Abstract
Background. The intensive use of insecticides in public health and agriculture has led to the development of insecticide resistances in malaria vectors across sub-Saharan Africa countries in the last two decades. The kdr target site point mutation which is among the best characterised resistance mechanisms seems to be changing its distribution patterns on the African continent. The 1014F kdr mutation originally described only in West Africa is spreading to East Africa while the 1014S kdr mutation originally described in East Africa, is spreading to West and Central Africa. However, the East- kdr mutation has not been reported in Côte d'Ivoire so far.
Methods. Immature stages of Anopheles gambiae s.l. were collected from breeding sites at the outskirts of Yamoussoukro, Côte d'Ivoire. Emerging 3–5 day old adult female mosquitoes were tested for susceptibility to deltamethrin 0.05%, malathion 5%, bendiocarb 1% and dichlorodiphenyltrichloroethane (DDT) 4% according to WHO standard procedures. A total of 50 An. gambiae s.l. specimens were drawn at random for DNA extraction and identification down to the species level. A subsample of 30 mosquitoes was tested for the East-African kdr mutation using a Taqman assay.
Results. The tested mosquito population appeared to be strongly resistant to deltamethrin (1.03% mortality), bendiocarb (38.46% mortality) and DDT (0% mortality) with probable resistance observed for malathion (92.47%). Among the 41 mosquitoes that were successfully characterized, An. coluzzii was predominant (68.3%) followed by An. gambiae s.s. (19.5%) and a few hybrids (7.3%). Out of 30 specimens genotyped for East- kdr, a single hybrid mosquito appeared to be heterozygous for the mutation.
Conclusion. The present study revealed the presence of the East- kdr mutation in Côte d’Ivoire for the first time in An. gambiae and highlights the urgent need to start monitoring the allele and genotype frequencies.
Keywords: Malaria, Anopheles gambiae, Vector control, Insecticide resistance, kdr mutation
Introduction
The implementation of malaria control strategies, such as the spraying of residual insecticides and the use of insecticide-treated mosquito nets have led to enormous progress in the control of malaria in Sub-Saharan Africa ( Hemingway, 2014). However, only four classes of insecticides are available for public health purposes, namely pyrethroids, carbamates, organophosphates and organochlorines. Since the discovery of pyrethroids, the only compounds currently recommended for impregnation of mosquito nets owing to their efficacy and safety for humans, no alternative insecticides have been identified. This strong reliance on the same molecules is inevitably translated into a heavy pressure on the target mosquitoes, which consequently have developed resistances to these compounds. The role of the agricultural sector in this selection of resistances remains predominant since anopheline larvae breed in and around farm settings in rural areas. Rice and vegetable cultivation, where 90% of insecticides used are pyrethroids, are of particular concern ( Chouaïbou et al., 2016). Induced resistances may involve increased degradation of the insecticide (so-called metabolic resistance involving three families of enzymes: P-450, esterases and glutathion-S-transferase) ( Hemingway et al., 2004) or a modification of the target preventing the insecticide from reaching its site of action (resistance-based target site point mutation) ( Martinez-Torres et al., 1998; Ranson et al., 2000; Weill et al., 2004).
The kdr and Ace-1 mutations are among the best characterised point mutations. Previous studies aiming at estimating the frequency of the kdr mutation and its distribution across the African continent have shown that the 1014F kdr mutation first described only in West Africa ( Awolola et al., 2005; Diabate et al., 2004; Tripet et al., 2007; Yawson et al., 2004) has spread to East Africa ( Kulkharni et al., 2006; Ochomo et al., 2015; Verhaeghen et al., 2006). Vice versa, the 1014S kdr mutation originally described only in East Africa, was observed in West and Central Africa in recent years ( Badolo et al., 2012; Nwane et al., 2011). The underlying resistance mechanism of both East- and West- kdr mutation is responsible for cross-resistance to dichlorodiphenyltrichloroethane (DDT) and pyrethroids ( Martinez-Torres et al., 1998; Ranson et al., 2000).
In Côte d’Ivoire resistances to insecticides of four classes used for vector control are prevalent and involve multiple mechanisms (i.e. West- kdr, Ace-1, P450s) ( Edi et al., 2014). However, the East- kdr mutation has not been reported in this country so far.
