Abstract
Background
Xinfuli Granule (XG), a compound Chinese herbal medicine, has been effectively used in China for the treatment of heart failure for more than fifty years. This study aimed to investigate the effects and the underlying mechanisms of Xinfuli in rats with doxorubicin-induced cardiotoxicity.
Methods
Sprague–Dawley rats were treated with intraperitoneal injection of Doxorubicin (DOX, 2.5 mg/kg per week) for six weeks, and then randomly divided into four groups which received intragastrically administration of normal saline (control group) or different dosage of XG (0.675 g/kg per day, 1.35 g/kg per day, and 2.7g/kg per day, respectively) for six weeks. Transthoracic echocardiography was performed to evaluate the left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF) before and after the XG treatment and histopathologic changes were also examined. Myocardial cell apoptosis was detected by TUNEL staining. The expression of related genes and proteins were analyzed using immunohistochemical staining.
Results
Compared to those in the control group, rats in XG treated groups showed significantly improved cardiac function and milder cardiac histopathological changes, lower cardiomyocyte apoptosis index, higher expression of Bcl-2 and lower expression of Bax.
Conclusions
Administration of XG improves cardiac function and histopathological changes in rats with doxorubicin-induced cardiotoxicity. These effects are associated with inhibition of cardiomyocyte apoptosis, perhaps via regulation of Bcl-2 and Bax protein expression.
Keywords: Apoptosis, Doxorubicin, Heart failure, Herbal medicine
1. Introduction
Xinfuli Granule (XG) is a compound traditional Chinese medicine consists of extracts from Radix astragali, Radix ginseng, Salvia miltiorrhiza, Scirpus fluviatilis, Rhizoma Alismatis, Angelica sinensis, Semen lepidii, Fructus chaenomelis, Semen arecae, and Ophiopogon japonicusformulation, which has been clinically used for the treatment of chronic heart failure (HF) for more than 50 years in Fuwai Hospital (Beijing, China). Our previous studies have demonstrated that XG could improve cardiac function in patients with heart failure, particularly in those patients with idiopathic dilated cardiomyopathy (DCM).[1] However, the underlying mechanisms of the treatment effects of XG is not clear.
Apoptosis plays important role in the development and progress of HF. Both ischaemic and idiopathic DCM are associated with apoptosis.[2],[3] Recently, several studies have indicated that some traditional Chinese medicine (TCM) with similar formula as XG may attenuate ischemia/reperfusion injury and myocardial injury by suppressing apoptosis.[4],[5] Our present study used a rat model of doxorubicin induced cardiotoxicity to explore the cardioprotective effects of XG on cardiac function and the underlying mechanisms, focusing on the regulation of cell apoptosis.
2. Methods
2.1. Animal model and grouping
Adult male Sprague-Dawley rats (weighing 200–220 g) were provided by the Animal Center of Academy of Military Medical Sciences. The rats were kept in clean and quiet environment with room temperature 22 ± 1 °C, humidity 40 ± 5%, and a 12 h light/dark cycle, and had free access to standard diet and water. They were treated with intraperitoneal injection of DOX saline solution (2.5 mg/kg per week) for six weeks, then the live rats were randomly divided into four groups which received intragastrically administration of normal saline (control group) or low dosage of XG (0.675 g/kg per day, XG-L group, n = 12), medium dosage of XG (1.35 g/kg per day, XG-M group, n = 12) and high dosage of XG (2.7 g/kg per day, XG-H group, n = 12) for six weeks. Twelve normal rats were also selected for normal group. All experimental procedures were approved by the ethic committee for animal use of Academy of Military Medical Sciences and were performed in accordance with their guidelines.
2.2. Echocardiography measurements
Before and after the XG treatment, Transthoracic echocardiography was performed with a 12-MHz phased-array transducer (Sonos 7500, Phillips). Two-dimensional parasternal long axis views were obtained in M-mode at the papillary muscle level. Left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF) were calculated. The parameters were measured over three consecutive cardiac cycles.
2.3. Tissue sample preparation
Rats were fasted for 12 h and then were sacrificed under deep anesthesia. The hearts were excised and immediately, rinsed with pre-cooling saline. The hearts were weighed to calculate the ratio of heart weight to body weight. After fixed 18 h in 10% maldehyde solution, the anterior wall of the hearts were embed in paraffin wax and cut into 4 µm slices for subsequent apoptosis and immunohistochemical analysis.
