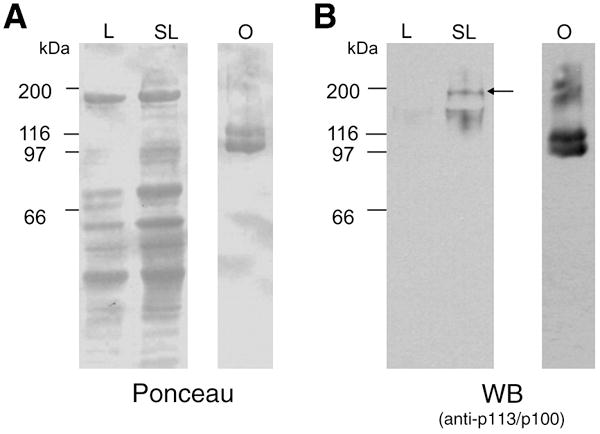
Liver extracts (15 μg total protein) from estradiol-stimulated (SL) and not stimulated (L) females were loaded in 8% SDS/PAGE and electrotranferred to nitrocellulose membrane. As a control, extract from ovulated oocytes (0.1 μg total protein) was loaded (lane O). A. Nitrocellulose membrane was stained with Ponceau as loading control. B. Western Blot using anti-baLvH antibodies (co-incubation, 1:1000 each). Molecular masses (kDa) are indicated on the left.
4. DISCUSSION
In almost all non-mammalian vertebrates, the oocytes size correlates with the amounts of yolk platelets in the cytoplasm. In fully grown amphibian oocytes, yolk proteins comprise at least 80% of total proteins (Benbow and Ford, 1975; Callen et al., 1980). The present study was aimed to determine the yolk protein composition in the amphibian Bufo arenarum and the stages associated to the Vtg uptake by the oocyte. Vitellogenin belongs to a family of multidomain lipoproteins, which are highly conserved throughout evolution. Fish, amphibian, reptile and avian vitellogenins are closely related not only functionally, but also structurally. Some epitopes of vitellogenins from different taxonomic groups have been conserved throughout evolution (Finn, 2007; Kristoffersen et al., 2009). Using heterologous antibodies against Vtg precursor, we identified three Vtg related proteins in Bufo arenarum mature oocytes extracts with molecular masses of 113, 100 and 30 kDa. By MS/MS spectrometry analyses, four abundant peptides sequences from p113 and seven abundant peptides from p100 were determined. Five of the eleven sequenced peptides presented high sequence homology with other amphibian Vtgs. P100 and p113 also share two peptide sequences between them and with Vtgs from other amphibian species. Specific antibodies were obtained for p113 and p100 respectively. The antisera were specific for each polypeptide suggesting that p113 and p100 belong to different Vtg isoforms. A weak cross-reaction was observed between them and could be related with the presence of conserve domains between both polypeptides. In vertebrates, a complete Vtg is composed of a signal peptide, a heavy chain lipovitellin (LvH), a phosvitin (Pv), a light chain lipovitellin (LvL), and a von Willebrand factor type D domain (Vwfd) (Romano et al., 2004). After removal of the signal peptide, the mature vertebrate Vtg is linearly organized as a quadrapartite NH2-LvH-Pv-LvLVwfd-COO-monomer. In early endosomes, Vtg is cleaved and it yields lipovitellins, phosvitin, and Vwfd (Finn, 2007). Protein sequence alignment between the sequenced peptides from Bufo arenarum oocytes and the already known Vtgs suggest that p113 and p100 could correspond to subdomains of different Vtg precursors. Finn (2007) has reported a consensus cleavage site “KKIL” for vertebrate Vtgs. Two years later, Kristoffersen (2009) reported a new consensus cleavage site “KIIL” or “KMIL”. Although the exact cleavage site of Xenopus LvH domain has not been determined, we found the consensus cleavage sites reported by Finn (768 amino acid position) and by Kristoffersen (1081 position) in both B2 and A1 Xenopus Vtgs (Fig. 2). The first cleavage site would render peptides of about 85 kDa, while the molecular mass predicted from the sequenced genes is 120 kDa considering the second cleavage site. Whatever the cleavage site is, all the Bufo Vtg-related sequenced peptides matched to both predicted amino terminal domains of Xenopus sp. Vtg precursors indicating that p113 and p100 would correspond to LvH domains (Fig. 2 A and B) from different Bufo Vtg precursors. In Xenopus laevis, four LvH isoforms were detected while in Bufo arenarum, like in Odontophrynus americanus (Winter et al., 1985), analysis of oocyte extracts by two dimensional gel electrophoresis showed that only two protein bands of LvH are the major yolk components of mature oocytes. The differences in the isoforms quantity may be ascribed to the tetraploid genome of Xenopus laevis comparing with the diploid genome of B. arenarum (Schmid and Guttenbach, 1988).
