Abstract
Addiction to alcohol and drugs is a major social and economic problem, and there is considerable interest in understanding the molecular mechanisms that promote addictive drives. A number of proteins have been identified that contribute to expression of addictive behaviors. NMDA receptors (NMDARs), a subclass of ionotropic glutamate receptors, have been of particular interest because their physiological properties make them an attractive candidate for gating induction of synaptic plasticity, a molecular change thought to mediate learning and memory. NMDARs are generally inactive at the hyperpolarized resting potentials of many neurons. However, given sufficient depolarization, NMDARs are activated and exhibit long-lasting currents with significant calcium permeability. Also, in addition to stimulating neurons by direct depolarization, NMDARs and their calcium signaling can allow strong and/or synchronized inputs to produce long-term changes in other molecules (such as AMPA-type glutamate receptors) which can last from days to years, binding internal and external stimuli in a long-term memory trace. Such memories could allow salient drug-related stimuli to exert strong control over future behaviors and thus promote addictive drives. Finally, NMDARs may themselves undergo plasticity, which can alter subsequent neuronal stimulation and/or the ability to induce plasticity. This review will address recent and past findings suggesting that NMDAR activity promotes drug- and alcohol-related behaviors, with a particular focus on GluN2B subunits as possible central regulators of many addictive behaviors, as well as newer studies examining the importance of non-canonical NMDAR subunits and endogenous NMDAR cofactors.
Addiction to drugs and alcohol extracts enormous physical, social and economic costs (Blincoe et al. 2002; Bouchery et al. 2011; CDC 2014; Sacks et al. 2013; SAMHSA 2015; WHO 2014). Clinical treatment of addictive drives is challenging, in part because of the high motivation for intoxicants, combined with the habitual drives that develop across long-term intake of abused substances. Thus, it is critically important to identify the brain regions and molecular signaling pathways that mediate motivation for intoxicants, since these could provide novel pharmacological and perhaps behavioral interventions to counteract the negative consequences associated with addictive behaviors.
NMDA receptors (NMDARs) have received considerable attention in relation to addictive behaviors (e.g. Burnett et al. 2015; Gass & Olive 2008; Landa et al. 2014; Tomek et al. 2013) and more generally for behavioral regulation and memory formation (Cull-Candy & Leszkiewicz 2004; Traynelis et al. 2010). Glutamate release is typically transient, leading to rapid activation of AMPA-type glutamate receptors (AMPARs) and depolarization of the neuron. In contrast, NMDARs are generally inactive at hyperpolarized resting membrane potentials due to block by magnesium ions on the inside of the receptors. As a consequence, NMDARs are only active after sufficient depolarization, which is often mediated by AMPARs. However, once active, NMDARs have long-lasting electrical currents and are permeable to calcium. Thus, NMDARs allow for prolonged neuronal stimulation under conditions where a cell is undergoing strong and/or synchronized activation, thus sustaining the impact of more potent stimulation.
Like many receptor families, there is some complexity related to different receptor subunits and other areas of regulation that can impact NMDAR function (for review, see Cull-Candy & Leszkiewicz 2004; Traynelis et al. 2010). NMDARs are heteromers composed of two GluN1 subunits, and two other subunits from among GluN2A–D and/or GluN3A–B. The functional properties of the resultant NMDARs can vary considerably in terms of decay time of the current, synaptic vs. extrasynaptic localization, trafficking, regulation by phosphorylation and other modifications, and calcium permeability. For example, while GluN1/2A and GluN1/2B NMDARs are both widely abundant across the brain, GluN1/2B NMDARs have slower decay time and greater localization to extrasynaptic sites. Recent studies have also underscored the importance of non-canonical NMDARs, containing GluN2C–D and/or GluN3A–B subunits, which are relatively resistant to the magnesium ion block and thus are active at hyperpolarized membrane potentials. This review will not attempt to address these many different factors, except as they pertain to NMDAR regulation of addictive behaviors.
More generally, NMDARs can modulate neuronal activity directly by acute depolarization of neurons, and also by regulating induction of synaptic plasticity. Since NMDARs are generally only active after a strong depolarization, this makes them an attractive mechanism to allow strong, synchronized stimulation to produce long-lasting functional changes. One such example is long-term potentiation (LTP) of AMPAR function, which occurs as a consequence of NMDAR-mediated calcium influx and activation of kinases and other signaling molecules (Park et al. 2013; Sweatt 2016). Such changes could mediate storage of memories that allow modification of future behavior based on previous experience. Importantly, addiction has been considered a maladaptive expression of memory, where drug-related stimuli produce powerful, enduring drug-related memories that allow drug-related cues to exert strong behavioral control and drive addiction. In this case, NMDARs might contribute to the development of future addictive drives but not acutely regulate behavior. However, NMDAR-mediated induction of synaptic plasticity can occur within minutes, e.g. where a rapidly developing NMDAR-dependent increase in AMPAR function promotes drug seeking (Gipson et al. 2013a, 2013b; Shen et al. 2011). Thus, NMDAR-mediated depolarization, induction of plasticity, or both may drive addictive behavior, and unless synaptic plasticity mechanisms are directly examined, the exact NMDAR mechanism would remain unclear.
Functional increases in NMDARs, which are observed after repeated exposure to many drugs or alcohol, could enhance NMDAR-mediated depolarization or plasticity induction. However, it can be challenging to definitively demonstrate whether an NMDAR adaptation is critical for driving a given behavior. There is often substantial NMDAR function in the control state, and the behavioral impact of an NMDAR blocker may reflect the NMDAR adaptation or just a requirement for NMDARs in general. In some cases, studies compare a related control behavior (e.g. self-administration of a natural reward) where the NMDAR blocker has no effect, suggesting that the NMDAR adaptation does not promote behavior more generally. However, this still may not be conclusive, since the control behavior may not require the brain region in which NMDAR plasticity is observed. Thus, it can be difficult to verify that NMDAR plasticity is essential for a given behavior, especially when there is strong NMDAR function in the control condition.
One important question is whether there are common patterns of NMDAR regulation across different intoxicants, e.g. through particular NMDAR subunits. NMDARs are likely critical for forming many types of intoxicant-related memories, perhaps through neuroadaptations in other signaling molecules, which would then drive future addictive behaviors. It is also likely that strong, sustained neuronal activity would lead to NMDAR activation, and allow NMDARs to directly promote expression of behavior. For example, continuous proximity to a drug-related cue could cause sustained activation of glutamatergic areas that process salient cues (such as the cortex and amygdala), which in turn would lead to prolonged NMDAR-mediated depolarization of downstream target regions that directly activate behavior, such as the nucleus accumbens (NAc). Finally, the contribution of NMDARs may be defined by the circuitry recruited during expression of a behavior. In particular, if a common circuit promotes addiction to different intoxicants, then NMDARs within a particular region (such as the NAc) may drive intake for all these intoxicants.
In addition, while a number of studies have examined the impact of NMDAR inhibitors that do not have clear subunit selectivity, many studies of addiction implicate GluN2B subunits. GluN2B subunits are interesting in that there can be a sizable population of extrasynaptic receptors, which can be rapidly trafficked into the synapse, and can produce differential regulation of plasticity induction relative to synaptic NMDARs (deBacker et al. 2015; Newpher & Ehlers 2009; Traynelis et al. 2010). There are also relatively selective antagonists for GluN2B subunits (ifenprodil, Ro 04–5595, deBacker et al. 2015, but see Trovero et al. 2016). The increasing evidence for a central role for GluN2B might, in part, reflect the presence of selective tools to interrogate GluN2B subunits, and subunits other than GluN2B may be assessed as more selective antagonists for other subunits are developed.
Given the vast literature, this review will not attempt to comprehensively address the many facets of NMDAR biology. Instead, we will address issues most relevant to NMDARs and addictive behaviors. We will examine the NMDAR contribution for each class of intoxicants, with a focus on particular brain regions (cortical, striatal and others). We will also address possible common and divergent roles for NMDARs, and identify where particular experiments could be valuable for enhancing our overall understanding of NMDAR regulation of addictive behavior. In addition, we seek to highlight studies which identify a central role for GluN2B subunits, as well as recent findings which underscore the importance of non-canonical NMDAR subunits and endogenous NMDAR cofactors for controlling addictive behaviors.
Brief synopsis of behavioral models of addiction and brain areas
Preclinical behavioral models of addiction
Before addressing the contribution of NMDARs to addiction, we must first understand the different behavioral paradigms used to model different aspects of intoxicant exposure, reward, intake and motivation. In general, there are two main types of addiction paradigms, involving passive vs. active drug exposure (for review, see Sanchis-Segura & Spanagel 2006; Koob & Volkow 2010; Weiss 2010; Belin-Rauscent et al. 2016; Everitt & Robbins 2016).
Passive, experimenter-administered drug exposure allows precise control over the timing and dose of intoxicant experience. There are two main paradigms, place conditioning and behavioral sensitization. During place conditioning, the potential rewarding or aversive properties of a compound can be assessed by pairing the compound with a particular context, while vehicle is paired with a second adjacent context. For rewarding substances, this results in conditioned place preference (CPP), where the individual spends more time in the reward-paired context in future sessions ( Tzschentke 2007). Conditioned place preference can be extinguished by repeated exposure to the context without drug reward, and then re-established (‘reinstated’) by drug exposure or a drug-paired cue. Behavioral sensitization is characterized by progressively increasing drug-induced locomotor activation which develops after repeated exposure to nearly every type of intoxicant (Robinson & Berridge 2001). Interestingly, sensitization to psychostimulants has been observed in humans (Landa et al. 2014). In addition, sensitization often persists long after the end of the drug exposure (Robinson & Berridge 2001), and has been associated with greater drug self-administration (de Vries et al. 1998; Piazza et al. 1990; Zapata et al. 2003) or CPP (Ahn et al. 2010; Bernier et al. 2011). Thus, sensitization may reflect a long-lasting enhancement of incentive salience of an intoxicant and associated cues, increasing the ability of these factors to drive intake and relapse after protracted abstinence. Similar to sensitization, CPP likely reflects enduring reward memories, except evoked by a specific drug-paired context. Also, many studies utilizing repeated passive administration may be studying a comparable state, even if behavioral effects are not measured.
Addiction in humans involves voluntary intake. Thus, animal models of drug self-administration have been developed where an animal makes operant responses (typically a lever press or nose poke) in order to gain access to a reward, which may be consumed orally (alcohol, sweet or fatty food) or delivered intravenously (cocaine, heroin, nicotine). Some studies compare the impact of brief (1–2 h) vs. extended (6–8 h) daily intake sessions, since extended access leads to escalation of intake and thus may model the escalation in humans that is indicative of development of addiction (Koob & Volkow 2010). Also, after operant self-administration has been established, animals can undergo repeated extinction sessions, where the operant response occurs but no reward is delivered, which eventually extinguishes responding. Relapse can then be modeled via reinstatement, where responding resumes after exposure to a drug-paired cue or context, a stressor, or by a small dose of the drug itself, all of which are strong contributors to relapse in humans (Epstein et al. 2006; Venniro et al. 2016). Thus, these preclinical models help to examine mechanisms driving voluntary intake and different aspects of relapse.
Since alcohol drinking occurs through oral intake in humans, bottle-based oral intake paradigms are often used in rodents. In many cases, this involves two-bottle choice, where animals have concurrent access to a bottle containing alcohol and a second bottle of water. In part, changes in water intake provide an internal control for possible non-specific effects on consumption. In addition, one or more weeks of abstinence from alcohol can lead to a dramatic enhancement of intake when access to alcohol is restored, which is known as the Alcohol Deprivation Effect, a model for relapse (Sanchis-Segura & Spanagel 2006). Finally, since alcohol is easily volatilized, a number of studies have used repeated, passive exposure to alcohol vapor, interspersed with withdrawal from alcohol (often termed chronic intermittent alcohol, CIE). Chronic intermittent alcohol allows for very high blood levels of alcohol, leading to dependence, withdrawal symptoms, and elevated alcohol drinking, and may model dependence in human alcoholics (Becker & Hale 1993; O’Dell et al. 2004; Radke et al. 2015a).
Compulsive aspects of addiction where voluntary intake persists despite negative physical, social and economic consequences, are a major obstacle to treatment in humans, (for review and complete discussion, see Hopf & Lesscher 2014). Thus, there is considerable interest in understanding the mechanisms that allow continued intake despite negative consequences. Several rodent paradigms have been developed to model compulsive aspects of human addiction, where self-administration persists even when paired with an aversive consequence. Intake despite footshock is considered a robust indicator of compulsion-like intake, and has been observed for both cocaine and alcohol (see Hopf & Lesscher 2014). Also, many studies utilize a quinine-resistant alcohol intake paradigm; a similar cortico-NAc circuitry mediates alcohol intake paired with shock or paired with the bitter taste of quinine (see below, Seif et al. 2013), validating the many studies utilizing the quinine-resistant model. Thus, these aversion-resistant intake models may provide valuable insights into the molecular and circuit mechanisms that promote continued intake in the face of negative consequences.
Finally, it is important to point out that addictive behavior can be driven by different underlying motivational phenomenon. For example, intoxicant intake during earlier sessions (across weeks of intake) may be driven by the acute rewarding, reinforcing impact of the intoxicant, which is generally called positive reinforcement. In contrast, after many sessions of exposure, self-administration may be driven by negative reinforcement, where intake relieves the negative affect or physical symptoms which accumulate across repeated exposure (see Koob & Volkow 2010). Thus, reduced expression of addictive behaviors by NMDAR blockers could reflect decreased addictive drives involving either positive or negative reinforcement. For example, in some cases, NMDAR inhibition can reduce both CPP and self-administration of a given intoxicant. However, since CPP can involve fewer exposure sessions than self-administration, the motivational mechanisms mediated by NMDARs may be different after a fewer or greater number of exposure sessions.
Brain areas implicated in addictive behaviors
Behaviors are generated through concerted activity within a series of brain circuits, and thus it is critical to understand how particular brain areas act to drive specific aspects of behavior. A complete description of brain areas implicated in addiction-related behaviors is beyond the scope of this review (see Gremel & Lovinger 2016; Koob & Volkow 2010; Saunders et al. 2015), but we briefly mention the most-studied regions. The NAc, the ventral portion of the striatum, promotes goal-directed and motivated behaviors, and the majority of studies we describe below address NMDAR adaptations in the NAc. Further, the NAc core subregion has been implicated more in regulation of behaviors by conditioned stimuli, while the NAc shell is implicated more in control of behavior by primary rewards, as well as inhibition of certain types of responding. Dorsal striatal (DStr) regions also play a central role in addiction, with the lateral DStr implicated in habitual behavior, and the medial DStr contributing to goal-directed behaviors where actions occur in relation to the outcomes they produce. Also, striatal regions have two largely distinct populations of neurons, the direct-pathway cells and indirect-pathway cells, which have been considered to promote and reduce behavioral activity, respectively.
These striatal regions often act in concert with cortical areas that regulate the execution of many types of behavior. The medial prefrontal cortex (mPFC) has received considerable attention for its role in decision making, error and conflict processing and goal-directed action, while the orbitofrontal cortex (OFC) is implicated in behavioral flexibility and encoding value of stimuli and actions. Finally, midbrain neurons from the ventral tegmental area (VTA) provide inputs to striatal, cortical, and other regions, and can promote addictive behaviors through diverse mechanisms including invigoration of behavior. These connections in part contain dopamine, but recent studies have underscored the importance of VTA neurons that release other neurotransmitters (Morales & Root 2014).