2.4. Histopathological examination
The Paraffin-embedded slices were stained with hematoxylin-eosin (HE) for morphologic examination. The extent of myocardial damage was evaluated in 200-fold magnification by light microscopy (Leica, Germany).
2.5. Terminal deoxynucleotidyl transferase dUTP Nick end labeling (TUNEL)
The cell apoptosis rate was determined by TUNEL according to manufacturer's instructions (Boster Biological Engineering Co. Ltd, Wuhan, China). Four micrographs were randomly selected. To assess the fraction of apoptotic cells, the count of TUNEL-positive cells were divided to do ratio with the total number of hematoxylin-positive cardiac myocyte nuclei.
2.6. Assessment of the expression of Bax and Bcl-2
The expression of Bax and Bcl-2 was assessed by immunohistochemical staining using corresponding antibodies (Santa Cruz, California, USA), according to the manufacture' instruction. Briefly, CMIAS image analyzer was used to calculate the average optical density value of each field under 400 microscope magnification in 10 random fields, and then the mean values were calculated as representatives of the expression levels of Bcl-2 and Bax.
2.7. Statistical analysis
All data were presented as mean ± SD. Numerical data were analyzed using One-Way analysis of variance (ANOVA) followed by least significant difference (LSD) for multiple comparisons between groups. Statistical analysis was performed with SPSS 16.0 (Santa Cruz, California, USA). A P value of less than 0.05 was considered as statistically significant.
3. Results
3.1. XG improved cardiac function
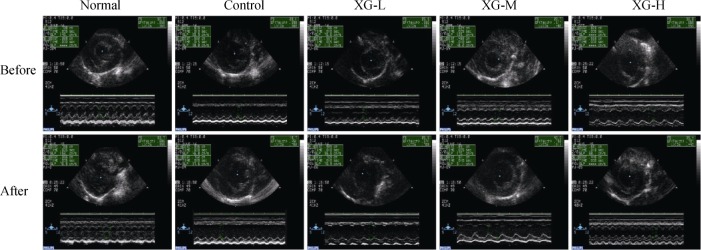
Echocardiography showed that, before XG treatment the rats in control, XG-L, XG-M and XG-H groups displayed significant systolic dysfunction with declined LVEF and LVFS as compared with the normal group (All P < 0.01). After XG treatment, both LVEF and LVFS in XG-M group significantly improved, whereas in the XG-H group, only LVEF increased significantly in comparison with the control group (All P < 0.05), indicating that medium-dose XG treatment could remarkably improve cardiac dysfunction (Figure 1 and Table 1).
Figure 1. Representative echocardiography assessment of a left ventricle from each group.
XG-H: high-dose of Xinfuli Granule group; XG-L: low-dose of Xinfuli Granule group; XG-M: medium-dose of Xinfuli Granule group.
Table 1. Echocardiography results in all groups before and after treatment.
| Groups | Normal | Control | XG-L | XG-M | XG-H | |
| LVEF | Before treatment | 83.38 ± 6.40 | 63.8 ± 6.22** | 62.73 ± 4.37** | 62.88 ± 6.51** | 61.68 ± 9.47** |
| After treatment | 85.8 ± 3.67 | 50.67 ± 8.65** | 52.43 ± 6.62** | 75.38 ± 6.85*, ## | 69.27 ± 11.17*, ## | |
| LVFS | Before treatment | 47.6 ± 5.94 | 30.65 ± 4.26** | 29.88 ± 2.87** | 30.22 ± 4.50** | 29.62 ± 5.98** |
| After treatment | 49.88 ± 4.32 | 28.08 ± 16.44* | 23.88 ± 3.81**, | 39.88 ± 7.02# | 35.53 ± 9.72* |
Data were presented as mean ± SD. **P < 0.01 and *P < 0.05 vs. the normal group; ##P < 0.01 and #P < 0.05 vs. the control group; (b): before XG treatment; (a): after XG treatment. LVEF: left ventricular ejection fraction; LVFS: left ventricular fractional shortening; XG: Xinfuli Granule; XG-H: high-dose of Xinfuli Granule group; XG-L: low-dose of Xinfuli Granule group; XG-M: medium-dose of Xinfuli Granule group.