The nature of the 30 kDa Vtg-related band is uncertain. Wiley and Wallace (1981) described the presence of three apo-LvL polypeptides with 34, 31.5 and 30.5 kDa and two phosvitins with 34 and 33 kDa in X. laevis oocytes. In contrast, Winter et al. (1985) reported bands of 31.7, 29.7 and 27.8 kDa for Odontophrynus americanus LvL and of 37.4, 27.7 and 26.1 kDa for phosphoproteins. Regarding to the identity of B. arenarum 30 kDa band recognized by O. mossambicus Vtg antiserum, our results suggest that the 30 kDa protein could be a LvL since phosvitins do not stain with Coomassie blue (Wallace, 1985).
Vtg is selectively taken up by growing oocytes, cleaved into yolk proteins and stored in the form of yolk platelets or granules. The insoluble material in the granules is mainly lipovitellins (Redshaw and Follett, 1971). We determined that Bufo lipovitellins are soluble only under high-ionic strength solutions.
In order to analyze the presence of baLvH during oogenesis we decided to subdivide stage II (Valdez Toledo and Pisanó, 1980) oocytes in three different periods (early, mid and late stage II oocytes) based on morphological differences within stage II. Previous morphological and histological studies determined that vitellogenesis started in stage II (primary vitellogenic) in Bufo oocytes (Valdez Toledo and Pisanó, 1980) and in stage III oocytes in X. laevis (Dumont, 1972). By Western blot and microscopy studies, we have shown that the Vtg uptake begins at stage I previtellogenic oocytes and is highest between stages III and IV, since stage IV oocytes increase several times the volume of stage III counterparts while the concentration of both LvHs does not change. Another focus of our research was to localize LvH during oogenesis. It is known that antibodies against yolk proteins may cross-react with their precursor; in fact, we demonstrated that baLvH antibodies recognized the precursor vitellogenin. It has been shown that Xenopus Vtg enters the capillary network of the follicle and passes through the connective tissue layer and follicular epithelium to the oocyte surface, where it is presumably sequestered by a pinocytosis mechanism (Wallace and Dumont, 1968). Interestingly, we found a bright fluorescent staining pattern in the border and in endocytic vesicles of stage I previtellogenic oocytes suggesting that Vtg protein trafficking occurs during the earliest stage in Bufo oogenesis. This result is in agreement with our immunodetection results on oocyte extracts. The uptake of Vtg into growing oocytes occurs by receptor-mediated endocytosis. Receptors for Vtg (VtgR) have been identified at the oocyte cell surface in several vertebrate groups, including birds (Stifani et al., 1988), amphibians (Opresko and Wiley, 1987) and fish (Stifani et al., 1990). In rainbow trout the VtgR is present in oocytes throughout the major part of ovarian development and that it can be detected even before the onset of vitellogenesis (Perazzolo et al., 1999). However, studies on VtgR expression in the Bufo arenarum are lacking and the mechanism of Vtg uptake in previtellogenic oocytes is still unknown.
Vtg uptake during previtellonic stage was also confirmed by immunoelectron studies which showed massive endocytosis of extracellular content with black gold particles inside the endocytic vesicles. Vtg internalization studies have been analyzed in late stages oocytes. However, nothing was known until now about Vtg internalization at earlier stages. Analyses of radiolabeled Vtg transport in Xenopus laevis vitellogenic oocytes showed that the pathway involves internalization of Vtg via coated pits and coated vesicles, entry into endosomes and then appeared in multivesicular bodies (analogous of primordial yolk platelets) (Dumont, 1972; Wall and Patel, 1987). Villecco et al. (1999) reported that the transport of Vtg in B. arenarum proceeds from oolemma via coated vesicles and multivesicular endosomes to primordial yolk platelets and finally to fully grown yolk platelets. Our immunohistochemical and immunoelectron microscopy studies in primary vitellogenic oocytes provide evidence on the transitionally different electro-dense structures and small nascent platelets peripherally. We also found multivesicular bodies in primary vitellogenic oolema and, as described by Villecco (1999), these structures are the nascent yolk precursors that would become fused with each other to form primordial yolk platelets. At the final stages of oogenesis, the amphibian oocytes acquire an animal-vegetal polarity, showing pigment granules in the animal pole and the yolk platelets in the vegetal hemisphere (Danilchik and Gerhart, 1987). In fully grown oocytes, we observed immunoreactive stain in the perivitellin space suggesting that the yolk precursor had been incorporated. The same features were found in our ultrastructure studies. We observed gold particles between the perivitelline space and the microvilli of the fully-grown oocytes; no differences were found between oocytes hemispheres surfaces. Danilchik and Gerhart (1987) reported that X. laevis polarized yolk distribution cannot be attributed to regional differences in the uptake of Vtg. Instead, they determined that a directed intracellular translocation of platelets may account for the animal-vegetal distribution of yolk in the oocyte.