It is also important to note that the large number of studies within a particular region, such as the NAc, does not per se imply that the region is ‘more important’ than lesser-studied areas. Brain regions act within interconnected circuits (Kalivas & McFarland 2003), and adaptations and/or basal signaling in many other brain regions are likely critical for promoting addictive behaviors, whether they have been identified yet or not.
NMDAR contributions to psychostimulant addiction
As described below, NMDAR action within particular brain regions regulates a number of psychostimulant-related behaviors. In some cases, such as psychostimulant sensitization, systemic administration of NMDAR blockers has produced mixed results across the large literature (for review, see Bespalov et al. 2000a,2000b; Landa et al. 2014). However, systemic NMDAR inhibition can alter the acute locomotor response to cocaine (Carmack et al. 2013), which complicates the interpretation of NMDAR effects on sensitization. Thus, we focus primarily on studies of intracranial interrogation of NMDARs and psychostimulant-related addictive behaviors.
The NAc is critical for expression of many motivated behaviors, and NAc NMDARs, especially GluN2B subunits, are altered by repeated, passive psychostimulant exposure. Repeated methamphetamine reduces synaptic GluN2B in the NAc, and inhibiting NAc GluN2B with shRNA results in greater locomotion with acute Meth treatment and occludes the development of sensitization, suggesting that greater GluN2B mediates the expression of sensitization (Mao et al. 2009). However, cocaine sensitization has been associated with both decreased GluN2B in the NAc (Liu et al. 2016a,2016b) and increased GluN2B in the NAc shell subregion (Zhang et al. 2007), and drug treatments that reverse sensitization also undo the NAc shell GluN2B increase, again suggesting that greater GluN2B drives sensitized responses (Zhang et al. 2007). In addition, passive cocaine exposure has also been associated with a transient increase in GluN2B–enriched synapses in the NAc which are ‘silent’ because they lack AMPAR currents (Dong & Nestler 2014; Huang et al. 2015), and may represent an immature-like population of silent synapses which mature into AMPAR-containing synapses devoted to drug-related memories.
Genetic knockout (KO) studies have also implicated striatal NMDARs in behaviors related to passive psychostimulant exposure, especially within specific striatal cell populations. Cocaine CPP and sensitization are prevented by expression of mutant NMDARs that do not pass calcium within direct-pathway striatal neurons (Heusner & Palmiter 2005), in agreement with the importance of NMDAR-derived calcium for LTP formation. Also, KO of GluN1 in all striatal neurons prevents cocaine CPP (Agatsuma et al. 2010). However, GluN1 KO in only direct-pathway striatal cells does not prevent cocaine CPP, but does reduce cue-primed reinstatement of cocaine CPP (Joffe et al. 2016), while KO in indirect-pathway striatal cells has no effects on cocaine behaviors (Joffe et al. 2016) but can impair a number of other forms of learning (Lambot et al. 2016). The reason for these discrepant findings remains unclear, although one caveat of KO studies is the possibility of homeostatic changes after removing NMDARs throughout the animal’s lifetime. For example, KO of GluN1 in all striatal projection neurons could have different compensatory effects from KO only in a subset of projection neurons. Also, since these studies involve KO across the striatum, these findings may reflect NMDAR action within the NAc, dorsal striatum or both. However, repeated methamphetamine reduces synaptic GluN2B in DStr as well as NAc (Mao et al. 2009), suggesting possible importance of both NAc and DStr NMDARs.
Together, there is evidence that striatal NMDARs are required for forming different conditioned associations related to psychostimulant exposure, which would agree with the importance of NMDARs for memory formation in general. In addition, systemic NMDAR blockers alter the development of cocaine CPP (Carmack et al. 2013; Lin et al. 2011), but there are mixed results regarding NMDAR antagonists and expression of cocaine CPP (Lin et al. 2011; Maldonado et al. 2007). Divergent results may in part relate to differences across studies in the number of drug exposures, drug dose, and/or number of withdrawal days before testing (e.g. Turchan et al. 2003). Thus, studies systematically varying different dosing regimens and withdrawal durations would be very helpful. In fact, a robust relationship between the appearance of NMDAR adaptations and the expression of sensitization or CPP, and retention of NMDAR changes across protracted withdrawal, would support the importance of NMDAR adaptations as a causal mechanism.
In contrast to behaviors related to passive psychostimulant exposure, NAc NMDARs are considered unnecessary for the expression of voluntary psychostimulant intake. For example, systemic inhibition of GluN2B subunits does not reduce responding for cocaine (deBacker et al. 2015). Also, NAc NMDARs are not required for the expression of different forms of cocaine self-administration (Suto et al. 2009; Vanderschuren et al. 2005), although extrasynaptic NMDAR function in the NAc shell is altered (Ortinski et al. 2013). In contrast, NMDAR blockers in the NAc (Backstrom & Hyytia 2007) and systemically (Bespalov et al. 2000a,2000b) inhibit cue-induced reinstatement for cocaine. An NMDAR role during reinstatement but not self-administration could reflect the different brain circuits recruited, e.g. where cued reinstatement may be driven more by conditioned associations, while self-administration is also driven, at least in part, by primary reinforcing effects (Kalivas & McFarland 2003). There is also little information at present about NAc NMDAR changes related to voluntary psychostimulant intake, as seen in the cortex (below). Since NAc NMDAR do not seem to regulate self-administration, it would be interesting if NAc NMDAR adaptations only appear after protracted withdrawal, an ‘incubation’ effect that has been observed for AMPAR adaptations (Chen et al. 2008; Conrad et al. 2008).
When trying to understand whether similar or different mechanisms promote addiction-related behaviors involving passive vs. active exposure models, it is also important to note that passive and active exposure paradigms often involve different numbers of drug exposures and dose, which could lead to qualitatively different types of conditioned associations, as well as differential adaptations. However, studies where passive drug dose and timing is carefully matched for active exposure still observe divergent neuro-adaptive changes (Chen et al. 2008). Nonetheless, it is interesting that extant studies implicate NAc/striatal NMDARs in regulation of sensitization, CPP and reinstatement, all of which may be more dependent on conditioned associations relative to self-administration. Thus, the brain circuitry recruited for the expression of a given behavior may be particularly important in determining whether striatal NMDARs promote that behavior, in addition to any NMDAR adaptations that may occur.
Cortical regions are critical for expression of many forms of behaviors, and cortical NMDAR adaptations are likely to profoundly influence the expression of addiction. In this regard, repeated methamphetamine exposure decreases GluN2B levels in the mPFC (Lominac et al. 2016) (see also Gonzalez et al. 2016). Furthermore, both extended and brief cocaine self-administration decrease mPFC GluN1 and GluN2B levels and tyrosine phosphorylation of GluN2B, which is required for surface expression of GluN2Bs (McGinty et al. 2015). Protracted extinction after cocaine self-administration is also associated with decreased cortical GluN1 levels (Crespo et al. 2002). However, another study found elevated mPFC GluN2B levels after 2 weeks withdrawal from extended but not brief daily cocaine access (Ben-Shahar et al. 2009). Also, both decreased (Crespo et al. 2002; McGinty et al. 2015) and increased (Ben-Shahar et al. 2007) mPFC NMDARs have been observed just after self-administration.
Although there are some mixed results, one comprehensive interpretation is that self-administration leads to long-lasting decreases in cortical NMDARs, including GluN2B, although excessive intake can increase NMDAR function under some conditions. Decreased NMDAR function could contribute to the cortical hypofunction that can promote cocaine seeking, likely by decreasing cortical control over behavioral inhibition and regulation (Chen et al. 2013; McGinty et al. 2015; Murphy et al. 2012). However, systemic GluN2B inhibition does not reduce lever-pressing for cocaine (deBacker et al. 2015), which might not be predicted if reducing cortical NMDAR function promotes self-administration. One possibility is that more moderate GluN2B changes (after drug intake) could have different effects relative to more complete GluN2B inhibition (by pharmacological blockers). Alternately, inhibiting GluN2B within multiple brain regions could produce offsetting or opposing effects (see, e.g. ‘NMDAR Contributions to Food Addiction’ below). Thus, it would be valuable to examine mPFC NMDAR adaptations and the behavioral impact of lower doses of NMDAR antagonists at different time points across self-administration, extinction and reinstatement. In fact, moderate GluN2B inhibition in the mPFC might only enhance intake at times when reductions in GluN2B are not yet observed (perhaps early in self-administration training). Also, while OFC NMDAR adaptations are observed with alcohol exposure (see below), the importance of OFC NMDARs for psychostimulant-related behaviors remains largely unknown.
Given the behavioral importance of VTA neurons, including dopaminergic projections, it is also interesting that NMDAR inhibition within the VTA suppresses both acquisition and expression of cocaine CPP (Zhou et al. 2012). This concurs with studies using KO of NMDARs within midbrain dopamine cells (with KO in both VTA and nearby substantia nigra), which overall observe impaired cocaine CPP and sensitization (although with some mixed results, Engblom et al. 2008; Zweifel et al. 2008). These studies are consistent with the importance of VTA dopamine and NMDARs for learning, and also suggest that VTA NMDAR activity is acutely required for expression of CPP. In addition, repeated methamphetamine increases NMDAR function within VTA neurons, facilitating LTP induction, which is related to magnitude of the methamphetamine-induced CPP (see below in ‘Interaction of NMDARs with other receptor types’). Thus, VTA NMDARs can promote expression of some psychostimulant-related addictive behaviors, and future studies using subunit-specific shRNAs and antagonists can address the importance of particular NMDAR subunits.
Recent studies have identified cocaine-related NMDAR changes in the bed nucleus of the stria terminalis (BNST), a subcortical region which plays a critical role in anxiety and other emotion-related behaviors. Cocaine self-administration increases the decay time of NMDAR currents in BNST, suggesting an increase in GluN2B subunits (deBacker et al. 2015), and also impairs NMDAR-dependent LTD induction in the BNST. Interestingly, impaired LTD induction could be reversed by blocking GluN2B function, indicating that greater GluN2B function can actually impair some forms of synaptic plasticity. However, systemic inhibition of GluN2B subunits did not reduce responding for cocaine (deBacker et al. 2015), suggesting that BNST GluN2B adaptations do not regulate ongoing self-administration. An interesting alternate possibility for this study, and other studies where adaptations are observed but do not seem to have behavioral consequences, is that GluN2B regulate the re-forming of drug-related memories during intake. In this case, GluN2B inhibition would impact intake on future sessions rather than the current session (see Keiflin et al. 2013). This also underscores the importance of not taking a too simplistic perspective when interpreting NMDAR adaptations. Adaptations certainly can occur which have little behavioral impact. However, our animal behavioral models also tend to focus on specific aspects of the broad behavioral repertoire. Thus, assessment of different aspects of addictive behaviors may uncover a more selective contribution of a given adaptation that is not apparent in simple measures of intake.
Finally, it is notable that preclinical studies find limited evidence for NMDARs regulation of cocaine self-administration. In this regard, the NMDAR inhibitor memantine, which is FDA approved for Alzheimer’s disease, is of great interest as a possible NMDAR intervention for other human conditions including addiction. However, studies from human addicts (Bisaga et al. 2010; Collins et al. 1998) and primates ( Newman & Beardsley 2006) show little impact of memantine on cocaine intake. Nonetheless, given that preclinical studies implicating NMDARs in CPP and reinstatement, it would be interesting if NMDAR blockers did reduce other addiction-related phenomenon in humans, such as human variants of place conditioning (e.g. Childs & de Wit 2009) and relapse. This would give greater validity to preclinical findings, especially that NMDAR inhibitors are effective against more specific aspects of addiction (e.g. relapse).
NMDAR contributions to alcohol addiction
A substantial literature has examined the importance of NMDARs for alcohol-related physiology and behavior. As described below, alcohol has a more unique relationship to NMDARs, since it is the only widely used intoxicant that directly inhibits NMDARs. Also, alcohol increases NMDAR function across many brain areas. In addition, NMDARs can directly promote several types of alcohol intake and relapse. Thus, although alcohol is known to interact with a number of cellular targets (see other reviews within this volume), NMDARs play a prominent role in regulating alcohol behaviors, and vice versa.
Direct inhibitory effect of alcohol on NMDARs
Unlike many other intoxicants, alcohol has direct, inhibitory effects on NMDARs (Ron & Wang 2009), although phencycline (PCP) and ketamine are NMDAR blockers that are also abused by humans. Alcohol inhibition of NMDARs was first identified in cultured neurons (Lovinger et al. 1990), and has since been observed in many brain regions, and can be mediated through GluN2B subunits (Wang et al. 2007; Wills et al. 2012). Importantly, alcohol inhibition of NMDARs is thought to contribute to the rewarding or intoxicating effects of alcohol (Hodge & Cox 1998). For example, animals can learn to press a lever when the interoceptive impact of a given compound is similar to the interoceptive effect of alcohol, indicating that they ‘feel’ the same (i.e. they have similar discriminative properties). Systemic NMDAR blockers substitute for the discriminative stimulus properties of alcohol (Grant & Colombo 1993) but not cocaine (Sanger et al 1992). Furthermore, infusion of noncompetitive NMDAR inhibitors such as MK-801 within the NAc substitutes for alcohol, suggesting that NAc inhibition in this manner ‘feels’ similar to alcohol, while the competitive NMDAR antagonist (RS)-CPP in the NAc does not (Hodge & Cox 1998, but see Kostowski & Bieńkowski 1999). It is also noteworthy that humans report alcohol-like effects of NMDAR blockers such as ketamine and memantine (Dickerson et al. 2010; Krupitsky et al. 2007; Krystal et al. 1998, 2006). Thus, NMDAR inhibition by alcohol may contribute to the rewarding and/or intoxicating effects of alcohol.
The ability of NMDAR inhibition within the NAc to mimic alcohol’s interoceptive effects is interesting, since animals will self-administer NMDAR blockers within the NAc, suggesting that this inhibition may be directly rewarding. In particular, animals will self-administer several different types of NMDAR blockers [MK-801, (RS)-CPP, PCP] directly into the NAc shell, although not into the NAc core (Carlezon & Wise 1996a). In agreement with a direct rewarding effect, the same NMDAR inhibitors within the NAc shell also enhance the reward elicited by medial forebrain bundle stimulation (Carlezon & Wise 1996b). Also, animals will self-administer these same NMDAR blockers directly into the mPFC (Carlezon & Wise 1996a), suggesting that cortical NMDAR inhibition may also be rewarding. However, these effects are quite different from NMDAR inhibition and alcohol. Alcohol’s discriminative stimulus effects occur within the NAc core but not the mPFC, and only a subclass of NMDAR blockers within the NAc substitute for systemic alcohol (Hodge & Cox 1998). Also, NMDAR inhibition by alcohol is more moderate (<50% reduction of currents observed ex vivo) relative to pharmacological blockers. Thus, strong NMDAR inhibition within the NAc shell and cortex can be rewarding, but a different mechanism, within the NAc core, allows alcohol to inhibit NMDARs and produce rewarding and/or intoxicating effects.