3.2. XG treatment improves cardiac histopathological changes
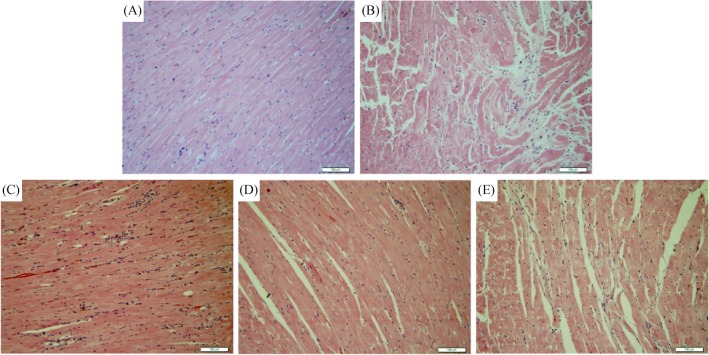
We evaluated the morphological changes associated with doxorubicin-induced myocardial damage using HE staining. As shown in Figure 2, serious myocardial damage characterized by disorganization of myofibrillar arrays, interstitial fibrosis, massive cardiomyocyte loss, cytoplasmic vacuolization, eosinophilic degeneration and intense infiltration of neutrophil granulocytes can be observed in rats of the control group, as shown in. XG treatment, especially when using the medium-dose (1.35 g/kg per day) appeared to alleviate the pathological injuries.
Figure 2. Histopathological changes of the myocardial tissue by HE staining (10 × 20).
(A): Normal group; (B): control group; (C): XG-L group; (D): XG-M group; and (E): XG-H group. XG-H: high-dose of Xinfuli Granule group; XG-L: low-dose of Xinfuli Granule group; XG-M: medium-dose of Xinfuli Granule group.
3.3. XG treatment inhibits myocardial apoptosis
To determine the effect of XG treatment on myocardial apoptosis, TUNEL staining was conducted. In the normal rats, few TUNEL-positive cells were seen in the myocardium (Figure 3A). On the contrary, a large number of TUNEL-positive cells were visible (Figure 3B), and cardiomyocyte apoptosis was markedly induced in the control group (apoptotic rate: 17.78 ± 1.56% vs. 3.37 ± 0.39, P < 0.01). Noticeably, cardiomyocyte apoptosis was signifi cantly inhibited by the administration of XG, for the number of TUNEL-positive cells and the apoptotic rate (Figure 3F) in the three XG groups were significantly reduced (all P < 0.01, as compared with the control group). Meanwhile, the apoptotic rate of the XG-M group was significantly lower than the other two treatment groups (both P < 0.05).
Figure 3. The effect of XG on myocardial cells apoptosis using TUNEL from each group (× 400).
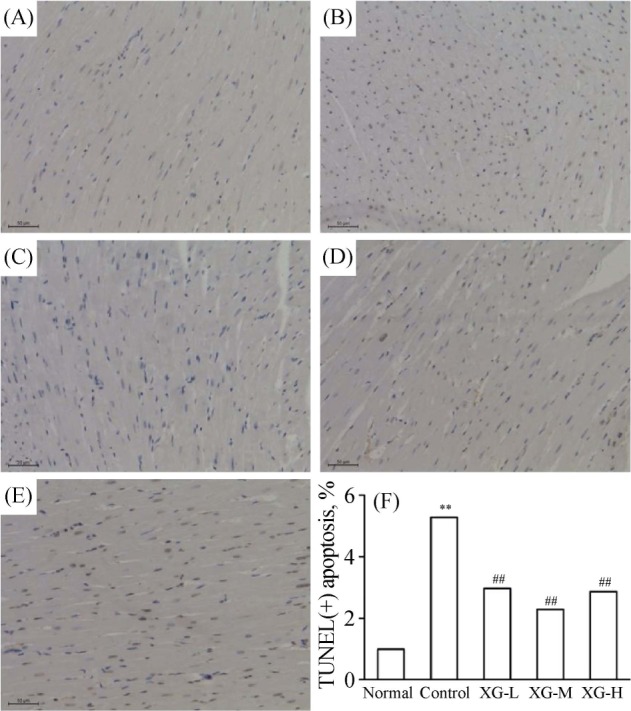
**P < 0.01 vs. normal group; ##P < 0.01 vs. control group. (A): Normal group; (B): control group; (C): XG-L group; (D): XG-M group; (E): XG-H group; (F): Bars present the percentage of TUNEL positive cells relative to total cells. TUNEL: transferase dUTP Nick end labeling; XG: Xinfuli Granule; XG-H: high-dose of Xinfuli Granule group; XG-L: low-dose of Xinfuli Granule group; XG-M: medium-dose of Xinfuli Granule group.