Vitellogenin is a sex-restricted yolk precursor protein in oviparous vertebrates and injection of oestradiol-17β into amphibian females enhances the synthesis of the yolk precursor in the liver. After B. arenarum female stimulation with estradiol, we were able to identify the yolk precursor only in stimulated liver extracts. Wiley and Wallace (1981) found three X. laevis Vtg forms of 197, 188 and 182 kDa. In Odontophrynus americanus, Winter et al. (1985) detected two Vtg forms of 207.5 kDa and 202.4 kDa. In our studies, we only detected one protein of ~190 kDa, corresponding to B. arenarum vitellogenin.
Summary
We describe here the presence of two LvH isoforms in Bufo arenarum mature oocytes. Using specific antibodies, we determined that Vtg uptake begins early during oogenesis, at the previtellogenic stage, and continues until oocytes have reached their mature status. In addition, we found that large endocytic vesicles mediate Vtg uptake in stage I oocytes, and that the size of the endocytic vesicles declines with oogenesis progression. Also in terms of Vtg protein trafficking, we detected Vtg precursor in the liver of estradiol-stimulated females but not in their oocytes, suggesting that proteolytic cleavage occurs as soon as Vtg is internalized. Finally, we propose a subclassification of B. arenarum stage-II oocytes in three physiologically and morphologically distinct periods (early, mid and late), based on specific characteristics observed in each one.
Acknowledgments
We wish to thank Alejandra Martinez and Dr. Beatriz Winik for their help with immunohistochemical and immunoelectron microscopy studies. This work was supported by grants from ANPCyT (PICT 15/31660) and CONICET (PIP-Marcelo Cabada and Silvia Arranz). Work by Dr. Salicioni was supported by NIH grants HD38082 and HD44044.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armas P, Cabada MO, Calcaterra NB. Primary structure and developmental expression of Bufo arenarum cellular nucleic acid-binding protein: changes in subcellular localization during early embryogenesis. Dev Growth Differ. 2001;43:13–23. doi: 10.1046/j.1440-169x.2001.00551.x. [DOI] [PubMed] [Google Scholar]
- Arranz SE, Albertali IE, Cabada MO. Bufo arenarum egg jelly coat: purification and characterization of two highly glycosylated proteins. Biochem J. 1997;323:307–312. doi: 10.1042/bj3230307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisone GA, Hedrick JL, Cabada MO. Vitelline envelope of Bufo arenarum: biochemical and biological characterization. Biol Reprod. 2002;66:1203–1209. doi: 10.1095/biolreprod66.4.1203. [DOI] [PubMed] [Google Scholar]
- Benbow RM, Ford CC. Cytoplasmic control of nuclear DNA synthesis during early development of Xenopus laevis: a cell-free assay. Proc Natl Acad Sci USA. 1975;72:2437–2441. doi: 10.1073/pnas.72.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabada MO, Sanchez Riera AN, Genta HD, Sanchez SS, Barisone GA. Vitelline envelope formation during oogenesis in Bufo arenarum. Biocell. 1996;20:77–86. [PubMed] [Google Scholar]
- Callen JC, Dennebouy N, Mounolou JC. Development of the mitochondrial mass and accumulation of mtDNA in previtellogenic stages of Xenopus laevis oocytes. J Cell Sci. 1980;41:307–320. doi: 10.1242/jcs.41.1.307. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilchik MV, Gerhart JC. Differentiation of the animal-vegetal axis in Xenopus laevis oocytes. I. Polarized intracellular translocation of platelets establishes the yolk gradient. Dev Biol. 1987;122:101–112. doi: 10.1016/0012-1606(87)90336-8. [DOI] [PubMed] [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972;136:153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Finn RN. Vertebrate yolk complexes and the functional implications of phosvitins and other subdomains in vitellogenins. Biol Reprod. 2007;76:926–935. doi: 10.1095/biolreprod.106.059766. [DOI] [PubMed] [Google Scholar]