Alcohol inhibition of NMDARs can complicate the interpretation of experiments examining NMDAR blockers and alcohol drinking. NMDAR antagonists could suppress alcohol-related behavior simply because NMDAR activity is required to drive intake. Alternately, if an NMDAR inhibitor mimics the intoxicating effect of alcohol, this could act as a satiety signal, indicating that an individual has already consumed alcohol, which could reduce the drive to drink more. For example, memantine decreases alcohol intake but not responding for alcohol under a progressive ratio, a measure of motivation (Alaux-Cantin et al. 2015). This has been interpreted as memantine substituting for interoceptive effects of alcohol, rather than reducing motivation for alcohol (see also Holter et al. 2000). However, it is also clear that competitive NMDAR antagonists such as AP-5 can decrease alcohol consumption under particular paradigms (see below), and AP-5 within the NAc also inhibits expression of alcohol CPP (Gremel & Cunningham 2009). Thus, NMDARs may directly promote alcohol reward and drinking behaviors, separate from or in addition to possible interoceptive and/or rewarding effects of NMDAR inhibition by alcohol itself. These multiple and perhaps opposite effects of NMDARs on alcohol behaviors may have very different implications relative to other abused drugs (addressed in the following sections).However, it may also allow NMDAR inhibitors to have greater clinical efficacy for decreasing excessive alcohol intake in humans.
Alcohol-related increases in NMDARs across many brain regions
A number of studies of alcohol exposure and intake have converged on the idea that NMDAR function increases across repeated exposure. This has been thought to occur as a compensation for direct alcohol inhibition of NMDARs, and has been observed in many brain regions. Greater NMDAR function can have a number of functional implications, including elevated neuronal activity and withdrawal symptoms (reviewed in Stuber et al. 2010), and perhaps greater consumption. Here, we will primarily examine recent studies of alcohol-related NMDAR changes in particular brain regions, especially GluN2B–related adaptations, and their impact on induction of synaptic plasticity.
Original studies in cultured hippocampal and cortical neurons and brain slices found that chronic alcohol exposure upregulates NMDAR function and synaptic targeting of GluN1 and GluN2B but not GluN2A subunits; also, no changes were observed in AMPAR currents, suggesting a receptor-specific effect (Carpenter-Hyland et al. 2004; Qiang et al. 2007). These simpler models have allowed the discovery of important aspects of alcohol exposure, especially that intermittent alcohol exposure, with intervening periods of withdrawal, can have greater effects than continuous alcohol exposure, such as larger and more persistent changes in GluN2B (Sheela Rani & Ticku 2006). Since then, alcohol exposure in the intact animal has been shown to increase NMDAR function in a number of brain areas, often with changes in GluN2Bs when subunits are examined (for review, see Wills et al. 2015).
In the NAc, repeated injection of alcohol increases GluN2B (McGuier et al. 2015). In addition, shorter term CIE (repeated alcohol vapor, see above) increases NMDAR currents and facilitates LTP induction in direct-pathway NAc cells, while CIE decreases NMDAR function and facilitates LTD induction in indirect-pathway NAc cells (Renteria et al. 2016). Thus, increased GluN2B in NAc direct-pathway cells may promote AMPAR plasticity and activity of these neurons, which could increase alcohol intake (cf. Beckley et al. 2016).
Alcohol also alters NMDAR function in several cortical regions. In the medial OFC, CIE increases NMDAR currents and mRNA levels for GluN1 and GluN2A but not GluN2B in the medial OFC, with no changes in NMDAR mRNA in vmPFC or DStr (Radke et al. 2015a). These medial OFC adaptations are accompanied by increased willingness to drink alcohol when paired with shock, considered a measure of compulsion-like intake, and this is specific to alcohol since CIE does not change punished food-seeking. In the lateral OFC, CIE decreases GluN2B levels, although plasticity induction is enhanced (Nimitvilai et al. 2016). These findings are in interesting contrast to those described below in ‘NMDAR Contributions to Food Addiction,’ where GluN2B inhibition in the OFC impairs shifting between alternate choices (Brigman et al. 2013), and genetic KO of cortical GluN2Bs promotes compulsion-like food intake (Radke et al. 2015b). Thus, both reduced and enhanced GluN2B seem to be able to impair adaptive choice. This could reflect the different rewards, alcohol vs. food. However, other aspects of cortical function and behavior are known to exhibit a U-shaped response in relation to cortical activity, with optimal function at intermediate activity levels (Cools & D’Esposito 2011).
In the mPFC, GluN1, GluN2B and GluN2A protein levels are enhanced by continuous, chronic alcohol exposure, which returns to control within 2 days of withdrawal (Kalluri et al. 1998). In addition, CIE exposure and 1 week withdrawal increases NMDAR function in the mPFC and impairs cognitive flexibility, an mPFC-dependent task (Kroener et al. 2012). This is an example of how intermittent alcohol exposure can have more protracted effects than continuous exposure. However, greater NMDAR function in the mPFC with CIE is associated with greater GluN1 and GluN2B levels just after a drinking session, but protein levels are normal after 1 week withdrawal even though synaptic NMDAR currents are greater. While this seems paradoxical, it may instead be an example of the relative specificity of electrophysiology and western blot methods. In particular, electrophysiological recordings allow great precision in determining functional changes within a particular cell (in this case, layer V), while western blot measures changes across a larger tissue area with diverse cell types and may miss molecular changes present in a specific cells. Nonetheless, these studies do agree overall that cortical regions consistently demonstrate strong NMDAR adaptations after alcohol exposure.
In addition to alcohol-related changes in NMDARs in striatal and cortical regions, CIE increases BNST LTP via extrasynaptic GluN2Bs (Wills et al. 2012). Chronic intermittent alcohol also produces a number of changes in GluN2B–associated binding partners in the hippocampus for both synaptic and non-synaptic populations of GluN2Bs (Wills et al. 2015). Thus, GluN2B adaptations with alcohol seem to occur widely across the brain.
Overall, these studies illustrate how repeated, passive alcohol exposure can enhance NMDAR function, especially GluN2B, across many brain regions, and that NMDAR changes have functional implications for induction of synaptic plasticity. It is likely that at least some of these NMDAR changes will alter expression of alcohol-related behavior(see below). However, one significant limitation of these studies is that alcohol exposure is passive, given that active and passive exposure produces very different adaptations for other drugs (e.g. Chen et al. 2008). Thus, it is critical that future work identify NMDAR adaptations associated with voluntary alcohol drinking (e.g. Seif et al. 2013, 2015).
Finally, human studies have also demonstrated that the alcohol-like acute effects of NMDAR blockers are reduced in alcohol-dependent individuals, which has been interpreted as evidence that people with AUDs have greater NMDAR function (Krystal et al. 2011). While this would be consistent with preclinical findings, reduced sensitivity to alcohol-like effects of NMDAR blockers may precede the development of AUDs, since non-alcoholic relatives of individuals with AUDs also show reduced responses to NMDAR inhibition (Petrakis et al. 2004). However, it may be difficult to rule out that relatives of people with AUDs have greater risk of developing NMDAR changes even with moderate alcohol consumption. Nonetheless, these human studies support the observation in animals that repeated alcohol exposure elevates NMDAR levels.
NMDARs and addiction-related alcohol behaviors
Increased NMDAR levels after repeated alcohol have the potential to strongly impact the expression of addictive behaviors. Although acquisition of alcohol CPP does not require GluN2Bs (Boyce-Rustay & Cunningham 2004), NMDAR inhibition in the NAc prevents expression of alcohol CPP (Gremel & Cunningham 2009). NMDARs also regulate expression of alcohol sensitization. Greater alcohol sensitization is associated with reduced mRNA levels for GluN1 and GluN2A in NAc and GluN2B in hippocampus, but higher GluN2A mRNA levels in BNST (Nona et al. 2014). In addition, individual mice with greater alcohol sensitization exhibit greater voluntary alcohol drinking; in parallel the NAc core of sensitized mice show reduced NMDAR function, NMDAR-dependent LTD and GluN1 and GluN2A protein levels, although greater GluN2B, after several weeks withdrawal (Abrahao et al. 2013). Reduced NMDAR levels in NAc core and mPFC in sensitized mice are also found after one day withdrawal (Quadros et al. 2002). Thus, overall greater NMDAR function within the NAc, and perhaps other brain areas, seems to suppress sensitization for alcohol and protect against increased alcohol drinking (Abrahao et al. 2013). In agreement, passive drug exposure increases NAc CREB (cAMP response element-binding protein) and NMDAR synaptic function, which decreases cocaine reward (Huang et al. 2008) and would be protective inasmuch as reward promotes development of addictive behaviors. However, there are some differences in early and later withdrawal from alcohol. In early withdrawal, sensitized mice show similar NMDAR levels as controls, while non-sensitized show elevated NMDARs, consistent with a protective function (Quadros et al. 2002). After several weeks of withdrawal, non-sensitized mice show similar NMDAR function as controls, while sensitized mice have reduced NMDARs (Abrahao et al. 2013). Thus, repeated alcohol exposure may cause opposing adaptations, an increase in NMDARs that decreases across withdrawal and is protective, and also a decrease in NMDAR levels only in sensitized animals. Nonetheless, these studies support the idea that alcohol exposure can strongly regulate NMDAR function, and that these NMDAR adaptations impact the expression of addiction-related behaviors.
In contrast to studies of passive alcohol exposure, several lines of evidence suggest that NMDARs promote voluntary alcohol drinking. Systemic NMDAR blockers decrease alcohol drinking under some paradigms (see Sabino et al. 2013; Vengeliene et al. 2005). Also, high doses of the NMDAR blocker AP-5 within the NAc inhibit operant alcohol intake in rats (Rassnick et al. 1992), although memantine in the NAc does not reduce operant intake in mice (Eisenhardt et al. 2015). Also, lower but still effective AP-5 doses do not reduce two-bottle choice alcohol drinking in rats (Seif et al. 2013), nor does memantine or NMDAR inhibition in direct pathway cells within the NAc in mice (Eisenhardt et al. 2015). In contrast, NMDAR inhibition within the NAc does strongly inhibit compulsion-like alcohol consumption that is driven by NAc adaptations in non-canonical NMDARs (Seif et al. 2013, 2015; see below). Furthermore, NAc NMDARs do not regulate cue-induced alcohol reinstatement in rats (Backstrom & Hyytia 2004) or mice (Eisenhardt et al. 2015). In contrast, pharmacological NMDAR inhibition or genetic NMDAR knockdown in direct pathway cells within the NAc strongly reduces the Alcohol Deprivation Effect, a model of relapse where consumption is greatly enhanced by protracted abstinence (Eisenhardt et al. 2015). Overall, these studiessuggest that NAc NMDARs are not required for simpler forms of intake, similar to what is observed for cocaine (see above), but are critical for states of higher motivation, including compulsion-like drinking and the Alcohol Deprivation Effect. What remains unclear is the lack of role for cue-primed reinstatement, since NMDARs in the NAc promote reinstatement for cocaine and heroin. Additional studies comparing NMDAR inhibition for different types of reinstatement for alcohol would be helpful.
In addition the NAc NMDARs, excessive, voluntary alcohol drinking is driven by GluN2B adaptations within the medial DStr. Repeated intake increases GluN2B levels and phosphorylation and AMPAR LTP within the medial DStr, which ultimately promotes alcohol intake (Wang et al. 2007, 2010, 2012). Interestingly, no GluN2B changes are observed in the NAc, and inhibition of GluN2Bs within NAc has no effect on excessive drinking (Seif et al. 2013; Wang et al. 2007). Thus, voluntary alcohol drinking is driven in part by subregion-specific striatal adaptations in different NMDAR subunits (see also Acosta et al. 2010). It is also notable that passive alcohol exposure does increase NAc GluN2B levels (McGuier et al. 2015), another example of how passive and active exposure can produce very different adaptations.
Finally, although there are limited studies, inhibition of NMDARs within the VTA decreases both the expression of alcohol CPP (Pina & Cunningham 2016) and the Alcohol Deprivation Effect (Eisenhardt et al. 2015). In contrast, neither operant nor two-bottle choice alcohol self-administration are altered (Eisenhardt et al. 2015).
Taken together, the extant preclinical studies indicate that NMDARs in several mesolimbic regions play a critical role in promoting voluntary alcohol consumption. These findings strongly support the use of NMDAR antagonists to address human AUDs, although there may be limitations, as described below in ‘Non-Canonical NMDARs and Cocaine and Alcohol Addiction.’
NMDAR contributions to opiate addiction
Studies of opiate-related addictive behaviors also implicate GluN2B subunits, especially in the NAc. Morphine CPP increased NAc GluN2B levels (Ma et al. 2006) and GluN1 mRNA (Jacobs et al. 2005; see also Turchan et al. 2003), and morphine-induced sensitization increases NAc GluN2B levels and phosphorylation, and NMDAR currents (Liu et al. 2014). However, GluN2B knockdown with siRNA in the NAc suppresses morphine CPP but not sensitization (Kao et al. 2011). Since these siRNAs would impact both development and expression of sensitization, it is notable that systemic GluN2B or NMDAR inhibition prevents the development of morphine sensitization (Aguilar et al. 2009; Liu et al. 2014). In addition, intracerebroventricular (i.c.v.) infusion of GluN2B inhibitors prevents both acquisition and expression of morphine CPP (Ma et al. 2006), and intra-NAc shell GluN2B inhibition suppresses reinstatement of morphine CPP (Ma et al. 2007). Also, repeated morphine increases NAc GluN1 surface levels and phosphorylation which lasts for months of withdrawal (Anderson et al. 2015), suggesting that NMDAR changes are long-lasting. Finally, since systemic and i.c.v. administration will impact all brain regions, we note that GluN2Bs in other brain areas can regulate opiate-related behaviors, e.g. where GluN2B inhibition within the hippocampus suppresses reinstatement of morphine CPP (Ma et al. 2007). Taken together, it is clear that GluN2B adaptations in NAc mediate expression of morphine CPP, while the role of NMDARs in the expression of opiate sensitization remains to be tested directly.
Interestingly, studies of voluntary intake have also demonstrated critical contributions of GluN2Bs. In particular, heroin exposure induces reinstatement of heroin seeking, and also leads to a rapid increase in surface expression of NAc GluN2B subunits and to LTP of AMPAR function in cortico-accumbens synapses (Shen et al. 2011). Importantly, blocking GluN2B within the NAc prevents both AMPAR LTP and the ability of heroin exposure to cause reinstatement. Thus, increased function of NAc GluN2Bs seem central for driving this form of relapse for opiates, in addition to promoting the expression of passively conditioned reward for morphine.
Given the importance of NMDARs in other brain regions, such as cortex and BNST, for psychostimulant and alcohol behaviors, future work should examine whether NMDARs in such regions also promote opiate addiction. For example, morphine CPP increases GluN2B protein levels in the hippocampus (Ma et al. 2006). In addition, NMDAR inhibition within the mPFC increases acute opiate reward via a VTA/dopamine mechanism (Bishop et al. 2011; Tan et al. 2014), and low dose memantine decreases the methadone dose required during maintenance treatment in opiate addicts (Lee et al. 2015). Thus, cortical NMDARs seem to regulate primary opiate reward, which would likely have a profound and perhaps clinically relevant impact on opiate addiction.