Methods
Mosquito sampling and susceptibility testing
Immature stages of An. gambiae s.l. were collected from breeding sites in October 2015 in rice fields at the outskirts of the city of Yamoussoukro (6°49′13″ N/5°16′36″ W) as part of a large insecticide resistance monitoring study across several cities in Côte d'Ivoire. Larvae sampled from different breeding sites were pooled and allowed to emerge as adults at the insectary of the Centre Suisse de Recherches Scientifiques en Côte d’Ivoire (CSRS). Emerging 3–5 day old adult female mosquitoes were tested for insecticide susceptibility. WHO standard procedures ( WHO, 2013) were followed to monitor the susceptibility of populations to the four chemical groups of insecticides commonly used in public health and agriculture including pyrethroids (deltamethrin 0.05%), organophosphates (malathion 5%), carbamates (bendiocarb 1%) and organochlorines (DDT 4%). Batches of 25 unfed mosquitoes were exposed to insecticide impregnated filter papers obtained from the WHO reference centre at the University Sains Malaysia (Penang, Malaysia). For each test session, 100 mosquitoes (four batches of 25 mosquitoes) were exposed to each insecticide and 50 mosquitoes (two batches of 25 mosquitoes) were exposed to untreated filter papers to serve as controls. The same procedure was carried out using reference susceptible An. gambiae mosquitoes (Kisumu strain) obtained from Liverpool School of Tropical Medicine in order to assess the quality of the papers used. All bioassays were conducted at insectary conditions (temperature of 25–27°C and 70–90% relative humidity) and WHO criteria were used as a guideline to assess the phenotypic resistance status of the tested mosquito populations ( WHO, 2013). According to those criteria, resistance is demonstrated by mortality rates <90%, 90–98% suggests increased tolerance (resistance has to be confirmed) and mortality rates >98% are indicative of susceptibility.
Molecular identification and kdr genotyping
For molecular identification, 50 An. gambiae s.l. specimens were drawn at random from the pool of unexposed mosquitoes (bioassay controls) for DNA extraction. Individual mosquitoes were identified down to their species level using the Sine-PCR method ( Santolamazza et al., 2008). Among these, 30 mosquitoes were tested for the East-African kdr mutation applying a Taqman assay ( Bass et al., 2010). The reaction was performed using an Agilent Stratagene MX3005 qPCR thermal cycler in 10 μl final volume containing SensiMix from Bioline, a primer coupled to a probe and water. The cycling conditions used were 10 min at 95°C, 40 cycles of 10 s at 95°C and 45 s at 60°C. Two probes labelled with fluorochromes FAM and HEX were used to detect the mutant allele and the wild type susceptible allele, respectively. Genotypes were scored after real time amplification as dual colour scatter plots produced by the MX3005P v4.10 software.
Results
Resistance status
The An. gambiae Kisumu reference strain exhibited full susceptibility (100% mortality) to all the insecticide treated papers confirming appropriate quality. Mortality in the control groups was consistently below 5%, and thus did not require any correction. Based on WHO criteria ( WHO, 2013), the wild mosquito population of Yamoussoukro appeared to be strongly resistant to deltamethrin (1.03% mortality), bendiocarb (38.46% mortality) and DDT (0% mortality). A probable resistance was, in addition, seen for malathion with a mortality rate of 92.47%.
Species identification and kdr genotyping
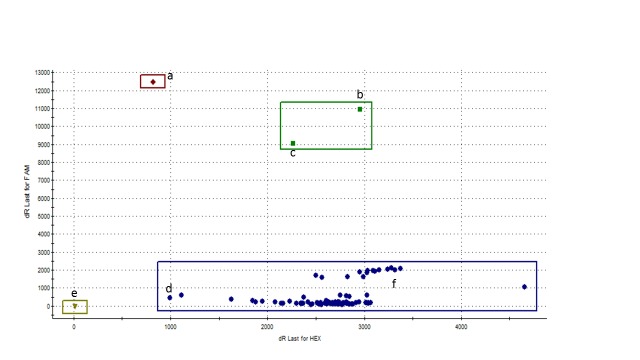
Out of the 50 mosquitoes that were used for DNA extraction, the species was successfully characterised in 41. Among these, An. coluzzii (former M molecular form) was predominant (n=28; 68.3%) followed by An. gambiae s.s. (former S molecular form) (n=8; 19.5%) and a few hybrids (M/S) (n=3; 7.3%). From the 30 specimens genotyped for East- kdr, a single hybrid (M/S) mosquito appeared to be heterozygous for the mutation ( Figure 1). Based on the fact that this specimen was heterozygous, it was further characterized for West- kdr to verify that it was not a double mutation West-East- kdr. However, it appeared to be negative for the West- kdr mutation.