- Frost DR, Grant T, Faivovich J, Bain R, Haas A, Haddad C, DeS R, Channing A, Wilkinson M, Donnellan S, Raxworthy C, Campbell J, Blotto B, Moler P, Drewes R, Nussbaum R, Lynch J, Green D, Wheeler W. The amphibian tree of life. Bull Am Museum Nat Hist. 2006;297:370–441. [Google Scholar]
- Gerber-Huber S, Nardelli D, Haefliger JA, Cooper DN, Givel F, Germond JE, Engel J, Green NM, Wahli W. Precursor-product relationship between vitellogenin and the yolk proteins as derived from the complete sequence of a Xenopus vitellogenin gene. Nucleic Acids Res. 1987;15:4737–4760. doi: 10.1093/nar/15.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilone SA, Arranz SE, Cabada MO. Detection of highly glycosylated proteins in polyacrylamide gels. Anal Biochem. 1998;261:224–227. doi: 10.1006/abio.1998.2747. [DOI] [PubMed] [Google Scholar]
- Kristoffersen BA, Nerland A, Nilsen F, Kolarevic J, Finn RN. Genomic and proteomic analyses reveal non-neofunctionalized vitellogenins in a basal clupeocephalan, the Atlantic herring, and point to the origin of maturational yolk proteolysis in marine teleosts. Mol Biol Evol. 2009;26:1029–1044. doi: 10.1093/molbev/msp014. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Molla A, Cartaud A, Lazaro R, Ozon R. Xenopus lipovitellin, a new target protein for calmodulin. FEBS Lett. 1983;154:101–104. doi: 10.1016/0014-5793(83)80883-7. [DOI] [PubMed] [Google Scholar]
- Opresko L, Wiley HS, Wallace RA. Differential postendocytotic compartmentation in Xenopus oocytes is mediated by a specifically bound ligand. Cell. 1980;22:47–57. doi: 10.1016/0092-8674(80)90153-1. [DOI] [PubMed] [Google Scholar]
- Opresko LK, Wiley HS. Receptor-mediated endocytosis in Xenopus oocytes. I. Characterization of the vitellogenin receptor system. J Biol Chem. 1987;262:4109–4115. [PubMed] [Google Scholar]
- Perazzolo LM, Coward K, Davail B, Normand E, Tyler CR, Pakdel F, Schneider WJ, Le MF. Expression and localization of messenger ribonucleic acid for the vitellogenin receptor in ovarian follicles throughout oogenesis in the rainbow trout, Oncorhynchus mykiss. Biol Reprod. 1999;60:1057–1068. doi: 10.1095/biolreprod60.5.1057. [DOI] [PubMed] [Google Scholar]
- Plaxton WC. Molecular and immunological characterization of plastid and cytosolic pyruvate kinase isozymes from castor-oil-plant endosperm and leaf. Eur J Biochem. 1989;181:443–451. doi: 10.1111/j.1432-1033.1989.tb14745.x. [DOI] [PubMed] [Google Scholar]
- Redshaw MR, Follett BK. The crystalline yolk-platelet proteins and their soluble plasma precursor in an amphibian, Xenopus laevis. Biochem J. 1971;124:759–766. doi: 10.1042/bj1240759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M, Rosanova P, Anteo C, Limatola E. Vertebrate yolk proteins: a review. Mol Reprod Dev. 2004;69:109–116. doi: 10.1002/mrd.20146. [DOI] [PubMed] [Google Scholar]
- Schmid M, Guttenbach M. Evolutionary diversity of reverse (R) fluorescent chromosome bands in vertebrates. Chromosoma. 1988;97:101–114. doi: 10.1007/BF00327367. [DOI] [PubMed] [Google Scholar]
- Stifani S, George R, Schneider WJ. Solubilization and characterization of the chicken oocyte vitellogenin receptor. Biochem J. 1988;250:467–475. doi: 10.1042/bj2500467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifani S, Le Menn F, Rodriguez JN, Schneider WJ. Regulation of oogenesis: the piscine receptor for vitellogenin. Biochim Biophys Acta. 1990;1045:271–279. doi: 10.1016/0005-2760(90)90130-p. [DOI] [PubMed] [Google Scholar]
- Tata JR, Smith DF. Vitellogenesis: a versatile model for hormonal regulation of gene expression. Recent Prog Horm Res. 1979;35:47–95. doi: 10.1016/b978-0-12-571135-7.50006-0. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez Toledo, C.L., Pisan¢, A., 1980. In: Vol. 4. pp. 315–330
