Abstract
Background and Purpose
Oral anticoagulants (OAC) substantially reduce risk of stroke in atrial fibrillation (AF), but uptake is suboptimal. Electronic health records (EHRs) enable automated identification of people at risk but not receiving treatment. We investigated the effectiveness of a software tool (AURAS-AF) designed to identify such individuals during routine care, through a cluster-randomised trial.
Methods
Screen reminders appeared each time the EHR of an eligible patient was accessed until a decision had been taken over OAC treatment. Where OAC was not started, clinicians were prompted to indicate a reason. Control practices continued usual care. The primary outcome was the proportion of eligible individuals receiving OAC at six months. Secondary outcomes included rates of cardiovascular events and reports of adverse effects of the software on clinical decision making.
Results
Forty-seven practices were randomised. The mean proportion prescribed OAC at 6 months was 66.3% (SD=9.3) in the intervention arm and 63.9% (9.5) in the control arm, adjusted difference: 1.21% (95% CI -0.72, 3.13). Incidence of recorded transient ischaemic attack (TIA) was higher in the intervention practices (median 10.0 versus 2.3 per 1000 patients with AF, P=0.027), but at twelve months we found a lower incidence of both all cause stroke (p=0.06) and haemorrhage (p=0.054). No adverse effects of the software were reported.
Conclusions
No significant change in OAC prescribing occurred. A greater rate of diagnosis of TIA (possibly due to improved detection or over-diagnosis) was associated with a reduction (of borderline significance) in stroke and haemorrhage over 12 months.
Clinical trial registration
ISRCTN55722437.
Indexing terms: Atrial fibrillation, Anticoagulants, Stroke, Reminder systems, Electronic Health Records
Subject terms: Atrial Fibrillation, Primary Prevention, Health Services
Introduction
Atrial fibrillation (AF) is a major risk factor for thromboembolic stroke.[1] Oral anticoagulants (OACs) reduce stroke risk in AF by 60-70%[2–3] but their uptake is suboptimal.[4–5] Risk factors for stroke are generally well recorded in UK primary care electronic health records (EHRs), providing an opportunity for automated risk assessment. We developed a software tool, AURAS-AF within a web-based EHR system and conducted a cluster-randomised trial to measure its impact and confirm its safety.[6]
Methods
This was a cluster-randomised trial across practices in South East and Central England.
Intervention
The AURAS-AF tool drew on the data in the records of patients with AF, and identified those fulfilling the eligibility criteria for OAC at that time.[7]
It functioned in two ways:
At the start of the trial, practices were asked to invite a list of eligible patients for a discussion over anticoagulants.
If a patient identified as eligible for but not using OAC was seen at the practice by a clinician, a screen reminder message would appear.
The tool was designed to challenge clinicians to justify treatment decisions at the point of care, when the patient would be present. There was no requirement imposed by the trial to adhere to guidelines or follow any specific treatment pathway.
Control practices
Practices allocated to the control arm continued to provide usual care to AF patients, including the requirements of the Quality and Outcomes Framework (QOF) funding system.[8]
Outcome measures
Primary outcome
The primary outcome was the proportion of patients eligible for OAC who were currently prescribed an OAC at the end of the 6 months intervention period.
Secondary outcomes
1) Proportion with CHADS2 score ≥2 currently prescribed OAC at 6 months; 2) Practices were asked to report instances of inappropriate clinical or prescribing decisions related to anticoagulation in patients with AF; Incidences of: 3) Thromboembolic stroke, TIA, or systemic (arterial) thromboembolism; 4) Haemorrhagic stroke and other haemorrhagic events; Incidence rates for the following events were added on the advice of the Data Monitoring Committee: 5) Thromboembolic stroke; 6) TIA; 7) Systemic thromboembolism; 8) Haemorrhagic stroke; 9) Other haemorrhagic events; 10) Unspecified stroke events; 11) All cause stroke (thromboembolic, haemorrhagic or unspecified).