Figure 1. East African kdr genotype of wild Yamoussoukro Anopheles gambiae s.l. population.
The ‘a’, ‘b’, ‘d’ and ‘e’ on the figure are the positive controls respectively for the homozygous mutant allele, heterozygous mutant/susceptible allele, homozygous susceptible allele and blank. One single mosquito (‘c’) displayed the heterozygous mutant/susceptible allele as it appeared to be susceptible for West- kdr mutation. All the other samples (‘f’) displayed the susceptible alleles.
Discussion
At least 64 countries with on-going malaria transmission around the globe have reported resistance to at least one insecticide in at least one vector ( WHO, 2012). In Côte d'Ivoire, resistance to the four families of insecticides was described within the same vector population simultaneously (Behi Fodjo and Mouhamadou Chouaibou, personal communication; Edi et al., 2012) involving several mechanisms at the same time ( Edi et al., 2012). The present study describes the East- kdr mutation for the first time in Côte d'Ivoire and delivers further proof for a pan-African propagation of the kdr resistance phenomena. The alarming new occurrence of this mutation in Ivorian anopheles mosquito populations represents a major threat to on-going vector control activities as the current strategy of the National Malaria Control Program in the country is heavily based on the use of long lasting nets. According to WHO, with the current level of global vector control coverage, about 220 000 children under the age of five are saved each year ( WHO, 2012); if pyrethroids were to lose their effectiveness due to resistances this number could decrease significantly. Moreover, some laboratory studies have shown that female anopheline mosquitoes with insecticide resistance alleles affect vector competence by increasing susceptibility to plasmodium ( Alout et al., 2013; Ndiath et al., 2014). If the same applies to field populations, then we are facing a potential dramatic increase in malaria transmission. Thus, there is an urgent need to carry out concrete actions for resistance management by using completely new mode of action insecticides and not reformulating insecticides already in use for agriculture. These insecticides could be alternated in time (rotation) and in space (mosaic) or simultaneously (mixture). The rotation and mosaic methods are based on the principles of limiting the duration of exposure of each insecticide to the target. The strategy of mixing insecticides is based on the assumption that the compounds could act in synergy, or that each insecticide in the mixture will be able to eliminate those individuals that are susceptible to it. The Innovative Vector Control Consortium (IVCC) has promised to deliver three new insecticides with totally novel and different modes of action by 2022. Using insecticides with different modes of action in rotation, mixture or mosaic could break the cycle of insecticide resistance and underpin a global malaria elimination and eradication program.
Conclusion
The present study confirms the presence of the East- kdr mutation in Côte d’Ivoire for the first time in An. gambiae. Even though the mutation was observed in a single mosquito at the heterozygous state it is of utmost importance to start monitoring the allele and genotype frequencies. This study should further aid the rational planning of insecticide resistance management deployed in Côte d’Ivoire and more widely in Africa.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2017 Chouaïbou M et al.
Data from: First report of the East African kdr mutation in an Anopheles gambiae mosquito in Côte d’Ivoire DOI: 10.6084/m9.figshare.4564831.v1 (Chouaïbou et al., 2017).
Acknowledgement
We thank Jasmina Saric (Grants and Publications Support Unit, CSRS) for the revision of the manuscript. We are grateful to Laurette DJOSSOU (from Institut Régional de Santé Publique/Université d’Abomey-Calavi) for here technical support.