- Valz-Gianinet JN, del Pino EJ, Cabada MO. Glycoproteins from Bufo arenarum vitelline envelope with fertility-impairing effect on homologous spermatozoa. Dev Biol. 1991;146:416–422. doi: 10.1016/0012-1606(91)90243-v. [DOI] [PubMed] [Google Scholar]
- Villecco EI, Aybar MJ, Sanchez Riera AN, Sanchez SS. Comparative study of vitellogenesis in the anuran amphibians Ceratophrys cranwelli (Leptodactilidae) and Bufo arenarum (Bufonidae) Zygote. 1999;7:11–19. doi: 10.1017/s0967199499000349. [DOI] [PubMed] [Google Scholar]
- Wahli W, Germond JE, ten Heggeler B, May FE. Vitellogenin genes A1 and B1 are linked in the Xenopus laevis genome. Proc Natl Acad Sci USA. 1982;79:68326836. doi: 10.1073/pnas.79.22.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall DA, Patel S. The intracellular fate of vitellogenin in Xenopus oocytes is determined by its extracellular concentration during endocytosis. J Biol Chem. 1987;262:14779–14789. [PubMed] [Google Scholar]
- Wallace RA. Studies on amphibian yolk. IX. Xenopus vitellogenin. Biochim Biophys Acta. 1970;215:176–183. doi: 10.1016/0304-4165(70)90400-9. [DOI] [PubMed] [Google Scholar]
- Wallace RA. Vitellogenesis and oocyte growth in nonmammalian vertebrates. Dev Biol (NY 1985) 1985;1:127–177. doi: 10.1007/978-1-4615-6814-8_3. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Dumont JN. The induced synthesis and transport of yolk proteins and their accumulation by the oocyte in Xenopus laevis. J Cell Physiol. 1968;72(Suppl-89) doi: 10.1002/jcp.1040720407. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Ho T. Protein incorporation by isolated amphibian oocytes. II. A survey of inhibitors. J Exp Zool. 1972;181:303–317. doi: 10.1002/jez.1401810303. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Hoch KL, Carnevali O. Placement of small lipovitellin subunits within the vitellogenin precursor in Xenopus laevis. J Mol Biol. 1990;213:407–409. doi: 10.1016/S0022-2836(05)80203-7. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Jared DW, Dumont JN, Sega MW. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973;184:321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Selman K. Oogenesis in Fundulus heteroclitus. II. The transition from vitellogenesis into maturation. Gen Comp Endocrinol. 1980;42:345–354. doi: 10.1016/0016-6480(80)90165-3. [DOI] [PubMed] [Google Scholar]
- Wang D, Kalb SR, Cotter RJ. Improved procedures for N-terminal sulfonation of peptides for matrix-assisted laser desorption/ionization post-source decay peptide sequencing. Rapid Commun Mass Spectrom. 2004;18:96–102. doi: 10.1002/rcm.1289. [DOI] [PubMed] [Google Scholar]
- Ward RT. The origin of protein and fatty yolk in Rana pipiens. IV. Secondary vesicular yolk formation in frog oocytes. Tissue Cell. 1978;10:525–534. doi: 10.1016/s0040-8166(16)30346-9. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Wallace RA. The structure of vitellogenin. Multiple vitellogenins in Xenopus laevis give rise to multiple forms of the yolk proteins. J Biol Chem. 1981;256:8626–8634. [PubMed] [Google Scholar]
- Winter CE, Floeter-Winter LM, Affonso MH, Ioshimoto LM, Becak W. Yolk proteins and their plasmatic precursor in the tetraploid Odontophrynus americanus (Amphibia, Anura) Comp Biochem Physiol B. 1985;82:515–524. doi: 10.1016/0305-0491(85)90016-1. [DOI] [PubMed] [Google Scholar]
- Yoshitome S, Nakamura H, Nakajo N, Okamoto K, Sugimoto I, Kohara H, Kitayama K, Igarashi K, Ito S, Sagata N, Hashimoto E. Mr 25 000 protein, a substrate for protein serine/threonine kinases, is identified as a part of Xenopus laevis vitellogenin B1. Dev Growth Differ. 2003;45:283–294. doi: 10.1046/j.1524-4725.2003.696.x. [DOI] [PubMed] [Google Scholar]