All outcomes were measured at 6 months and repeated at 12 months (6 months following the end of the intervention period).
Audit of cardiovascular events
Each thromboembolic or haemorrhagic event occurring during the intervention period was investigated to identify whether it might have resulted from use of the software, for instance through inappropriate prescribing decisions.
Allocation
Practices were randomised with an allocation ratio of 1:1, minimised on practice list size and proportion of eligible patients with AF prescribed OACs at baseline.
Data collection
Anonymised outcome data from the practices were extracted via a virtual private network (VPN) linked to Oxford University. This source includes all electronically coded information, including diagnoses and medication.
Sample size
We estimated that a sample of 46 practices would be needed for 95% power to detect a relative difference of 25% in the primary outcome with 5% significance.[6]
Statistical analysis
Statistical analyses were undertaken using SPSS version 22 and Stata 13 on an intention to treat basis. Cluster summary measures were analysed using a weighted linear regression model with the minimisation variables fitted as covariates. If assumptions of linear regression were violated, a Mann-Whitney U test was applied.
Results
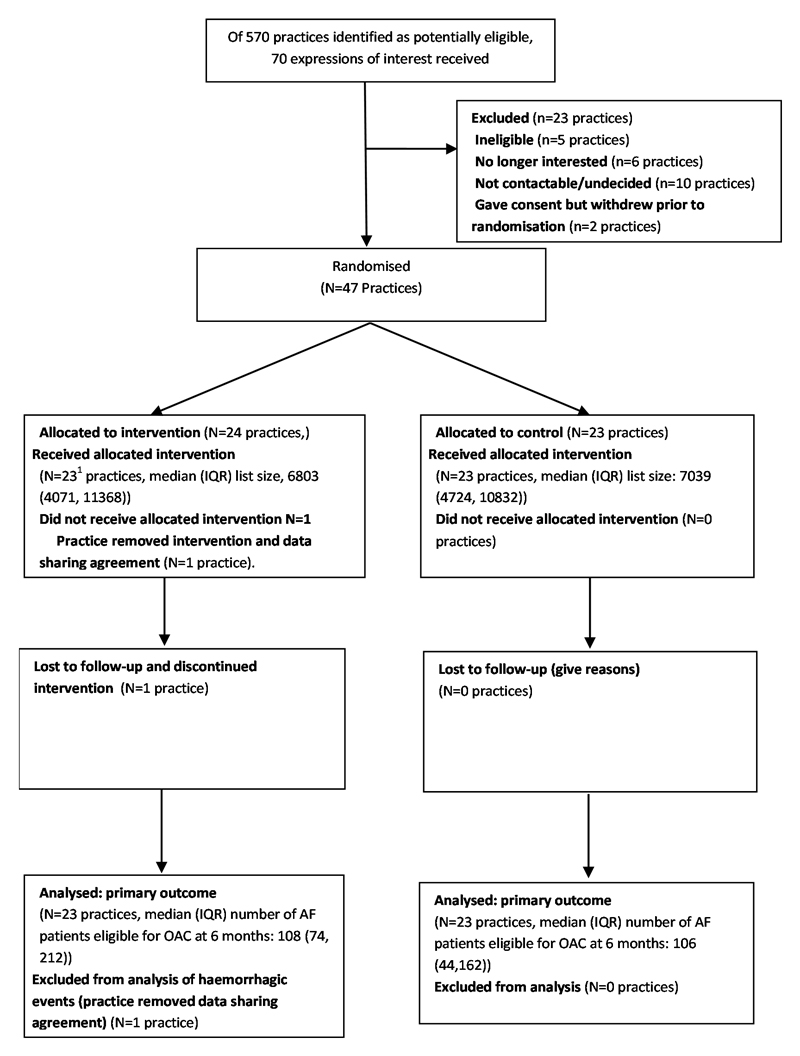
We approached 570 potentially eligible practices, 70 expressed interest, and 47 were randomised (Figure). One withdrew during the first three months of the trial, leaving 46 in the intention to treat sample. These provided a combined patient population of 359,937 with 6,429 patients with AF at baseline (20th February 2014), of which 5,339 (83%) were eligible for OAC and of these, 3,340 (62.6%) were already treated. The population characteristics were similar in each arm (Table).