3.4. XG treatment regulate Bcl-2 and Bax expression in myocardial tissues
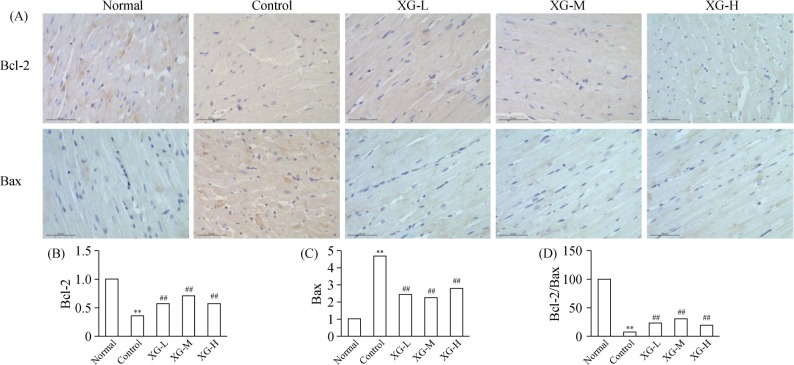
To further confirm the anti-apoptosis of XG, we use the immunohistochemistry to quantitate the expression of Bcl-2 and Bax protein in the left ventricle myocardial tissues from all the groups. As shown in Figure 4, the expression of Bcl-2 (0.068 ± 0.015 vs. 0.360 ± 0.080) and ratio of Bcl-2/Bax (0.188 ± 0.009 vs. 4.812 ± 0.851) were significantly decreased in control group as compared with those of the normal rats (both P < 0.01), whereas Bax protein expression was significantly increased (0.188 ± 0.013 vs. 0.04 ± 0.008, P < 0.01). XG treatment significantly up-regulate the Bcl-2 expression while down-regulate the Bax expression, (Figure 4B–D).
Figure 4. The expression of Bcl-2 and Bax protein in the left ventricle myocardial tissues (× 400).
(A): The representative protein products of Bcl-2 and Bax protein in normal, control, XG-L, XG-M and XG-H group were measured by immunohistochemistry analysis; (B): the expression of Bcl-2 protein in each group; (C): Bax protein expression in each group; (D): the ratio of Bcl-2/Bax in each group. **P < 0.01 vs. normal group; ##P < 0.01 vs. control group. XG-H: high-dose of Xinfuli Granule group; XG-L: low-dose of Xinfuli Granule group; XG-M: medium-dose of Xinfuli Granule group.
4. Discussion
In the present study, we showed that in rats with doxorubicin-induced cardiotoxicity, administration of XG improves cardiac function and histopathological changes and these effects are associated with lower cardiomyocyte apoptosis index, higher expression of Bcl-2 and lower expression of Bax.
The protective effects of XG on doxorubicin-induced cardiotoxicity observed in our present study may involve multiple mechanisms. For example, Ginsenosides, often found in ginseng, may modulate the Na+/K+-ATPase channels.[6] In a study of Qiliqiangxin, a TCM extracted from 11 herbs, including Radix astragali and Radix ginseng, Tao, et al.[7] showed that this TCM formula can ameliorate adverse cardiac remodeling, decreased apoptosis and reduced fibrosis in mice with acute myocardial infarction. Tong, et al.[5] had reported that the myocardial protection function of Qishen Yiqi formula (QSYQ), which consists Radix Salvia miltiorrhiza, Panax notoginseng, Dalbergia odorifera, and Astragalus membranaceus, may relate to the reduction of myocardial cell apoptosis in adriamycin-induced cardiomyopathy animal model. Ling, et al.[8] reported that a pharmaceutical preparation of Salvia miltiorrhiza, which is also a competent of XG, can protect cardiomyocytes from tumor necrosis factor-induced apoptosis and reduces angiotensin II-stimulated collagen synthesis in fibroblasts.