Finally, preclinical studies of opiate-related behaviors seem to be very encouraging for the possibility that NMDAR inhibition could be therapeutically useful in human drinkers. However, memantine has shown limited effectiveness in treating opiate dependence in humans, except for counteracting physical withdrawal symptoms (Bisaga et al. 2001, 2011; Jain et al. 2011). While this is discouraging, it may be that, as discussed above for cocaine, NMDAR inhibitors may be efficacious against particular aspects of addiction (such as withdrawal and relapse) rather than as a general treatment for intake per se.
NMDAR contributions to nicotine addiction
Nicotine studies also suggest an important role for NMDARs. NMDAR changes after passive nicotine exposure have shown varied results that seem to reflect differences in nicotine exposure. Nicotine sensitization, with 14 days exposure but no withdrawal, increases NMDAR currents in NAc core and mPFC but not NAc shell, with no changes in GluN2B (Ávila-Ruiz et al. 2014). In contrast, briefer nicotine exposure (7 days) increases GluN2B phosphorylation in both NAc and striatum (Nakajima et al. 2012). Also, GluN2B but not GluN2A levels are decreased across withdrawal in the striatum (Pistillo et al. 2016). In contrast, across withdrawal, GluN1 and GluN2A but not GluN2B levels increase in the VTA, while GluN2A but not GluN2B levels are decreased in the mPFC. Thus, there are no actual discrepancies among these studies, and they all support the ability of nicotine exposure to produce NMDAR adaptations.
Like other intoxicants, NAc NMDARs are implicated in aspects of voluntary intake of nicotine. NMDAR inhibition within the NAc shell (and lateral septum), but not the NAc core, increases nicotine self-administration (D’Souza & Markou 2014), which is not due to alpha7-nAChR–NMDAR direct interactions (see below). Increased intake with NMDAR inhibition seems to suggest that NAc NMDARs actually suppress intake, which might occur via NAc connections to other brain regions (D’Souza & Markou 2014). An alternate possibility is that this dose of the NMDAR inhibitor produces moderate inhibition. This could reduce the reward value of nicotine and enhance self-administration in response to the decreased reward, which has been observed for nicotine in primates (Kohut & Bergman 2016). However, nicotine-NMDAR interactions in the NAc are also complicated by the presence of nAChR-regulated NMDARs on presynaptic dopamine and glutamate terminals (Salamone et al. 2014; Zappettini et al. 2014). Thus, the interaction of nicotine and NMDARs within the NAc are likely to be complex.
Studies of cued reinstatement for nicotine have also implicated NAc NMDARs. Withdrawal from nicotine increases GluN2A and GluN2B levels within the NAc, and cued reinstatement increases AMPAR and NMDAR currents, and also slows NMDAR current decay which may indicate greater GluN2B levels (Gipson et al. 2013b). Importantly, inhibition of either GluN2A or GluN2Bs within the NAc reduces cued reinstatement for nicotine (Gipson et al. 2013b). In addition, i.c.v. disruption of alpha7-nAChR-NMDAR interactions reduces reinstatement of nicotine seeking (see below). However, another study found that cued reinstatement for nicotine is actually enhanced by NMDAR inhibition within the NAc core but not shell (D’Souza & Markou 2014), similar to the increased nicotine intake noted above. Thus, although there are some divergent findings, all studies agree that NAc NMDARs are potent regulators of cued reinstatement for nicotine.
Finally, NMDARs have been tested in human smokers. The intoxicating effects of smoking are reduced by memantine (Jackson et al. 2009) and the partial agonist D-cycloserine (Nesic et al. 2011). However, another study found that memantine did not influence the acute effects of nicotine and also did not reduce smoking (Thuerauf et al. 2007). An NMDAR glycine site antagonist also had no effect on craving or smoking levels (Evins et al. 2011). Thus, like cocaine and heroin, human studies of NMDAR inhibition have produced disappointing results, which is in strong contrast to the promising preclinical findings. It may that the intravenous method for nicotine administration in animals does not match the experience of inhaled nicotine in humans. Alternately, systemic D-cycloserine only reduces intake in animals with lower levels of self-administration (Levin et al. 2011). Thus, NMDAR blockers may be efficacious only in a subset of human smokers.
NMDAR contributions to food addiction
GluN2B subunits within the cortex may also play a particular role in addiction-like food intake. After genetic KO of GluN2B only in cortical glutamatergic neurons, mice continue responding for food even when paired with footshock (Radke et al. 2015b). This suggests that cortical GluN2Bs normally suppress compulsion-like responding for food. Removing GluN2Bs from cortical principal neurons did not alter progressive ratio or extinction responding, also considered measures of compulsion-like responding (see Hopf & Lesscher 2014), thus indicating a specific role for cortical GluN2Bs in punishment-resistant aspects of motivation for food. Also, GluN2B KO in cortical interneurons or striatal neurons has no effects on any of these addiction-related behaviors. In addition, GluN2B inhibition in the OFC (and perhaps other cortical areas) impairs shifting between alternate choices (Brigman et al. 2013). Thus, GluN2Bs in glutamatergic cortical neurons may facilitate adaptive behavioral choices and cognitive flexibility, including switching away from a preferred reward when it is paired with punishment, which is consistent with an overall role for cortical regions in behavioral control.
NMDARs also regulate pathological drives for food through regions other than cortex. NMDAR inhibitors within the NAc shell but not core reduce binge eating of a highly palatable food, but not lab chow intake, and also suppress responding in the presence of an aversive bright light, another compulsion-like model (Smith et al. 2015). However, another study found that inhibiting NMDARs within the NAc core or shell reduced operant responding for food pellets (D’Souza & Markou 2014). Nonetheless, these studies together suggest that NMDARs in different regions may have different impacts on pathological food intake, being protective in cortical areas but driving maladaptive responding in the NAc. These findings also partially concur with studies (described below) that cortico-accumbens inputs and NAc NMDARs support compulsion-like responding for alcohol (Seif et al. 2013, 2015).
Non-canonical NMDARs and cocaine and alcohol addiction
One interesting area of modern addiction research has focused on non-canonical NMDARs containing GluN2C/D and/or GluN3 subunits, which are unusual due to greater activity at hyperpolarized membrane potentials relative to other NMDAR subtypes (Cull-Candy & Leszkiewicz 2004; Traynelis et al. 2010). Thus, these non-canonical NMDARs can provide a sustained depolarizing influence even at hyperpolarized potentials, and dramatically enhance neuronal firing (Seif et al. 2013; Zhang et al. 2009). Some non-canonical NMDARs also have reduced currents and calcium permeability, and, in the most extreme case, GluN1/3 receptors lacking GluN2 subunits are not activated by glutamate (Smothers & Woodward 2007). Although these receptors have received relatively little attention, recent studies have uncovered the potential critical role that these unusual NMDAR subunits can play in cocaine sensitization and compulsion-like alcohol consumption.
It has long been observed that a single cocaine exposure increases AMPAR function in the VTA (Ungless et al. 2001), and recent studies suggest that this requires the insertion of NMDARs containing GluN3A as well as GluN2B (Yuan et al. 2013). Although this reduces NMDAR current levels, it does indicate that non-canonical NMDARs are critical for the induction of AMPAR plasticity. Interestingly, mGluR activation after cocaine exposure reverses the cocaine-related changes in both GluN3A and AMPARs (Yuan et al. 2013), in agreement with a reversal in the expression of cocaine sensitization by mGluRs (Bellone & Luscher 2006). Thus, AMPAR plasticity mediated by non-canonical NMDARs promotes expression of sensitization. Furthermore, by acting as a target for mGluRs, these non-canonical NMDARs might represent a mechanism whereby reversal of cocaine-related plastic changes could reduce addictive behaviors.
In addition to these cocaine findings, our recent studies have implicated non-canonical NMDARs in the expression of compulsion-like alcohol drinking in rats, where intake persists even when paired with aversive consequences (see above). We discovered that longer-term, intermittent alcohol drinking leads to the appearance of GluN2C/3-containing NMDARs under specific cortical (mPFC and anterior insula) synapses within the NAc core (Seif et al. 2013, 2015). Importantly, inhibition of these non-canonical NMDARs within the NAc significantly reduces compulsion-like alcohol drinking, but has little effect on ‘regular’ alcohol drinking (intake without overt aversive consequences). Our preclinical results agree with hypotheses from several clinical groups (Tiffany & Conklin, 2000; Naqvi & Bechara, 2010; Naqvi et al. 2014) that compulsive intake is characterized by conflict, e.g. between the desire for alcohol and the fear of adverse consequences, which then recruits cortical areas that process conflict and exert behavioral control. In contrast, these groups hypothesize that intake in the absence of conflict is mediated by more dorsal-striatal, habit-based mechanisms. In our studies, ‘regular’ drinking does not require cortical projections to the NAc or NAc NMDARs, while compulsion-like alcohol drinking is critically dependent on cortico-NAc projections and non-canonical NMDARs in the NAc. In fact, our study (Seif et al. 2013) is the first direct evidence for the hypotheses of Tiffany, Naqvi and colleagues. However, further work is required to address the relationship between the level of conflict and recruitment of different cortical or striatal regions. In particular, some aspects of conflict may occur during what we have called regular alcohol drinking, such as overcoming the aversive taste of alcohol. Also, we note that non-canonical NMDARs are present under cortical but not amygdala and other abundant inputs to the NAc, suggesting that these NMDARs are selectively targeted to cortico-NAc terminals (Seif et al. 2013). It is tempting to speculate that these non-canonical NMDARs bias NAc activation by cortical signals, which are likely essential for driving compulsion-like drinking in the face of conflict.
In agreement with these preclinical findings and clinical hypotheses, a recent study found that the NMDAR blocker memantine reduces alcohol cravings but not alcohol drinking in humans (Krishnan-Sarin et al. 2015; see also Krupitsky et al. 2007). Since this study involved heavy drinkers that were not treatment-seeking, they were ostensibly not in conflict about drinking; the only conflict they would experience would be during craving, reflecting a desire for alcohol when it was not available (see also Bisaga & Evans 2004). In addition, memantine may preferentially inhibit GluN2C- and 2D–containing NMDARs (Kotermanski & Johnson 2009). These results concur with the idea that non-canonical NMDARs drive alcohol behaviors that involve conflict. Other clinical studies have shown that memantine reduces intake and cravings in treatment-seeking AUD patients with co-morbid depression (Muhonen et al. 2008), although it had no effect in another study of treatment-seekers (Evans et al. 2007). Nonetheless, a GluN2C single nucleotide polymorphism linked to risk for alcoholism is associated with greater activation of several cortical areas by alcohol cues, and where brain activity is related to both craving and future relapse (Bach et al. 2015). Taken together, these findings suggest that non-canonical NMDARs can play a significant role in regulating addiction to alcohol, and perhaps other drugs of abuse (Sedaghati et al. 2010), particularly under conditions where there is conflict regarding intake.
One additional finding from our studies is that d-serine, either systemically or within the NAc, selectively reduces compulsion-like drinking in a manner similar to the NMDAR blocker AP-5 (Seif et al. 2015). Although D-serine is canonically considered an NMDAR activator at the glycine site (see following section), it can also inhibit non-canonical NMDARs containing the GluN3 subunit (Chatterton et al. 2002; Takarada et al. 2009, 2012), although at higher concentrations than those that promote NMDAR activity (Takarada et al. 2009, 2012). In this regard, aversion-resistant alcohol drinking is inhibited by 50–100 µg but not 25 µg D-serine within the NAc core (Seif et al. 2015), while 4 µg D-serine within the NAc enhances NMDAR function and promotes memory formation (Curcio et al. 2013). Thus, D-serine could represent an important therapeutic intervention to counteract compulsive motivation for alcohol, which, at the same time, could increase the memory for not giving in to compulsive drives.
Plasticity and contributions of NMDAR co-agonists
NMDAR activity requires the presence of d-serine or d-glycine as a co-agonist at the NMDAR glycine-binding site. Thus, changes in the regulation of these factors can alter NMDAR function. For example, passive cocaine exposure increases expression of the d-serine degrading enzyme, d-amino acid oxidase (DAAO), and decreases expression of serine racemase, which mediates synthesis of d-serine, within the NAc core. Together, these adaptations reduce d-serine levels in the NAc core and impair induction of NMDAR-dependent LTP and LTD (Curcio et al. 2013). Interestingly, supplementing D-serine within the NAc restores plasticity induction, and also blocks the expression of sensitization (Curcio et al. 2013). More recently, Liu et al. (2016a, 2016b) demonstrated that the cocaine-related changes in NAc DAAO are long-lasting, and that supplementing D-serine within the NAc restores the ability to extinguish CPP and reverse cocaine-related decreases in GluN2B. Thus, decreased NMDAR function due to decreased agonism at the glycine site increases the impact of behavioral sensitization, and restoring normal NMDAR function seems to protect against this addiction-related behavior. However, genetic KO of serine racemase, and the associated NMDAR hypofunction, is associated with decreased cocaine locomotor sensitization and CPP reinstatement, while supplementation with D-serine restores CPP (Puhl et al. 2015).
Together, these results suggest that altered regulation of NMDAR cofactors can strongly influence addiction-related behaviors, and also that different levels of NMDAR function can have different effects. KO studies with very strong NMDAR inhibition indicate an absolute requirement for NMDARs for formation of associative drug memories. However, more moderate NMDAR hypofunction increases susceptibility to developing or expressing addiction-related behavior, and d-serine could represent a useful therapeutic intervention to restore normal plasticity and reduce the impact of intoxicant-related memories. In agreement, the Alcohol Deprivation Effect, where drinking increases after protracted abstinence, is greatly reduced by inhibition of a glycine transporter (Vengeliene et al. 2010) or by blocking the glycine site on NMDARs (Vengeliene et al. 2005). The former effect, which involves an increase in D-glycine levels, may reflect a normalization of alcohol-related changes in glutamatergic and glycinergic receptor function. Although preliminary, these studies support the idea that altering NMDAR cofactors might reflect a useful clinical intervention to ameliorate NMDAR dysfunctions that promote addictive behaviors.
Co-agonist NMDAR regulators could also reduce addictive behavior by other mechanisms. For example, intoxicant-related cues can be extinguished by repeatedly presenting the cues in the absence of reward, which decreases the ability of such cues to drive addictive behaviors. Since extinction represents new learning, it is likely that NMDARs are required for extinction (through their general role in memory formation), e.g. where GluN2Bs in the ventral mPFC regulate extinction of cocaine CPP (Otis et al. 2014). Thus, increasing NMDAR function during extinction training, e.g. with D-serine or the partial agonist D-cycloserine, could increase extinction learning and reduce the ability of intoxicant-related cues to drive addictive behaviors (Myers et al. 2010; Myers & Carlezon 2012; Hammond et al. 2013; Schade & Paulus 2015). In humans, pairing D-cycloserine with extinction of alcohol cues decreases cue-induced activation of dorsal and ventral striatum, where decreased cue-related brain activation correlates with lower craving and relapse risk (Kiefer et al. 2015). However, other studies find that D-cycloserine does not facilitate extinction training in human alcohol or cocaine users (Kamboj et al. 2011; Price et al. 2009). Although the use of NMDAR agonists to promote extinction of drug cues remains conceptually attractive, the lack of effectiveness in some human studies may reflect the difficulty of overcoming and suppressing very strong addictive drives, and the multiple cues and contexts that an addict may have paired with intoxicant intake.