Flow diagram of trial participants.
Table. Baseline characteristics and incidence of cardiovascular events in AF patients over 6 and 12 months.
| Control | Intervention | |||
|---|---|---|---|---|
| Baseline characteristics | Median (IQR) unless indicated | |||
| List size | 7039 (4724, 10832) | 6803 (4071, 11368) | ||
| Prevalence of AF per 100 patients | 1.71 (1.11, 2.20) | 1.92 (1.77, 2.21) | ||
| Proportion of AF patients who are female | 44.6% (40.9, 50.0) | 46.3% (42.4, 50.0) | ||
| Proportion of AF patients under 80 years | 56.5% (51.0, 62.3) | 56.9% (51.6, 59.8) | ||
| Proportion eligible for OAC and prescribed OAC at baseline1 | 61.9% (9.89) | 63.5% (8.85) | ||
| Events | Incidence (patients with at least one event per 1000 AF patients) | P Value2 | ||
| Thromboembolic stroke, TIA or other major thromboembolism | N=23 | N=23 | ||
| 6 months | 0 (0, 7.75) | 10.3 (0, 16.3) | 0.03 | |
| 12 months | 12.6 (0, 22.3) | 14.5 (4.2, 26.1) | 0.41 | |
| Haemorrhage (including haemorrhagic stroke)3 | 6 months | 26.5 (15.0)1 | 21.0 (14.7)1 | 0.414 |
| 12 months | 50.3 (33.4, 57.3) | 34.7 (27.4, 43.6) | 0.054 | |
| All cause stroke3 | 6 months | 8.5 (0, 17.7) | 7.9 (0, 13.4) | 0.43 |
| 12 months | 24.8 (19.3, 28.9) | 15 (9.1, 28.3) | 0.06 | |
| Transient ischaemic attack | 6 months | 0 (0,0) | 6.4 (0, 12.2) | 0.008 |
| 12 months | 2.3 (0, 9.0) | 10.0 (4.2, 18.2) | 0.027 | |
| Thromboembolic stroke | 6 months | 0 (0, 0) | 0 (0, 4.1) | 0.40 |
| 12 months | 0 (0, 12.8) | 0 (0, 5.0) | 0.36 | |
| Haemorrhagic stroke3 | 6 months | 0 (0,0)1 | 0 (0,0)1 | 0.82 |
| 12 months | 0 (0, 0.96) | 0 (0, 3.14) | 0.92 | |
| Unspecified stroke | 6 months | 5.2 (0, 16.4) | 3.2 (0, 9.4) | 0.26 |
| 12 months | 17.0 (11.4)1 | 13.3 (11.0)1 | 0.394 | |
Mean (SD)
Mann Whitney U test
N=22 in the control arm for haemorrhagic event searches (see Figure). The other searches were unaffected.
P value obtained from weighted linear regression.
Primary outcome
The mean proportion (SD) of eligible patients prescribed OAC at six months was 66.3% (9.25) in the intervention arm and 63.9% (9.46) in the control arm. The adjusted mean difference (95% CI) was 1.21% (-0.72, 3.13), P=0.213.
Secondary outcomes
The proportion in the subgroup with a CHADS2 score ≥2 prescribed anticoagulants at the end of the study was not significantly different between trial arms. There were no reports of inappropriate clinical or prescribing decisions or cardiovascular events triggered by use of the software. Practice based searches supporting the cardiovascular event audit confirmed the validity of the remote data extraction.