Apoptosis is a tightly regulated complex process which involves abundant pro- and anti-apoptotic molecules, of which Bcl-2 family of proteins is the most important.[9] The Bcl-2 family of proteins consists of anti-apoptotic (Bcl-2 and Bcl-xL) and pro-apoptotic (Bad, Bak, and Bax) proteins.[10],[11] The ratio (Bcl-2/Bax) is often used for showing the extent of myocardial apoptosis. Studies have confirmed that cardiac-specific over-expression of Bcl-2 can enhance the resistance to apoptosis and protect the cardiac function, which suggests the protective role of anti-apoptotic Bcl-2 in the heart.[12]–[14] Knockout of Bax in mice hearts also reduced cell apoptosis and improved myocardial function.[15] Upregulation of Bax/Bcl-2 ratio has been observed during the transition to pressure overload-induced heart failure.[16] Our study found that the expression of Bcl-2 protein and the ratio of Bcl-2/Bax in rats of the XG treatment groups were significantly increased while Bax protein decreased compared with the control group, which indicated that the inhibition of apoptosis of XG treatment was partly through increasing Bcl-2 protein expression and reducing expression of Bax protein.
In conclusion, our present study showed that administration of XG improves cardiac function and histopathological changes in rats with doxorubicin-induced cardiotoxicity. These effects are associated with inhibition of cardiomyocyte apoptosis, perhaps via regulation of Bcl-2 and Bax protein expression.
Acknowledgments
This study was supported by the grants from the “Ten Chinese Medicine for Ten Diseases” Project of Beijing, China (SBSY2013-005), National Science Foundation of China (81541010) and Capital Medical Development Scientific Research Fund (2014-4-4035).
References
- 1.Ma LH, Jiao ZM, Qu JZ, et al. Clinical observation of Xinfuli Granule for the treatment of dilated cardiomyopathy. Chin J Tradit Chin Med Pharm. 2006;18:126–128. [Google Scholar]
- 2.Abbate A, Biondi-Zoccai GG, Bussani R, et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41:753–760. doi: 10.1016/s0735-1097(02)02959-5. [DOI] [PubMed] [Google Scholar]
- 3.Kang PM, Izumo S. Apoptosis and heart failure: a critical review of the literature. Circ Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Xu L, Qiao Z, et al. YiXin-Shu, a ShengMai-San-based traditional Chinese medicine formula, attenuates myocardial ischemia/reperfusion injury by suppressing mitochondrial mediated apoptosis and upregulating liver-X-receptor alpha. Sci Rep. 2016;6:23025. doi: 10.1038/srep23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong JY, Xu YJ, Bian YP, et al. Effect and mechanism of Qishen Yiqi Pills on adriamycin-induced cardiomyopathy in mice. Chin J Nat Med. 2013;11:514–518. doi: 10.1016/S1875-5364(13)60093-X. [DOI] [PubMed] [Google Scholar]
- 6.Tang WH, Huang Y. Cardiotonic modulation in heart failure: insights from traditional Chinese medicine. J Am Coll Cardiol. 2013;62:1073–1074. doi: 10.1016/j.jacc.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao L, Shen S, Fu S, et al. Traditional Chinese medication Qiliqiangxin attenuates cardiac remodeling after acute myocardial infarction in mice. Sci Rep. 2015;5:8374. doi: 10.1038/srep08374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling S, Luo R, Dai A, et al. A pharmaceutical preparation of Salvia miltiorrhiza protects cardiac myocytes from tumor necrosis factor-induced apoptosis and reduces angiotensin II-stimulated collagen synthesis in fibroblasts. Phytomedicine. 2009;16:56–64. doi: 10.1016/j.phymed.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Kim NH, Kang PM. Apoptosis in cardiovascular diseases: mechanism and clinical implications. Korean Circ J. 2010;40:299–305. doi: 10.4070/kcj.2010.40.7.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 11.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Chua CC, Ho YS, et al. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 13.Mayorga M, Bahi N, Ballester M, et al. Bcl-2 is a key factor for cardiac fibroblast resistance to programmed cell death. J Biol Chem. 2004;279:34882–34889. doi: 10.1074/jbc.M404616200. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee S, Stewart AS, Bish LT, et al. Viral gene transfer of the antiapoptotic factor Bcl-2 protects against chronic postischemic heart failure. Circulation. 2002;106:I212–I217. [PubMed] [Google Scholar]
- 15.Hochhauser E, Cheporko Y, Yasovich N, et al. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007;47:11–20. doi: 10.1385/cbb:47:1:11. [DOI] [PubMed] [Google Scholar]
- 16.Garg S, Narula J, Chandrashekhar Y. Apoptosis and heart failure: clinical relevance and therapeutic target. J Mol Cell Cardiol. 2005;38:73–79. doi: 10.1016/j.yjmcc.2004.11.006. [DOI] [PubMed] [Google Scholar]