Interaction of NMDARs with other receptor types
While we have focused on the importance of NMDARs in addictive behaviors, it is important to note that functional plasticity of other receptors, including both glutamatergic AMPARs and mGluRs, can also be critical mediators of addiction-related behaviors (e.g. Chen et al. 2008; Conrad et al. 2008; Kasanetz et al. 2013; Meinhardt et al. 2013). In some cases, this occurs through interactions with NMDARs, which we will review here.
NMDAR function and plasticity induction can be regulated by direct interaction of NMDARs with other receptor types. For example, i.c.v. infusion of a peptide that disrupts direct interactions between alpha7-nAChRs and NMDARs prevents the nAChR enhancement of NMDAR currents and LTP, as well as object recognition memory and reinstatement of nicotine seeking, but not nicotine self-administration (Li et al. 2012, 2013). These studies are particularly interesting, since they suggest that endogenous acetylcholine and activation of nAChRs and NMDARs are all critical for driving nicotine reinstatement. In addition, NMDARs can interact with dopamine-type-1 receptors (D1Rs), both through indirect interactions through second messengers (Cepeda et al. 2009), and also through direct interactions that can be disrupted using a specific peptide. D1Rs and NMDARs interact in the NAc to promote methamphetamine reinstatement (Taepavarapruk et al. 2015), as well as activation of downstream signaling in the NAc in response to reward-predictive cues (Kirschmann et al. 2014). However, studies using the peptide to disrupt direct D1R–NMDAR interactions have produced mixed results, since D1R activation enhances NMDAR-dependent LTP in hippocampal cultures (Nai et al. 2009), while D1Rs inhibit NMDAR function (Lee et al. 2002) and NMDAR-dependent LTP at cortico-NAc inputs after in vivo morphine (Zheng et al. 2014). This is also in contrast to the DStr, where D1Rs are required for NMDAR-dependent LTP (Centonze et al. 2001). Thus, D1Rs can strongly regulate the function of NMDARs, although the direction of this impact may vary across brain regions.
NMDARs and mGluRs can also have interesting interactions in relation to addictive behaviors. Repeated cocaine exposure leads to NMDAR-dependent AMPAR LTP in cortico-NAc inputs (Bellone & Luscher 2006; Pascoli et al. 2011). This LTP can be reversed by mGluR agonists or patterned optogenetic stimulation, which also decrease expression of sensitization (Creed et al. 2015). Cocaine-induced AMPAR LTP in the VTA is also depressed by mGluR agonists (Mameli et al. 2009). While additional studies are required to fully understand the molecular mechanisms through which mGluRs and NMDARs interact, mGluRs may represent a novel and important therapeutic intervention for reducing NMDAR-related plasticity that drives addictive behaviors.
A different type of NMDAR-mGluR interaction in the VTA is observed after repeated alcohol or methamphetamine exposure. These intoxicants increase NMDAR function within VTA neurons, facilitating LTP induction (Ahn et al. 2010; Bernier et al. 2011). Greater NMDAR function is secondary to intoxicant-induced enhancement of mGluR-dependent increases in intracellular and action-potential-related calcium signals. Also, repeated alcohol increases cocaine CPP, and the magnitude of the methamphetamine CPP in vivo correlates with the mGluR increase in calcium in brain slice. Thus, increased VTA mGluR and NMDAR function can promote drug-related conditioning which drives addictive behaviors.
Conclusions and future directions
There is substantial preclinical evidence that NMDARs promote a number of addictive behaviors, including through plasticity of specific NMDAR subunits. One central question is whether there are common or divergent mechanisms for different types of intoxicants and addiction-related behaviors, which will have important implications for the therapeutic use of NMDAR modulators.
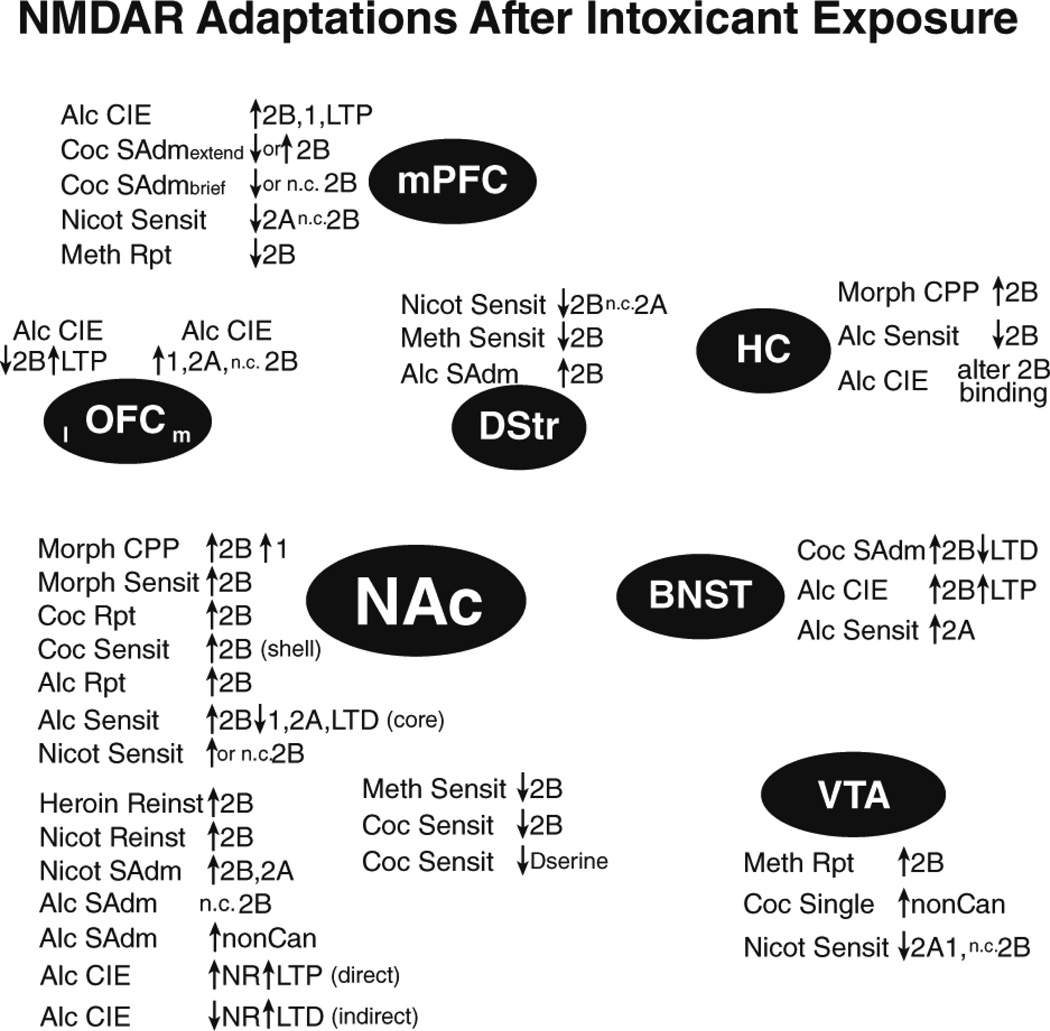
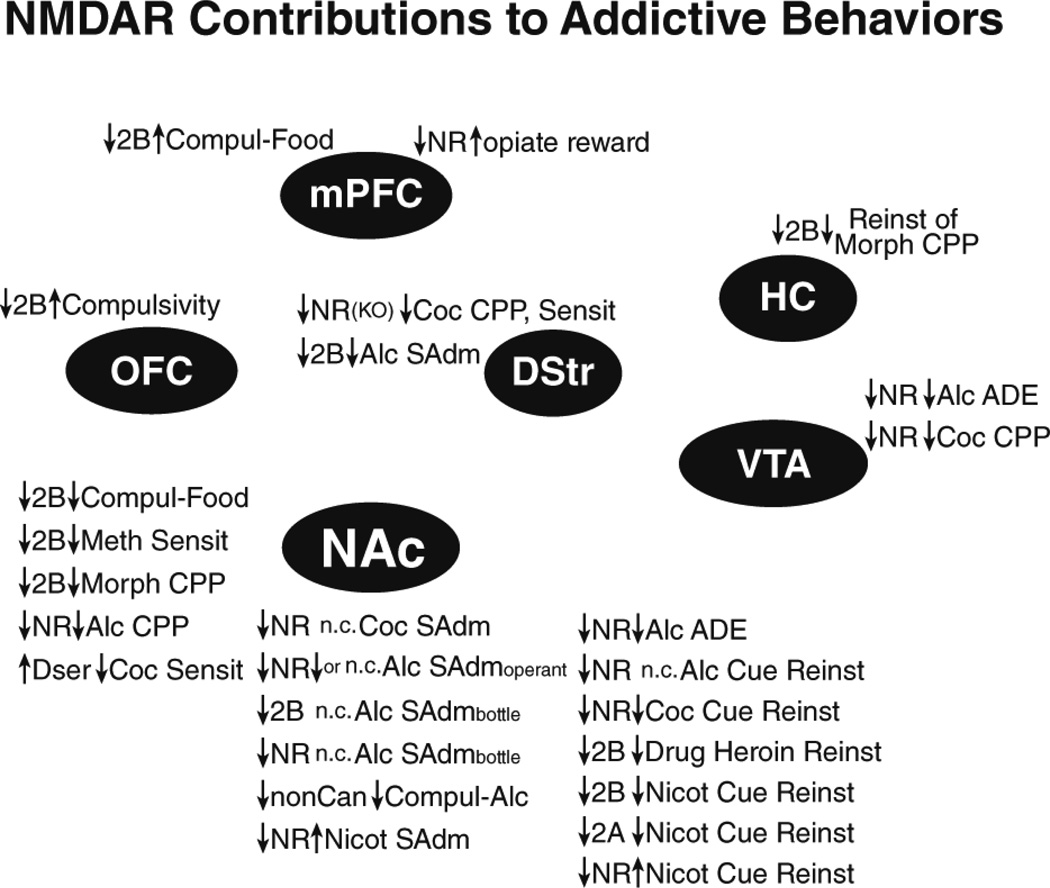
Converging evidence implicates GluN2Bs as central regulators of many addiction-related behaviors, including binge intake of food. Intoxicant-related GluN2B adaptations are observed across a number of brain regions (Fig. 1), although changes in other subunits are also observed. Furthermore, modulating GluN2Bs and NMDARs more generally suppresses many (although not all) addiction-related behaviors (Fig. 2). Also, the widespread contribution of NAc NMDARs (Fig. 1 2) is perhaps not surprising, given the central role of the NAc in mediating reward and goal-directed behaviors. However, the prevalence of NAc studies may also reflect a bias in studying this region. Thus, it will be important to determine the contribution of NMDARs across different brain regions and abused intoxicants. In fact, NMDARs, especially GluN2Bs, in a number of brain regions can undergo intoxicant-related adaptations and promote addiction-related behaviors, when tested. Another interesting possibility is that GluN2Bs are more prone to exhibit plasticity because they play a central role in homeostasis, and thus are designed to exhibit functional changes the face of repeated, strong challenge. Thus, it would be interesting if other forms of homeostatic challenge produce similar GluN2B adaptations, e.g. as seen with stress exposure (e.g. Kiselycznyk et al. 2011; Murphy et al. 2014; Quadir et al. 2016).
Figure 1.
NMDAR adaptations after exposure to addictive intoxicants. Summary of NMDAR adaptations observed after passive or active intoxicant exposure. Adaptations are grouped near the brain region they have been reported within. List of abbreviations: 1: GluN1; 2A: GluN2A; 2B: GluN2B; ADE: alcohol deprivation effect; Alc: alcohol; bottle: alcohol intake from a bottle; brief: brief daily access; CIE: chronic intermittent ethanol exposure; Coc: cocaine; Compul: compulsion-like; direct: direct-pathway (DA1R–containing) cells; Dser: d-serine; extend: extended daily access; indirect: indirect-pathway (DA2R–or adenosine-2-receptor-containing) cells; KO: knockout; l: lateral; m: medial; Meth: methamphetamine; Morph: morphine; n.c. no change; Nicot: nicotine; nonCan: non-canonical NMDARs; NR: NMDAR; operant: operant intake; Reinst: reinstatement; Reward: acute reward; Rpt: repeated; SAdm: self-administration; Sensit: sensitization; Single: single exposure.
Figure 2.
Behavioral impact of inhibiting NMDARs in different brain regions. Summary of studies where the impacts of NMDAR function within a specific brain region was directly addressed with intracranial NMDAR manipulation. Results are grouped near the brain region they have been reported within. Also, only studies examining expression (not development) of addictive behaviors are included, except for KO studies. See Fig. 1 for list of abbreviations.
Comprehensive experiments examining NMDARs in regions beyond the NAc are critical to understanding common and divergent aspects of addictive behaviors, which may relate at least in part to the very different primary mechanisms of action of addictive substances. Some theories of addiction have emphasized that a central brain circuit that may underlie addiction to many different drugs as well as non-intoxicants such as gambling and sex (Marchant et al. 2015; Volkow et al. 2013). However, the varied interoceptive nature of different drugs, including the intoxication, reward and aversion and time course of action, could result in very different conditioned associations, even within different circuits. The relative abundance of the molecular target(s) of a given drug across different brain regions also likely plays a role. Thus, there are likely to be both common and divergent circuits across different abused compounds (Badiani et al. 2011; Marchant et al. 2015), and the specific circuits and NMDAR adaptations recruited for a given drug will likely significantly impact the therapeutic use of NMDAR blockers.
A particular emphasis should also be placed on understanding NMDAR adaptations that occur after voluntary self-administration, and also after protracted withdrawal from voluntary intake. Overall, inhibition of NAc NMDARs suppresses a number of forms of cued reinstatement, with less of an impact on self-administration (Fig. 2). Thus, it would be very helpful to have studies from different intoxicants where the duration of intake, both access time per day and number of total intake sessions, were more comparable. We could then more precisely assess how different intoxicants alter NMDARs across voluntary intake, and across the weeks of withdrawal from intake. In particular, it may be that NMDAR adaptations become more pronounced across withdrawal, similar to the incubation of AMPAR plasticity (described above). Understanding the development and persistence of different NMDAR neuro-adaptations will likely be relevant to humans who are attempting to maintain abstinence across months and years of sobriety, which is a major therapeutic goal for human. However, given the large number of people who want to reduce intake or quit, but still actively engage in intake, intoxicant-related NMDAR changes associated with intake may also be critical therapeutic targets.
Studies of passive intoxicant exposure are also interesting, but sometimes more challenging to interpret. Phenomenon such as CPP and sensitization, which are widely studied in animal models, are also observed in humans. Furthermore, it is likely that these drug-related associations, which could occur with passive or active drug exposure, can contribute to cue-driven aspects of addictive behaviors, including those that drive relapse. However, it is clear from several examples that passive and active exposure can produce different receptor adaptations. In addition, although there is reasonable consensus for an increase in GluN2B in the NAc across passive exposure models, there are also some mixed results. It is difficult to be certain how much of this diversity reflects true differences among drugs, rather than technical differences in terms of dose, days of exposure or other variables.