The Table gives the incidence of cardiovascular events during the 6 and 12 month time periods following randomisation. The increased rate of thromboembolic events in the intervention arm is due to a higher rate of TIA diagnosis with no increase in thromboembolic stroke or unspecified stroke. In fact there is a reduction of borderline significance in strokes of all types (P=0.06) and of haemorrhage (p=0.054) at 12 months.
Discussion
Strengths and limitations
This was a pragmatic randomised trial involving a diverse range of practices across a wide geographical area using a modern, web-based EHR platform.
Comparison to other studies
The findings concur with other studies demonstrating small or modest impacts of reminder interventions on clinician behaviour.[9]
Interpretation
Since the trial was conceived there has been a refocussing of the identification problem away from those eligible for OAC and towards the minority who do not require it,[10] making stroke risk assessment less important compared with other more difficult barriers. Decisions over anticoagulation may take longer to make than our six month intervention period. A longer follow up might also be required to confirm the improvements in stroke and haemorrhage rates suggested by our data.
Conclusions
Use of the software was associated with no significant change in prescribing, but improved stroke and haemorrhage rates (of borderline significance) at 12 months.
Acknowledgements
We thank the National Institute for Health Research (NIHR) Clinical Research Network (Primary Care), participating practices, Egton Medical Information Systems, the Data Monitoring and Trial Steering Committees, and the charities Arrhythmia Alliance and the Atrial Fibrillation Association for their support.
Funding
Funded through a National Institute for Health Research (School for Primary Care Research) grant FR6/173. TH was partly funded by a NIHR Academic Clinical Lectureship. TM was partly funded by the National Institute for Health Research (NIHR) through the Collaborations for Leadership in Applied Health Research and Care for West Midlands (CLAHRC-WM). This article presents independent research funded by the NIHR. The views expressed in this publication are not necessarily those of the NIHR, the Department of Health, NHS Partner Trusts, University of Birmingham or the CLAHRC WM Management Group. FDRH was partly supported by the NIHR School for Primary Care Research, the Oxford Biomedical Research Centre, the NIHR CLAHRC Oxford, and Harris Manchester College, Oxford.
Footnotes
Ethical Approval
NRES Committee South Central – Berkshire, 11th February 2013, ref: 13/SC/0026.
Disclosures
None.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial-fibrillation as an independent risk factor for stroke - the Framingham-study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Edwards SJ. Stroke prevention with aspirin, warfarin and ximelagatran in patients with non-valvular atrial fibrillation: a systematic review and meta-analysis. Thromb Res. 2006;118:321–333. doi: 10.1016/j.thromres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 4.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Holt TA, Hunter TD, Gunnarsson C, Khan N, Cload P, Lip GY. Risk of stroke and oral anticoagulant use in atrial fibrillation: a cross-sectional survey. Brit J Gen Pract. 2012;62:e710–e717. doi: 10.3399/bjgp12X656856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt TA, Fitzmaurice DA, Marshall T, Fay M, Qureshi N, Dalton ARH, et al. AUtomated Risk Assessment for Stroke in Atrial Fibrillation (AURAS-AF) - an automated software system to promote anticoagulation and reduce stroke risk: study protocol for a cluster randomised controlled trial. [Accessed 30th September, 2016];Trials. 2013 14:385. doi: 10.1186/1745-6215-14-385. http://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-14-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence (NICE) NICE; London, England: 2012. [Accessed 30th September, 2016]. Dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation (TA249) p. 9. https://www.nice.org.uk/guidance/ta249?unlid=506042494201625122152. [Google Scholar]
- 8.Quality and Outcomes Framework. Health and Social Care Information Centre website. [Accessed 30th September, 2016]; http://qof.hscic.gov.uk/
- 9.Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database of Systematic Reviews. 2009;(3) doi: 10.1002/14651858.CD001096.pub2. Art. No.: CD001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atrial fibrillation: management. [Accessed 30th September, 2016];NICE Guideline CG180. National Institute for Health and Care Excellence. website. https://www.nice.org.uk/guidance/CG180 Published June 2014 (last updated August 2014)