Funding Statement
This work was supported by the Wellcome Trust [103995].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- Alout H, Ndam NT, Sandeu MM, et al. : Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates. PLoS One. 2013;8(5):e63849. 10.1371/journal.pone.0063849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolola TS, Oyewole IO, Amajoh CN, et al. : Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta Trop. 2005;95(3):204–209. 10.1016/j.actatropica.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Badolo A, Traore A, Jones CM, et al. : Three years of insecticide resistance monitoring in Anopheles gambiae in Burkina Faso: resistance on the rise? Malar J. 2012;11:232. 10.1186/1475-2875-11-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass C, Nikou D, Vontas J, et al. : The Vector Population Monitoring Tool (VPMT): High-Throughput DNA-Based Diagnostics for the Monitoring of Mosquito Vector Populations. Malar Res Treat. 2010;2010:190434. 10.4061/2010/190434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaïbou M, Fodjo BK, Fokou G, et al. : Influence of the agrochemicals used for rice and vegetable cultivation on insecticide resistance in malaria vectors in southern Côte d'Ivoire. Malar J. 2016;15:426. 10.1186/s12936-016-1481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabate A, Brengues C, Baldet T, et al. : The spread of the Leu-Phe kdr mutation through Anopheles gambiae complex in Burkina Faso: genetic introgression and de novo phenomena. Trop Med Int Health. 2004;9(12):1267-1273. 10.1111/j.1365-3156.2004.01336.x [DOI] [PubMed] [Google Scholar]
- Edi CA, Koudou BG, Bellai L, et al. : Long-term trends in Anopheles gambiae insecticide resistance in Côte d’Ivoire. Parasit Vectors. 2014;7:500. 10.1186/s13071-014-0500-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edi CV, Koudou GB, Jones CM, et al. : Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Côte d'Ivoire. Emerg Infect Dis. 2012;18(9):1508–1511. 10.3201/eid1809.120262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Hawkes NJ, McCarroll L, et al. : The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34(7):653-65. 10.1016/j.ibmb.2004.03.018 [DOI] [PubMed] [Google Scholar]
- Hemingway J: The role of vector control in stopping the transmission of malaria: threats and opportunities. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130431. 10.1098/rstb.2013.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkharni MA, Rowland M, Alifrangis M, et al. : Occurrence of the leucine-to-phenylalanine knockdown resistance ( kdr) mutation in Anopheles arabiensis populations in Tanzania, detected by a simplified high-throughput SSOP-ELISA method. Malar J. 2006;5:56. 10.1186/1475-2875-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torres D, Chandre F, Williamson MS, et al. : Molecular characterization of pyrethroid knockdown resistance ( kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7(2):179–184. 10.1046/j.1365-2583.1998.72062.x [DOI] [PubMed] [Google Scholar]
- Mouhamadou C: RTPCR_ekdr_Ykro_Chouaibou.txt. Figshare. 2017. Data Source [Google Scholar]
- Ndiath MO, Cailleau A, Diedhiou SM, et al. : Effects of the kdr resistance mutation on the susceptibility of wild Anopheles gambiae populations to Plasmodium falciparum: a hindrance for vector control. Malar J. 2014;13:340. 10.1186/1475-2875-13-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwane P, Etang J, Chouaїbou MS, et al. : Kdr-based insecticide resistance in Anopheles gambiae s.s populations in Cameroon: spread of the L1014F and L1014S mutations. BMC Res Notes. 2011;4:463. 10.1186/1756-0500-4-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochomo E, Subramaniam K, Kemei B, et al. : Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An. arabiensis in western Kenya. Parasite Vector. 2015;8:616. 10.1186/s13071-015-1223-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, Jensen B, Vulule JM, et al. : Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9(5):491–497. 10.1046/j.1365-2583.2000.00209.x [DOI] [PubMed] [Google Scholar]
- Santolamazza F, Mancini E, Simard F, et al. : Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163. 10.1186/1475-2875-7-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet F, Wright J, Cornel A, et al. : Longitudinal survey of knockdown resistance to pyrethroid ( kdr) in Mali, West Africa, and evidence of its emergence in the Bamako form of Anopheles gambiae s.s. Am J Trop Med Hyg. 2007;76(1):81–87. [PubMed] [Google Scholar]
- Verhaeghen K, Van Bortel W, Roelants P, et al. : Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malar J. 2006;5:16. 10.1186/1475-2875-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill M, Malcolm C, Chandre F, et al. : The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol. 2004;13:1–7. 10.1111/j.1365-2583.2004.00452.x [DOI] [PubMed] [Google Scholar]
- WHO: Global plan for insecticide resistance management in malaria vectors (GPIRM).World Health Organization, Geneva;2012. Reference Source [Google Scholar]
- WHO: Test procedures for insecticide resistance monitoring in malaria vector mosquitoes.World Health Organization, Geneva;2013. Reference Source [Google Scholar]
- Yawson AE, McCall PJ, Wilson MD, et al. : Species abundance and insecticide resistance of Anopheles gambiae in selected areas of Ghana and Burkina Faso. Med Vet Entomol. 2004;18(4):372–377. 10.1111/j.0269-283X.2004.00519.x [DOI] [PubMed] [Google Scholar]