It is also important to understand the mechanism by which NMDAR antagonists acutely reduce addictive behaviors. While NMDARs can directly depolarize neurons, they also play a central role in induction of AMPAR plasticity, which can occur very rapidly and contribute critically to expression of addiction-related behaviors, including reinstatement of heroin (Shen et al. 2011), cocaine (Gipson et al. 2013a) and nicotine (Gipson et al. 2013b). NMDAR-dependence of AMPAR plasticity and reinstatement behavior were directly verified for heroin and nicotine. This raises the interesting possibility that rapidly-developing NMDAR-dependent plasticity contributes to expression of many addiction-related behaviors. Functional plasticity of NMDARs adds an additional wrinkle, since greater NMDAR function can enhance plasticity induction as well as direct depolarization. In order to have the greatest therapeutic benefit, it would be best to have a complete understanding of the molecular mechanisms through which NMDARs regulate a given behavior.
Another important caveat when considering NMDAR studies is that different classes of NMDAR antagonists can have varied behavioral effects. For example, uncompetitive inhibitors (e.g. memantine, ketamine) only bind to open NMDARs, while competitive inhibitors (e.g. AP-5) can more potently inhibit NMDARs by preventing their activation. Thus, uncompetitive inhibitors can exert more mild NMDAR inhibition, with qualitatively different effects relative to more complete blockade. For example, memantine and ketamine increase impulsive choice in rats with low basal impulsivity, while AP-5 does not (Cottone et al. 2013). In addition, uncompetitive NMDAR inhibitors within the NAc produce interoceptive actions which mimic the moderate alcohol inhibition of NMDARs, while competitive inhibitors do not (Hodge & Cox 1998), even though competitive NMDAR inhibitors within the NAc can impact alcohol reward and intake (Gremel & Cunningham 2009; Rassnick et al. 1992; Seif et al. 2013). Thus, NMDARs could play multiple functional roles, even within the same brain region. Even memantine and ketamine can have differential effects on alcohol drinking (Sabino et al. 2013), which may reflect differences in affinity and kinetics of binding and unbinding, as well as memantine action at non-canonical NMDARs (see above). Thus, a complete understanding of NMDAR contributions to a given behavior, and the possible clinical implications of the NMDAR effects, likely requires careful dose–response analysis of multiple types of NMDAR inhibitors.
Recent studies have also identified that less appreciated aspects of NMDAR signaling, including plasticity of non-canonical NMDAR subunits and NMDAR co-agonists, can also be crucial mediators of addiction-related behaviors. Non-canonical NMDARs provide a depolarizing influence even at hyperpolarized resting potentials, and can potently enhance the influence of synapses containing such receptors. Also, there is some concurrence of animal and human studies regarding the contribution of non-canonical NMDARs to alcohol intake. Thus, the ability to inhibit non-canonical NMDARs with FDA-approved compounds such as d-serine and memantine may provide immediately accessible therapeutic interventions for alcohol and perhaps cocaine addiction. Furthermore, other addiction-related behaviors may also be regulated by non-canonical NMDARs and glycine-site modulators. These new forms of NMDAR plasticity also remind us that much remains to be learned about the contribution of NMDARs to addiction-related behaviors.
One common thread across the reviewed studies is the possibility of using NMDAR blockers or agonists to intervene therapeutically in human addiction. Greater NMDAR function may promote pathological intake, although increased NMDAR function could actually protect against some behaviors. It is also possible that systemic NMDAR antagonists might have complex effects if there are multiple NMDAR adaptations across different brain regions that may have diverse behavioral roles. However, NMDAR inhibitors have limited effectiveness for some aspects of human addiction (cocaine and opiate intake), with perhaps better evidence for alcohol. Given the many adaptations and behavioral effects observed in preclinical studies, this may suggest that animal findings are of limited relevance to humans. On the other hand, given the evidence for NAc NMDARs driving reinstatement, and NMDARs in NAc and cortex regulating compulsion-like intake, it may be that NMDAR blockers would be effective against specific aspects of addiction in humans, such as relapse and intake despite conflicting motivations, rather than simply reducing ongoing intake.
Since NMDARs play a central role in learning and memory, some clinical strategies have also focused on NMDAR agonists to enhance extinction of drug memories. However, as described above, human studies using this strategy have had little success so far. In addition, NMDAR-dependent drug memories could be disrupted during reconsolidation, a period of time where a particular memory trace is reactivated, e.g. by the presence of drug-related cues. Since memory reforms (‘re-consolidates’) in this critical period, blocking NMDARs during this time can persistently disrupt the conditioned associations that drive addiction (Feltenstein & See 2007; Li et al. 2016; Milton et al. 2008, 2012; Vengeliene et al. 2015; von der Goltz et al. 2009). Interfering at the time of reconsolidation is beginning to be attempted in humans (Lonergan et al. 2016), although not yet with NMDAR blockers to our knowledge.
It is also important to note that targeting NMDARs as therapy for human addiction presents some significant challenges. NMDAR inhibitors can have a number of adverse side effects, including memory deficits, difficulties in concentration, agitation, catatonia, as well as psychotomimetic effects including hallucinations (Heresco-Levy et al. 2013; Holmes et al. 2013; Millan 2005; Parsons et al. 1999). Such adverse side-effects could be reduced by NMDAR inhibitors with subunit specificity (Heresco-Levy et al. 2013; Holmes et al. 2013) or with more moderate inhibition (Parsons et al. 1999). Thus, it is interesting that memantine may preferentially target non-canonical NMDARs, and may specifically target aspects of alcohol addiction where conflict is present. Also, given the extensive evidence for GluN2B subunits in many addiction-related behaviors, antagonists selective for these subunits could be of great clinical benefit. However, NMDAR inhibitors may have effects that were not originally expected, e.g. where ketamine acts as an antidepressant, perhaps due to a rapidly-developing compensatory glutamatergic enhancement (Abdallah et al. 2016), or where memantine can produce adaptations across subsequent days, e.g. changes in Brain-Derived Neurotrophic Factor, which can then alter alcohol drinking even after NMDARs are no longer inhibited (see Alaux-Cantin et al. 2015). While this may make ketamine and memantine even more useful as therapeutic interventions, with potential positive benefits from both direct and indirect effects, it can also complicate the interpretation of studies using them. However, some NMDAR antagonists, such as PCP and ketamine, are themselves abused substances (Liu et al. 2016a, 2016b). However, abuse liability may depend on the compound, as there seems to be little evidence for addictive potential for memantine (Vosburg et al. 2005). Nonetheless, despite these caveats, extensive preclinical and some human evidence suggests that NMDARs can drive addiction-related behaviors, and NMDAR antagonists continue to be of great clinical and therapeutic interest.
Acknowledgments
I thank Dr. Kelly Lei and Scott Wegner for their critical review of this manuscript. Supported by NIH/NIAAA AA021445 and AA017072.
Footnotes
The author declares no conflict of interest.
References
- Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33:689–697. doi: 10.1002/da.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahao KP, Ariwodola OJ, Butler TR, Rau AR, Skelly MJ, Carter E, Alexander NP, McCool BA, Souza-Formigoni ML, Weiner JL. Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. J Neurosci. 2013;33:4834–4842. doi: 10.1523/JNEUROSCI.5839-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta G, Hasenkamp W, Daunais JB, Friedman DP, Grant KA, Hemby SE. Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys. Brain Res. 2010;1318:144–154. doi: 10.1016/j.brainres.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatsuma S, Dang MT, Li Y, Hiroi N. N-methyl-d-aspartic acid receptors on striatal neurons are essential for cocaine cue reactivity in mice. Biol Psychiatry. 2010;67:778–780. doi: 10.1016/j.biopsych.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar MA, Manzanedo C, Do Couto BR, Rodriguez-Arias M, Minarro J. Memantine blocks sensitization to the rewarding effects of morphine. Brain Res. 2009;1288:95–104. doi: 10.1016/j.brainres.2009.06.100. [DOI] [PubMed] [Google Scholar]
- Ahn KC, Bernier BE, Harnett MT, Morikawa H. IP3 receptor sensitization during in vivo amphetamine experience enhances NMDA receptor plasticity in dopamine neurons of the ventral tegmental area. J Neurosci. 2010;30:6689–6699. doi: 10.1523/JNEUROSCI.4453-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux-Cantin S, Buttolo R, Houchi H, Jeanblanc J, Naassila M. Memantine reduces ethanol drinking but not relapse in ethanol-dependent rats. Addict Biol. 2015;20:890–901. doi: 10.1111/adb.12177. [DOI] [PubMed] [Google Scholar]
- Anderson EM, Reeves T, Kapernaros K, Neubert JK, Caudle RM. Phosphorylation of the N-methyl-d-aspartate receptor is increased in the nucleus accumbens during both acute and extended morphine withdrawal. J Pharmacol Exp Ther. 2015;355:496–505. doi: 10.1124/jpet.115.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila-Ruiz T, Carranza V, Gustavo LL, Limon DI, Martinez I, Flores G, Flores-Hernandez J. Chronic administration of nicotine enhances NMDA-activated currents in the prefrontal cortex and core part of the nucleus accumbens of rats. Synapse. 2014;68:248–256. doi: 10.1002/syn.21726. [DOI] [PubMed] [Google Scholar]
- Bach P, Kirsch M, Hoffmann S, Jorde A, Mann K, Frank J, Charlet K, Beck A, Heinz A, Walter H, Rietschel M, Kiefer F, Vollstadt-Klein S. The effects of single nucleotide polymorphisms in glutamatergic neurotransmission genes on neural response to alcohol cues and craving. Addict Biol. 2015;20:1022–1032. doi: 10.1111/adb.12291. [DOI] [PubMed] [Google Scholar]
- deBacker J, Hawken ER, Normandeau CP, Jones AA, Di Prospero C, Mechefske E, Gardner Gregory J, Hayton SJ, Dumont EC. GluN2B–containing NMDA receptors blockade rescues bidirectional synaptic plasticity in the bed nucleus of the stria terminalis of cocaine self-administering rats. Neuropsychopharmacology. 2015;40:394–405. doi: 10.1038/npp.2014.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of ethanol withdrawal ‘kindling’. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Beckley JT, Laguesse S, Phamluong K, Morisot N, Wegner SA, Ron D. The first alcohol drink triggers mTORC1-dependent synaptic plasticity in nucleus accumbens dopamine D1 receptor neurons. J Neurosci. 2016;36:701–713. doi: 10.1523/JNEUROSCI.2254-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin-Rauscent A, Fouyssac M, Bonci A, Belin D. How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biol Psychiatry. 2016;79:39–46. doi: 10.1016/j.biopsych.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Keeley P, Cook M, Brake W, Joyce M, Nyffeler M, Heston R, Ettenberg A. Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine. Brain Res. 2007;1131:220–228. doi: 10.1016/j.brainres.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier BE, Whitaker LR, Morikawa H. Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J Neurosci. 2011;31:5205–5212. doi: 10.1523/JNEUROSCI.5282-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Zvartau EE, Beardsley PM, Balster RL. Effects of NMDA receptor antagonists on cocaine-conditioned motor activity in rats. Eur J Pharmacol. 2000a;390:303–311. doi: 10.1016/s0014-2999(99)00927-9. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Zvartau EE, Balster RL, Beardsley PM. Effects of N-methyl-d-aspartate receptor antagonists on reinstatement of cocaine-seeking behavior by priming injections of cocaine or exposures to cocaine-associated cues in rats. Behav Pharmacol. 2000b;11:37–44. doi: 10.1097/00008877-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology (Berl) 2004;172:16–24. doi: 10.1007/s00213-003-1617-5. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Comer SD, Ward AS, Popik P, Kleber HD, Fischman MW. The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans. Psychopharmacology (Berl) 2001;157:1–10. doi: 10.1007/s002130100739. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Cheng WY, Levin FR, Mariani JJ, Raby WN, Nunes EV. A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug Alcohol Depend. 2010;111:97–104. doi: 10.1016/j.drugalcdep.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Sullivan MA, Cheng WY, Carpenter KM, Mariani JJ, Levin FR, Raby WN, Nunes EV. A placebo controlled trial of memantine as an adjunct to oral naltrexone for opioid dependence. Drug Alcohol Depend. 2011;119:e23–e29. doi: 10.1016/j.drugalcdep.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SF, Lauzon NM, Bechard M, Gholizadeh S, Laviolette SR. NMDA receptor hypofunction in the prelimbic cortex increases sensitivity to the rewarding properties of opiates via dopaminergic and amygdalar substrates. Cereb Cortex. 2011;21:68–80. doi: 10.1093/cercor/bhq060. [DOI] [PubMed] [Google Scholar]
- Blincoe L, Seay A, Zaloshnja E, Miller T, Romano E, Luchter S, Spicer R. The Economic Impact of Motor Vehicle Crashes, 2000. Washington, DC: U.S. Department of Transportation; 2002. pp. 1–82. [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prevent Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav Neurosci. 2004;118:822–834. doi: 10.1037/0735-7044.118.4.822. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci. 2013;16:1101–1110. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett EJ, Chandler LJ, Trantham-Davidson H. Glutamatergic plasticity and alcohol dependence-induced alterations in reward, affect and cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2015;65:309–320. doi: 10.1016/j.pnpbp.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996a;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacology (Berl) 1996b;128:413–420. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- Carmack SA, Kim JS, Sage JR, Thomas AW, Skillicorn KN, Anagnostaras SG. The competitive NMDA receptor antagonist CPP disrupts cocaine-induced conditioned place preference, but spares behavioral sensitization. Behav Brain Res. 2013;239:155–163. doi: 10.1016/j.bbr.2012.10.042. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Excessive Drinking Costs U.S. $223.5 Billion. Atlanta, GA: Center for Disease Control; 2014. [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13:1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Jocoy EL, Levine MS. NMDA and dopamine: diverse mechanisms applied to interacting receptor systems. In: Dongen Van AM, editor. Biology of the NMDA Receptor. Boca Raton, FL: CRC Press/Taylor and Francis; 2009. [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Amphetamine-induced place preference in humans. Biol Psychiatry. 2009;65:900–904. doi: 10.1016/j.biopsych.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins ED, Ward AS, McDowell DM, Foltin RW, Fischman MW. The effects of memantine on the subjective, reinforcing and cardiovascular effects of cocaine in humans. Behav Pharmacol. 1998;9:587–598. doi: 10.1097/00008877-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Iemolo A, Narayan AR, Kwak J, Momaney D, Sabino V. The uncompetitive NMDA receptor antagonists ketamine and memantine preferentially increase the choice for a small, immediate reward in low-impulsive rats. Psychopharmacology (Berl) 2013;226:127–138. doi: 10.1007/s00213-012-2898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Luscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–664. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Oliva JM, Ghasemzadeh MB, Kalivas PW, Ambrosio E. Neuroadaptive changes in NMDAR1 gene expression after extinction of cocaine self-administration. Ann N Y Acad Sci. 2002;965:78–91. doi: 10.1111/j.1749-6632.2002.tb04153.x. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Curcio L, Podda MV, Leone L, Piacentini R, Mastrodonato A, Cappelletti P, Sacchi S, Pollegioni L, Grassi C, D’Ascenzo M. Reduced d-serine levels in the nucleus accumbens of cocaine-treated rats hinder the induction of NMDA receptor-dependent synaptic plasticity. Brain. 2013;136:1216–1230. doi: 10.1093/brain/awt036. [DOI] [PubMed] [Google Scholar]
- D’Souza MS, Markou A. Differential role of N-methyl-d-aspartate receptor-mediated glutamate transmission in the nucleus accumbens shell and core in nicotine seeking in rats. Eur J Neurosci. 2014;39:1314–1322. doi: 10.1111/ejn.12491. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- Dickerson D, Pittman B, Ralevski E, Perrino A, Limoncelli D, Edgecombe J, Acampora G, Krystal JH, Petrakis I. Ethanol-like effects of thiopental and ketamine in healthy humans. J Psychopharmacol. 2010;24:203–211. doi: 10.1177/0269881108098612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci. 2014;35:374–383. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhardt M, Leixner S, Lujan R, Spanagel R, Bilbao A. Glutamate receptors within the mesolimbic dopamine system mediate alcohol relapse behavior. J Neurosci. 2015;35:15523–15538. doi: 10.1523/JNEUROSCI.2970-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, Parlato R, Sprengel R, Luscher C, Schutz G, Spanagel R. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Brooks DJ, Garawi F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol Clin Exp Res. 2007;31:775–782. doi: 10.1111/j.1530-0277.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Evins AE, Pachas G, Mischoulon D, Urbanoski K, Carlini S, Sousa J, Bentley K, Rigotti NA, Nino-Gomez J, Loebl T, Janes AC, Kaufman MJ, Fava M. A double-blind, placebo-controlled trial of the NMDA glycine site antagonist, GW468816, for prevention of relapse to smoking in females. J Clin Psychopharmacol. 2011;31:597–602. doi: 10.1097/JCP.0b013e31822bb390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013a;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci USA. 2013b;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Goltz C, Vengeliene V, Bilbao A, Perreau-Lenz S, Pawlak CR, Kiefer F, Spanagel R. Cue-induced alcohol-seeking behaviour is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacology (Berl) 2009;205:389–397. doi: 10.1007/s00213-009-1544-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez B, Rivero-Echeto C, Muniz JA, Cadet JL, Garcia-Rill E, Urbano FJ, Bisagno V. Methamphetamine blunts Ca(2+) currents and excitatory synaptic transmission through D1/5 receptor-mediated mechanisms in the mouse medial prefrontal cortex. Addict Biol. 2016;21:589–602. doi: 10.1111/adb.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-d-aspartate antagonists. J Pharmacol Exp Ther. 1993;264:1241–1247. [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Odlaug BL, Potenza MN, Kim SW. Memantine shows promise in reducing gambling severity and cognitive inflexibility in pathological gambling: a pilot study. Psychopharmacology (Berl) 2010;212:603–612. doi: 10.1007/s00213-010-1994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology. 2009;34:1443–1453. doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Lovinger DM. Associative and sensorimotor cortico-basal ganglia circuit roles in effects of abused drugs. Genes Brain Behav. 2016 doi: 10.1111/gbb.12309. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S, Seymour CM, Burger A, Wagner JJ. d-Serine facilitates the effectiveness of extinction to reduce drug-primed reinstatement of cocaine-induced conditioned place preference. Neuropharmacology. 2013;64:464–471. doi: 10.1016/j.neuropharm.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U, Shoham S, Javitt DC. Glycine site agonists of the N-methyl-d-aspartate receptor and Parkinson’s disease: a hypothesis. Mov Disord. 2013;28:419–424. doi: 10.1002/mds.25306. [DOI] [PubMed] [Google Scholar]
- Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference. J Neurosci. 2005;25:6651–6657. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 2013;229:539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter SM, Danysz W, Spanagel R. Novel uncompetitive N-methyl-d-aspartate (NMDA)-receptor antagonist MRZ 2/579 suppresses ethanol intake in long-term ethanol-experienced rats and generalizes to ethanol cue in drug discrimination procedure. J Pharmacol Exp Ther. 2000;292:545–552. [PubMed] [Google Scholar]
- Hopf FW, Lesscher HM. Rodent models for compulsive alcohol intake. Alcohol. 2014;48:253–264. doi: 10.1016/j.alcohol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Brown TE, Han MH, Saal DB, Neve RL, Zukin RS, Sorg BA, Nestler EJ, Malenka RC, Dong Y. CREB modulates the functional output of nucleus accumbens neurons: a critical role of N-methyl-d-aspartate glutamate receptor (NMDAR) receptors. J Biol Chem. 2008;283:2751–2760. doi: 10.1074/jbc.M706578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Schluter OM, Dong Y. Silent synapses speak up: updates of the neural rejuvenation hypothesis of drug addiction. Neuroscientist. 2015;21:451–459. doi: 10.1177/1073858415579405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Nesic J, Groombridge C, Clowry O, Rusted J, Duka T. Differential involvement of glutamatergic mechanisms in the cognitive and subjective effects of smoking. Neuropsychopharmacology. 2009;34:257–265. doi: 10.1038/npp.2008.50. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Wardeh G, Smit AB, Schoffelmeer AN. Morphine causes a delayed increase in glutamate receptor functioning in the nucleus accumbens core. Eur J Pharmacol. 2005;511:27–30. doi: 10.1016/j.ejphar.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Jain K, Jain R, Dhawan A. A double-blind, double-dummy, randomized controlled study of memantine versus buprenorphine in naloxone-precipitated acute withdrawal in heroin addicts. J Opioid Manag. 2011;7:11–20. doi: 10.5055/jom.2011.0044. [DOI] [PubMed] [Google Scholar]
- Joffe ME, Vitter SR, Grueter BA. GluN1 deletions in D1- and A2A–expressing cell types reveal distinct modes of behavioral regulation. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Massey-Chase R, Rodney L, Das R, Almahdi B, Curran HV, Morgan CJ. Changes in cue reactivity and attentional bias following experimental cue exposure and response prevention: a laboratory study of the effects of d-cycloserine in heavy drinkers. Psychopharmacology (Berl) 2011;217:25–37. doi: 10.1007/s00213-011-2254-z. [DOI] [PubMed] [Google Scholar]
- Kao JH, Huang EY, Tao PL. NR2B subunit of NMDA receptor at nucleus accumbens is involved in morphine rewarding effect by siRNA study. Drug Alcohol Depend. 2011;118:366–374. doi: 10.1016/j.drugalcdep.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, Fiancette JF, Renault P, Piazza PV, Manzoni OJ. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2013;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Reese RM, Woods CA, Janak PH. The orbitofrontal cortex as part of a hierarchical neural system mediating choice between two good options. J Neurosci. 2013;33:15989–15998. doi: 10.1523/JNEUROSCI.0026-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Kirsch M, Bach P, Hoffmann S, Reinhard I, Jorde A, von der Goltz C, Spanagel R, Mann K, Loeber S, Vollstadt-Klein S. Effects of d-cycloserine on extinction of mesolimbic cue reactivity in alcoholism: a randomized placebo-controlled trial. Psychopharmacology (Berl) 2015;232:2353–2362. doi: 10.1007/s00213-015-3882-5. [DOI] [PubMed] [Google Scholar]
- Kirschmann EK, Mauna JC, Willis CM, Foster RL, Chipman AM, Thiels E. Appetitive cue-evoked ERK signaling in the nucleus accumbens requires NMDA and D1 dopamine receptor activation and regulates CREB phosphorylation. Learn Mem. 2014;21:606–615. doi: 10.1101/lm.035113.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselycznyk C, Svenningsson P, Delpire E, Holmes A. Genetic, pharmacological and lesion analyses reveal a selective role for corticohippocampal GLUN2B in a novel repeated swim stress paradigm. Neuroscience. 2011;193:259–268. doi: 10.1016/j.neuroscience.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Bergman J. Reinforcing effectiveness of nicotine in nonhuman primates: effects of nicotine dose and history of nicotine self-administration. Psychopharmacology (Berl) 2016;233:2451–2458. doi: 10.1007/s00213-016-4293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostowski W, Bieńkowski P. Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol. 1999;17:63–80. doi: 10.1016/s0741-8329(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, O’Malley SS, Franco N, Cavallo DA, Morean M, Shi J, Pittman B, Krystal JH. N-methyl-d-aspartate receptor antagonism has differential effects on alcohol craving and drinking in heavy drinkers. Alcohol Clin Exp Res. 2015;39:300–307. doi: 10.1111/acer.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grinenko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, Stetson P, Trevisan LA, Charney DS. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Madonick S, Perry E, Gueorguieva R, Brush L, Wray Y, Belger A, D’Souza DC. Potentiation of low dose ketamine effects by naltrexone: potential implications for the pharmacotherapy of alcoholism. Neuropsychopharmacology. 2006;31:1793–1800. doi: 10.1038/sj.npp.1300994. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Nappi SK, Trevisan L, Pittman B, D’Souza DC, Suckow RF. Characterization of the interactive effects of glycine and d-cycloserine in men: further evidence for enhanced NMDA receptor function associated with human alcohol dependence. Neuropsychopharmacology. 2011;36:701–710. doi: 10.1038/npp.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambot L, Chaves Rodriguez E, Houtteman D, Li Y, Schiffmann SN, Gall D, de Kerchove d’Exaerde A. Striatopallidal neuron NMDA receptors control synaptic connectivity, locomotor, and goal-directed behaviors. J Neurosci. 2016;36:4976–4992. doi: 10.1523/JNEUROSCI.2717-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa L, Machalova A, Sulcova A. Implication of NMDA receptors in behavioural sensitization to psychostimulants: a short review. Eur J Pharmacol. 2014;730:77–81. doi: 10.1016/j.ejphar.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology. 2011;61:1060–1069. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, Wang LJ, Lee IH, Wang TY, Chen KC, Yang YK, Hong JS, Lu RB. Low-dose memantine attenuated methadone dose in opioid-dependent patients: a 12-week double-blind randomized controlled trial. Sci Rep. 2015;5:10140. doi: 10.1038/srep10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Petro A, Rose JE. D-cycloserine selectively decreases nicotine self-administration in rats with low baseline levels of response. Pharmacol Biochem Behav. 2011;98:210–214. doi: 10.1016/j.pbb.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li Z, Pei L, Le AD, Liu F. The alpha7nACh-NMDA receptor complex is involved in cue-induced reinstatement of nicotine seeking. J Exp Med. 2012;209:2141–2147. doi: 10.1084/jem.20121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Nai Q, Lipina TV, Roder JC, Liu F. alpha7nAchR/NMDAR coupling affects NMDAR function and object recognition. Mol Brain. 2013;6:58. doi: 10.1186/1756-6606-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ge S, Li N, Chen L, Zhang S, Wang J, Wu H, Wang X, Wang X. NMDA and dopamine D1 receptors within NAc-shell regulate IEG proteins expression in reward circuit during cocaine memory reconsolidation. Neuroscience. 2016;315:45–69. doi: 10.1016/j.neuroscience.2015.11.063. [DOI] [PubMed] [Google Scholar]
- Lin KY, Cherng CG, Yang FR, Lin LC, Lu RB, Yu L. Memantine abolishes the formation of cocaine-induced conditioned place preference possibly via its IL-6-modulating effect in medial prefrontal cortex. Behav Brain Res. 2011;220:126–131. doi: 10.1016/j.bbr.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Liu XS, Hou Y, Yan TL, Guo YY, Han W, Guan FL, Chen T, Li T. Dopamine D3 receptor-regulated NR2B subunits of N-methyl-d-aspartate receptors in the nucleus accumbens involves in morphine-induced locomotor activity. CNS Neurosci Ther. 2014;20:823–829. doi: 10.1111/cns.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZQ, Gu XH, Yang YJ, Yin XP, Xu LJ, Wang W. d-Serine in the nucleus accumbens region modulates behavioral sensitization and extinction of conditioned place preference. Pharmacol Biochem Behav. 2016a;143:44–56. doi: 10.1016/j.pbb.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lin D, Wu B, Zhou W. Ketamine abuse potential and use disorder. Brain Res Bull. 2016b;126:68–73. doi: 10.1016/j.brainresbull.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Quadir SG, Barrett HM, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Campbell RR, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Kippin TE, Phillips TJ, Szumlinski KK. Prefrontal glutamate correlates of methamphetamine sensitization and preference. Eur J Neurosci. 2016;43:689–702. doi: 10.1111/ejn.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan M, Saumier D, Tremblay J, Kieffer B, Brown TG, Brunet A. Reactivating addiction-related memories under propranolol to reduce craving: a pilot randomized controlled trial. J Behav Ther Exp Psychiatry. 2016;50:245–249. doi: 10.1016/j.jbtep.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibition of neuronal glutamate receptor function. Ann Med. 1990;22:247–252. doi: 10.3109/07853899009148935. [DOI] [PubMed] [Google Scholar]
- Ma YY, Guo CY, Yu P, Lee DY, Han JS, Cui CL. The role of NR2B containing NMDA receptor in place preference conditioned with morphine and natural reinforcers in rats. Exp Neurol. 2006;200:343–355. doi: 10.1016/j.expneurol.2006.02.117. [DOI] [PubMed] [Google Scholar]
- Ma YY, Chu NN, Guo CY, Han JS, Cui CL. NR2B–containing NMDA receptor is required for morphine-but not stress-induced reinstatement. Exp Neurol. 2007;203:309–319. doi: 10.1016/j.expneurol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Maldonado C, Rodriguez-Arias M, Castillo A, Aguilar MA, Minarro J. Effect of memantine and CNQX in the acquisition, expression and reinstatement of cocaine-induced conditioned place preference. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:932–939. doi: 10.1016/j.pnpbp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2015;1628:219–232. doi: 10.1016/j.brainres.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Zelek-Molik A, Sun WL. Cocaine self-administration causes signaling deficits in corticostriatal circuitry that are reversed by BDNF in early withdrawal. Brain Res. 2015;1628:82–87. doi: 10.1016/j.brainres.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuier NS, Padula AE, Mulholland PJ, Chandler LJ. Homer2 deletion alters dendritic spine morphology but not alcohol-associated adaptations in GluN2B–containing N-methyl-d-aspartate receptors in the nucleus accumbens. Front Pharmacol. 2015;6:28. doi: 10.3389/fphar.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33:2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. N-methyl-d-aspartate receptors as a target for improved antipsychotic agents: novel insights and clinical perspectives. Psychopharmacology (Berl) 2005;179:30–53. doi: 10.1007/s00213-005-2199-1. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Schramm MJ, Wawrzynski JR, Gore F, Oikonomou-Mpegeti F, Wang NQ, Samuel D, Economidou D, Everitt BJ. Antagonism at NMDA receptors, but not beta-adrenergic receptors, disrupts the reconsolidation of pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacology (Berl) 2012;219:751–761. doi: 10.1007/s00213-011-2399-9. [DOI] [PubMed] [Google Scholar]
- Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282:60–68. doi: 10.1016/j.neuroscience.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhonen LH, Lahti J, Sinclair D, Lonnqvist J, Alho H. Treatment of alcohol dependence in patients with co-morbid major depressive disorder—predictors for the outcomes with memantine and escitalopram medication. Subst Abuse Treat Prev Policy. 2008;3:20. doi: 10.1186/1747-597X-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology (Berl) 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JA, Stein IS, Lau CG, Peixoto RT, Aman TK, Kaneko N, Aromolaran K, Saulnier JL, Popescu GK, Sabatini BL, Hell JW, Zukin RS. Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J Neurosci. 2014;34:869–879. doi: 10.1523/JNEUROSCI.4538-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr D-cycloserine effects on extinction of conditioned responses to drug-related cues. Biol Psychiatry. 2012;71:947–955. doi: 10.1016/j.biopsych.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2010;36:274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Li S, Wang SH, Liu J, Lee FJ, Frankland PW, Liu F. Uncoupling the D1-N-methyl-d-aspartate (NMDA) receptor complex promotes NMDA-dependent long-term potentiation and working memory. Biol Psychiatry. 2009;67:246–254. doi: 10.1016/j.biopsych.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Kinugasa Y, Torii J, Hishinuma T, Tomioka Y, Yamada K, Yamakuni T. Repeated treatment with nicotine induces phosphorylation of NMDA receptor NR2B subunit in the brain regions involved in behavioral sensitization. Neurosci Lett. 2012;524:133–138. doi: 10.1016/j.neulet.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic J, Duka T, Rusted JM, Jackson A. A role for glutamate in subjective response to smoking and its action on inhibitory control. Psychopharmacology (Berl) 2011;216:29–42. doi: 10.1007/s00213-011-2189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JL, Beardsley PM. Effects of memantine, haloperidol, and cocaine on primary and conditioned reinforcement associated with cocaine in rhesus monkeys. Psychopharmacology (Berl) 2006;185:142–149. doi: 10.1007/s00213-005-0282-2. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 2009;19:218–227. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ. Chronic intermittent ethanol exposure enhances the excitability and synaptic plasticity of lateral orbitofrontal cortex neurons and induces a tolerance to the acute inhibitory actions of ethanol. Neuropsychopharmacology. 2016;41:1112–1127. doi: 10.1038/npp.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nona CN, Li R, Nobrega JN. Altered NMDA receptor subunit gene expression in brains of mice showing high vs. low sensitization to ethanol. Behav Brain Res. 2014;260:58–66. doi: 10.1016/j.bbr.2013.11.037. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Turner JR, Pierce RC. Extrasynaptic targeting of NMDA receptors following D1 dopamine receptor activation andcocaine self-administration. J Neurosci. 2013;33:9451–9461. doi: 10.1523/JNEUROSCI.5730-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Fitzgerald MK, Mueller D. Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference. J Neurosci. 2014;34:6057–6064. doi: 10.1523/JNEUROSCI.4980-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park P, Volianskis A, Sanderson TM, Bortolotto ZA, Jane DE, Zhuo M, Kaang BK, Collingridge GL. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philos Trans R Soc Lond B Biol Sci. 2013;369:20130131. doi: 10.1098/rstb.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-d-aspartate (NMDA) receptor antagonist—a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Luscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2011;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Pina MM, Cunningham CL. Involvement of ventral tegmental area ionotropic glutamate receptors in the expression of ethanol-induced conditioned place preference. Behav Brain Res. 2016;313:23–29. doi: 10.1016/j.bbr.2016.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistillo F, Fasoli F, Moretti M, McClure-Begley T, Zoli M, Marks MJ, Gotti C. Chronic nicotine and withdrawal affect glutamatergic but not nicotinic receptor expression in the mesocorticolimbic pathway in a region-specific manner. Pharmacol Res. 2016;103:167–176. doi: 10.1016/j.phrs.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Price KL, McRae-Clark AL, Saladin ME, Maria MM, DeSantis SM, Back SE, Brady KT. D-cycloserine and cocaine cue reactivity: preliminary findings. Am J Drug Alcohol Abuse. 2009;35:434–438. doi: 10.3109/00952990903384332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Berg AR, Bechtholt AJ, Coyle JT. Availability of N-methyl-d-aspartate receptor coagonists affects cocaine-induced conditioned place preference and locomotor sensitization: implications for comorbid schizophrenia and substance abuse. J Pharmacol Exp Ther. 2015;353:465–470. doi: 10.1124/jpet.115.223099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Denny AD, Ticku MK. Chronic intermittent ethanol treatment selectively alters N-methyl-d-aspartate receptor subunit surface expression in cultured cortical neurons. Mol Pharmacol. 2007;72:95–102. doi: 10.1124/mol.106.033043. [DOI] [PubMed] [Google Scholar]
- Quadir SG, Santos JR, Campbell RR, Wroten MG, Singh N, Holloway JJ, Bal SK, Camarini R, Szumlinski KK. Homer2 regulates alcohol and stress cross-sensitization. Addict Biol. 2016;21:613–633. doi: 10.1111/adb.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IM, Hipolide DC, Frussa-Filho R, De Lucca EM, Nobrega JN, Souza-Formigoni ML. Resistance to ethanol sensitization is associated with increased NMDA receptor binding in specific brain areas. Eur J Pharmacol. 2002;442:55–61. doi: 10.1016/s0014-2999(02)01503-0. [DOI] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, Holmes A. Chronic EtOH effects on putative measures of compulsive behavior in mice. Addict Biol. 2015a doi: 10.1111/adb.12342. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Nakazawa K, Holmes A. Cortical GluN2B deletion attenuates punished suppression of food reward-seeking. Psychopharmacology (Berl) 2015b;232:3753–3761. doi: 10.1007/s00213-015-4033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Renteria R, Maier EY, Buske TR, Morrisett RA. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.03.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Ron D, Wang J. The NMDA receptor and alcohol addiction. In: Dongen Van AM, editor. Biology of the NMDA Receptor. Boca Raton, FL: CRC Press/Taylor and Francis; 2009. [PubMed] [Google Scholar]
- Sabino V, Narayan AR, Zeric T, Steardo L, Cottone P. mTOR activation is required for the anti-alcohol effect of ketamine, but not memantine, in alcohol-preferring rats. Behav Brain Res. 2013;247:9–16. doi: 10.1016/j.bbr.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Roeber J, Bouchery EE, Gonzales K, Chaloupka FJ, Brewer RD. State costs of excessive alcohol consumption, 2006. Am J Prev Med. 2013;45:474–485. doi: 10.1016/j.amepre.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Salamone A, Zappettini S, Grilli M, Olivero G, Agostinho P, Tome AR, Chen J, Pittaluga A, Cunha RA, Marchi M. Prolonged nicotine exposure down-regulates presynaptic NMDA receptors in dopaminergic terminals of the rat nucleus accumbens. Neuropharmacology. 2014;79:488–497. doi: 10.1016/j.neuropharm.2013.12.014. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Risk and Protective Factors and Initiation of Substance Use: Results from the 2014 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015. [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Terry P, Katz JL. Memantine has phencyclidine-like but not cocaine-like discriminative stimulus effects in rats. Behav Pharmacol. 1992;3:265–268. [PubMed] [Google Scholar]
- Saunders BT, Richard JM, Janak PH. Contemporary approaches to neural circuit manipulation and mapping: focus on reward and addiction. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140210. doi: 10.1098/rstb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade S, Paulus W. d-Cycloserine in neuropsychiatric diseases: a systematic review. Int J Neuropsychopharmacol. 2015;19:1–7. doi: 10.1093/ijnp/pyv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghati M, Vousooghi N, Goodarzi A, Yaghmaei P, Mokri A, Zarrindast MR. Expression of NR3B but not NR2D subunit of NMDA receptor in human blood lymphocytes can serve as a suitable peripheral marker for opioid addiction studies. Eur J Pharmacol. 2010;633:50–54. doi: 10.1016/j.ejphar.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, Hopf FW. d-Serine and d-cycloserine reduce compulsive alcohol intake in rats. Neuropsychopharmacology. 2015;40:2357–2367. doi: 10.1038/npp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela Rani CS, Ticku MK. Comparison of chronic ethanol and chronic intermittent ethanol treatments on the expression of GABA(A) and NMDA receptor subunits. Alcohol. 2006;38:89–97. doi: 10.1016/j.alcohol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b–containing receptors. Proc Natl Acad Sci USA. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KL, Rao RR, Velazquez-Sanchez C, Valenza M, Giuliano C, Everitt BJ, Sabino V, Cottone P. The uncompetitive N-methyl-d-aspartate antagonist memantine reduces binge-like eating, food-seeking behavior, and compulsive eating: role of the nucleus accumbens shell. Neuropsychopharmacology. 2015;40:1163–1171. doi: 10.1038/npp.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-d-aspartate NR1 and NR3 subunits. J Pharmacol Exp Ther. 2007;322:739–748. doi: 10.1124/jpet.107.123836. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Curr Top Behav Neurosci. 2010;3:3–27. doi: 10.1007/7854_2009_23. [DOI] [PubMed] [Google Scholar]
- Suto N, Ecke LE, Wise RA. Control of within-binge cocaine-seeking by dopamine and glutamate in the core of nucleus accumbens. Psychopharmacology (Berl) 2009;205:431–439. doi: 10.1007/s00213-009-1553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Neural plasticity & behavior - sixty years of conceptual advances. J Neurochem. 2016 doi: 10.1111/jnc.13580. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Butts KA, Phillips AG. Dopamine and glutamate interaction mediates reinstatement of drug-seeking behavior by stimulation of the ventral subiculum. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarada T, Takahata Y, Iemata M, Hinoi E, Uno K, Hirai T, Yamamoto T, Yoneda Y. Interference with cellular differentiation by d-serine through antagonism at N-methyl-d-aspartate receptors composed of NR1 and NR3A subunits in chondrocytes. J Cell Physiol. 2009;220:756–764. doi: 10.1002/jcp.21821. [DOI] [PubMed] [Google Scholar]
- Takarada T, Takarada-Iemata M, Takahata Y, Yamada D, Yamamoto T, Nakamura Y, Hinoi E, Yoneda Y. Osteoclastogenesis is negatively regulated by d-serine produced by osteoblasts. J Cell Physiol. 2012;227:3477–3487. doi: 10.1002/jcp.24048. [DOI] [PubMed] [Google Scholar]
- Tan H, Rosen LG, Ng GA, Rushlow WJ, Laviolette SR. NMDA receptor blockade in the prelimbic cortex activates the mesolimbic system and dopamine-dependent opiate reward signaling. Psychopharmacology (Berl) 2014;231:4669–4679. doi: 10.1007/s00213-014-3616-0. [DOI] [PubMed] [Google Scholar]
- Thuerauf N, Lunkenheimer J, Lunkenheimer B, Sperling W, Bleich S, Schlabeck M, Wiltfang J, Kornhuber J. Memantine fails to facilitate partial cigarette deprivation in smokers—no role of Memantine in the treatment of nicotine dependency? J Neural Transm (Vienna) 2007;114:351–357. doi: 10.1007/s00702-006-0570-y. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–S153. doi: 10.1080/09652140050111717. Wiley Online Library |. [DOI] [PubMed] [Google Scholar]
- Tomek SE, Lacrosse AL, Nemirovsky NE, Olive MF. NMDA receptor modulators in the treatment of drug addiction. Pharmaceuticals (Basel) 2013;6:251–268. doi: 10.3390/ph6020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovero F, David S, Bernard P, Puech A, Bizot JC, Tassin JP. The combination of marketed antagonists of α1b–adrenergic and 5-HT2A receptors inhibits behavioral sensitization and preference to alcohol in mice: a promising approach for the treatment of alcohol dependence. PLoS One. 2016;11:e0151242. doi: 10.1371/journal.pone.0151242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan J, Maj M, Przewlocka B. The effect of drugs of abuse on NMDAR1 receptor expression in the rat limbic system. Drug Alcohol Depend. 2003;72:193–196. doi: 10.1016/s0376-8716(03)00193-5. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Leonardi-Essmann F, Sommer WH, Marston HM, Spanagel R. Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol Psychiatry. 2010;68:704–711. doi: 10.1016/j.biopsych.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Olevska A, Spanagel R. Long-lasting effect of NMDA receptor antagonist memantine on ethanol-cue association and relapse. J Neurochem. 2015;135:1080–1085. doi: 10.1111/jnc.13350. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Hart CL, Haney M, Foltin RW. An evaluation of the reinforcing effects of memantine in cocaine-dependent humans. Drug Alcohol Depend. 2005;79:257–260. doi: 10.1016/j.drugalcdep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Alcohol induces long-term facilitation of NR2B–NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B–containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D. Alcohol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Advances in animal models of relapse for addiction research. In: Kuhn CM, Koob GF, editors. Advances in the Neuroscience of Addiction. 2nd. Boca Raton, FL: CRC Press/Taylor & Francis; 2010. [PubMed] [Google Scholar]
- WHO. Global Status Report on Alcohol and Health-2014. World Health Organization; 2014. [Google Scholar]
- Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, Delpire E, Winder DG. GluN2B subunit deletion reveals key role in acute and chronic alcohol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci USA. 2012;109:E278–E287. doi: 10.1073/pnas.1113820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Baucum AJ, 2nd, Holleran KM, Chen Y, Pasek JG, Delpire E, Tabb DL, Colbran RJ, Winder DG. Chronic intermittent alcohol disrupts the GluN2B–associated proteome and specifically regulates group I mGlu receptor-dependent long-term depression. Addict Biol. 2015 doi: 10.1111/adb.12319. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Mameli M, O’Connor EC, Dey PN, Verpelli C, Sala C, Perez-Otano I, Luscher C, Bellone C. Expression of cocaine-evoked synaptic plasticity by GluN3A–containing NMDA receptors. Neuron. 2013;80:1025–1038. doi: 10.1016/j.neuron.2013.07.050. [DOI] [PubMed] [Google Scholar]
- Zapata A, Chefer VI, Ator R, Shippenberg TS, Rocha BA. Behavioural sensitization and enhanced dopamine response in the nucleus accumbens after intravenous cocaine self-administration in mice. Eur J Neurosci. 2003;17:590–596. doi: 10.1046/j.1460-9568.2003.02491.x. [DOI] [PubMed] [Google Scholar]
- Zappettini S, Grilli M, Olivero G, Chen J, Padolecchia C, Pittaluga A, Tome AR, Cunha RA, Marchi M. Nicotinic alpha7 receptor activation selectively potentiates the function of NMDA receptors in glutamatergic terminals of the nucleus accumbens. Front Cell Neurosci. 2014;8:332. doi: 10.3389/fncel.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology. 2007;32:377–387. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Llinas RR, Lisman JE. Inhibition of NMDARs in the nucleus reticularis of the thalamus produces delta frequency bursting. Front Neural Circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Liu Z, Wei C, Han J, Liu Y, Zhang X, Ren W. Activation of the D1 receptors inhibits the long-term potentiation in vivo induced by acute morphine administration through a D1-GluN2A interaction in the nucleus accumbens. Neuroreport. 2014;25:1191–1197. doi: 10.1097/WNR.0000000000000245. [DOI] [PubMed] [Google Scholar]
- Zhou SJ, Xue LF, Wang XY, Jiang WG, Xue YX, Liu JF, He YY, Luo YX, Lu L. NMDA receptor glycine modulatory site in the ventral tegmental area regulates the acquisition, retrieval, and reconsolidation of cocaine reward memory. Psychopharmacology (Berl) 2012;221:79–89. doi: 10.1007/s00213-011-2551-6. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]